The Effects of Bilirubin and Lumirubin on the Differentiation of Human Pluripotent Cell-Derived Neural Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Unconjugated BR and LR Preparation
2.2. Cell Cultivation and Differentiation to Neurons
2.3. Additional In Vitro Studies
2.3.1. Growth Curve Analysis
2.3.2. Cytotoxicity Assay
2.3.3. MTT Cell Viability/Metabolic Activity Test
2.3.4. Flow Cytometry Analyses
2.4. Western Blot Analysis
2.5. DNA Damage Analysis (Comet Assay)
2.6. Immunofluorescence and Cell Imaging
2.7. RNA Isolation and Quantitative Real-Time RT-PCR
2.8. Statistical Analyses
3. Results
3.1. The Effect of BR and LR on the Viability of NSC
3.2. The Effect of BR and LR on the Proliferation and Cell Cycle of NSC
3.3. LR Induces Morphological Changes and Rosette Formation in Self-Renewing NSC
3.4. The Effect of BR and LR Exposure on Terminal Differentiation of NSC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitek, L.; Tiribelli, C. Bilirubin: The yellow hormone? J. Hepatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.K.M.M.J.; Watchko, J.F. (Eds.) Care of the Jaundiced Neonate; McGraw-Hill: New York, NY, USA, 2012. [Google Scholar]
- Hegyi, T.; Goldie, E.; Hiatt, M. The protective role of bilirubin in oxygen-radical diseases of the preterm infant. J. Perinatol. 1994, 14, 296–300. [Google Scholar] [PubMed]
- Shekeeb, S.M.; Kumar, P.; Sharma, N.; Narang, A.; Prasad, R. Evaluation of oxidant and antioxidant status in term neonates: A plausible protective role of bilirubin. Mol. Cell. Biochem. 2008, 317, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Watchko, J.F.; Tiribelli, C. Bilirubin-induced neurologic damage-mechanisms and management approaches. N. Engl. J. Med. 2013, 369, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Brites, D.; Fernandes, A. Bilirubin-induced neural impairment: A special focus on myelination, age-related windows of susceptibility and associated co-morbidities. Sem. Fetal Neonatal Med. 2015, 20, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Maisels, M.J.; McDonagh, A.F. Phototherapy for neonatal jaundice. N. Engl. J. Med. 2008, 358, 920–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasprova, J.; Dvorak, A.; Vecka, M.; Lenicek, M.; Lacina, O.; Valaskova, P.; Zapadlo, M.; Plavka, R.; Klan, P.; Vitek, L. A novel accurate LC-MS/MS method for quantitative determination of Z-lumirubin. Sci. Rep. 2020, 10, 4411. [Google Scholar] [CrossRef] [Green Version]
- Lightner, D.A.; Linnane, W.P., III; Ahlfors, C.E. Bilirubin photooxidation products in the urine of jaundiced neonates receiving phototherapy. Pediatr. Res. 1984, 18, 696–700. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Guo, G.; Li, A.; Cai, W.Q.; Wang, X. Challenges of phototherapy for neonatal hyperbilirubinemia (Review). Exp. Ther. Med. 2021, 21, 231. [Google Scholar] [CrossRef]
- Faulhaber, F.R.S.; Procianoy, R.S.; Silveira, R.C. Side effects of phototherapy on neonates. Am. J. Perinatol. 2019, 36, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.F.; Reilly, M.; Sharp, F.R. Oxidation of bilirubin produces compounds that cause prolonged vasospasm of rat cerebral vessels: A contributor to subarachnoid hemorrhage-induced vasospasm. J. Cereb. Blood Flow Metab. 2002, 22, 472–478. [Google Scholar] [CrossRef] [Green Version]
- Jašprová, J.; Dal Ben, M.; Hurný, D.; Hwang, S.; Žížalová, K.; Kotek, J.; Wong, R.J.; Stevenson, D.K.; Gazzin, S.; Tiribelli, C.; et al. Neuro-inflammatory effects of photodegradative products of bilirubin. Sci. Rep. 2018, 8, 7444. [Google Scholar] [CrossRef]
- Dvorak, A.; Pospisilova, K.; Zizalova, K.; Capkova, N.; Muchova, L.; Vecka, M.; Vrzackova, N.; Krizova, J.; Zelenka, J.; Vitek, L. The effects of bilirubin and lumirubin on metabolic and oxidative stress markers. Front. Pharmacol. 2021, 12, 567001. [Google Scholar] [CrossRef]
- Bohaciakova, D.; Hruska-Plochan, M.; Tsunemoto, R.; Gifford, W.D.; Driscoll, S.P.; Glenn, T.D.; Wu, S.; Marsala, S.; Navarro, M.; Tadokoro, T.; et al. A scalable solution for isolating human multipotent clinical-grade neural stem cells from ES precursors. Stem Cell Res. Ther. 2019, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Falk, A.; Koch, P.; Kesavan, J.; Takashima, Y.; Ladewig, J.; Alexander, M.; Wiskow, O.; Tailor, J.; Trotter, M.; Pollard, S.; et al. Capture of neuroepithelial-like stem cells from pluripotent stem cells provides a versatile system for in vitro production of human neurons. PLoS ONE 2012, 7, e29597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, P.; Opitz, T.; Steinbeck, J.A.; Ladewig, J.; Brustle, O. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc. Natl. Acad. Sci. USA 2009, 106, 3225–3230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, S.H.; Martin, J.; Elia, J.; Flippin, J.; Paramban, R.I.; Hefferan, M.P.; Vidal, J.G.; Mu, Y.; Killian, R.L.; Israel, M.A.; et al. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS ONE 2011, 6, e17540. [Google Scholar] [CrossRef] [Green Version]
- McDonagh, A.F.; Assisi, F. The ready isomerization of bilirubin IX-a in aqueous solution. Biochem. J. 1972, 129, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Jasprova, J.; Dal Ben, M.; Vianello, E.; Goncharova, I.; Urbanova, M.; Vyroubalova, K.; Gazzin, S.; Tiribelli, C.; Sticha, M.; Cerna, M.; et al. The biological effects of bilirubin photoisomers. PLoS ONE 2016, 11, e0148126. [Google Scholar] [CrossRef]
- Fedorova, V.; Vanova, T.; Elrefae, L.; Pospisil, J.; Petrasova, M.; Kolajova, V.; Hudacova, Z.; Baniariova, J.; Barak, M.; Peskova, L.; et al. Differentiation of neural rosettes from human pluripotent stem cells in vitro is sequentially regulated on a molecular level and accomplished by the mechanism reminiscent of secondary neurulation. Stem Cell Res. 2019, 40, 101563. [Google Scholar] [CrossRef] [PubMed]
- McKelvey-Martin, V.J.; Green, M.H.; Schmezer, P.; Pool-Zobel, B.L.; De Meo, M.P.; Collins, A. The single cell gel electrophoresis assay (comet assay): A European review. Mutat. Res. 1993, 288, 47–63. [Google Scholar] [CrossRef]
- Chacon, E.; Acosta, D.; Lemasters, J.J. Primary cultures of cardiac myocytes as in vitro models for pharmacological and toxicological assessments. In In Vitro Methods in Pharmaceutical Research; Castell, J.V., Gomez-Lechon, M.J., Eds.; Academic Press: New York, NY, USA, 1997; pp. 209–223. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Sakane, F.; Hashimoto, K. N-cadherin-based adherens junction regulates the maintenance, proliferation, and differentiation of neural progenitor cells during development. Cell Adhes. Migr. 2015, 9, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Efthymiou, A.G.; Chen, G.; Rao, M.; Chen, G.; Boehm, M. Self-renewal and cell lineage differentiation strategies in human embryonic stem cells and induced pluripotent stem cells. Expert Opin. Biol. Ther. 2014, 14, 1333–1344. [Google Scholar] [CrossRef]
- Kamachi, Y.; Cheah, K.S.; Kondoh, H. Mechanism of regulatory target selection by the SOX high-mobility-group domain proteins as revealed by comparison of SOX1/2/3 and SOX9. Mol. Cell. Biol. 1999, 19, 107–120. [Google Scholar] [CrossRef] [Green Version]
- Kunath, T.; Saba-El-Leil, M.K.; Almousailleakh, M.; Wray, J.; Meloche, S.; Smith, A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development 2007, 134, 2895–2902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slusher, T.M.; Zamora, T.G.; Appiah, D.; Stanke, J.U.; Strand, M.A.; Lee, B.W.; Richardson, S.B.; Keating, E.M.; Siddappa, A.M.; Olusanya, B.O. Burden of severe neonatal jaundice: A systematic review and meta-analysis. BMJ Paediatr. Open 2017, 1, e000105. [Google Scholar] [CrossRef] [Green Version]
- Dal Ben, M.; Bottin, C.; Zanconati, F.; Tiribelli, C.; Gazzin, S. Evaluation of region selective bilirubin-induced brain damage as a basis for a pharmacological treatment. Sci. Rep. 2017, 7, 41032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silberberg, D.H.; Johnson, L.; Schutta, H.; Ritter, L. Effects of photodegradation products of bilirubin on myelinating cerebellum cultures. J. Pediatr. 1970, 77, 613–618. [Google Scholar] [CrossRef]
- Kim, Y.K.; Na, K.S.; Myint, A.M.; Leonard, B.E. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 277–284. [Google Scholar] [CrossRef]
- Kurt, A.; Aygun, A.D.; Kurt, A.N.; Godekmerdan, A.; Akarsu, S.; Yilmaz, E. Use of phototherapy for neonatal hyperbilirubinemia affects cytokine production and lymphocyte subsets. Neonatology 2009, 95, 262–266. [Google Scholar] [CrossRef]
- Gazzin, S.; Zelenka, J.; Zdrahalova, L.; Konickova, R.; Zabetta, C.C.; Giraudi, P.J.; Berengeno, A.L.; Raseni, A.; Robert, M.C.; Vitek, L.; et al. Bilirubin accumulation and Cyp mRNA expression in selected brain regions of jaundiced Gunn rat pups. Pediatr. Res. 2012, 71, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Genc, S.; Genc, K.; Kumral, A.; Baskin, H.; Ozkan, H. Bilirubin is cytotoxic to rat oligodendrocytes in vitro. Brain Res. 2003, 985, 135–141. [Google Scholar] [CrossRef]
- Silva, R.F.; Rodrigues, C.M.; Brites, D. Rat cultured neuronal and glial cells respond differently to toxicity of unconjugated bilirubin. Pediatr. Res. 2002, 51, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.M.P.; Sola, S.; Silva, R.F.M.; Brites, D. Aging confers different sensitivity to the neurotoxic properties of unconjugated bilirubin. Pediatr. Res. 2002, 51, 112–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhine, W.D.; Schmitter, S.P.; Yu, A.C.; Eng, L.F.; Stevenson, D.K. Bilirubin toxicity and differentiation of cultured astrocytes. J. Perinatol. 1999, 19, 206–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumral, A.; Genc, S.; Genc, K.; Duman, N.; Tatli, M.; Sakizli, M.; Ozkan, H. Hyperbilirubinemic serum is cytotoxic and induces apoptosis in murine astrocytes. Biol. Neonate 2005, 87, 99–104. [Google Scholar] [CrossRef]
- Rodrigues, C.M.; Sola, S.; Brites, D. Bilirubin induces apoptosis via the mitochondrial pathway in developing rat brain neurons. Hepatology 2002, 35, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Grojean, S.; Koziel, V.; Vert, P.; Daval, J.L. Bilirubin induces apoptosis via activation of NMDA receptors in developing rat brain neurons. Exp. Neurol. 2000, 166, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhu, W.; Diao, H.; Zhou, C.; Chen, F.F.; Yang, J. A comparative study of using comet assay and gammaH2AX foci formation in the detection of N-methyl-N’-nitro-N-nitrosoguanidine-induced DNA damage. Toxicol. In Vitro 2006, 20, 959–965. [Google Scholar] [CrossRef] [Green Version]
- El-Abdin, M.Y.Z.; El-Salam, M.A.; Ibrhim, M.Y.; Koraa, S.S.M.; Mahmoud, E. Phototherapy and DNA changes in full term neonates with hyperbilirubinemia. Egypt. J. Med. Hum. Genet. 2012, 13, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Mesbah-Namin, S.A.; Shahidi, M.; Nakhshab, M. An increased genotoxic risk in lymphocytes from phototherapy-treated hyperbilirubinemic neonates. Iran. Biomed. J. 2017, 21, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Ramy, N.; Ghany, E.A.; Alsharany, W.; Nada, A.; Darwish, R.K.; Rabie, W.A.; Aly, H. Jaundice, phototherapy and DNA damage in full-term neonates. J. Perinatol. 2016, 36, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Yahia, S.; Shabaan, A.E.; Gouida, M.; El-Ghanam, D.; Eldegla, H.; El-Bakary, A.; Abdel-Hady, H. Influence of hyperbilirubinemia and phototherapy on markers of genotoxicity and apoptosis in full-term infants. Eur. J. Pediatr. 2015, 174, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Afifi, M.F.; Abdel Mohsen, A.H.; Abdel Naeem, E.; Abdel Razic, M.I. Role of phototherapy, BAX gene expression in hyperbilirubinemia development in full-term neonates. Egypt. J. Med. Hum. Genet. 2020, 21, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Solecki, D.J.; Govek, E.E.; Tomoda, T.; Hatten, M.E. Neuronal polarity in CNS development. Genes Dev. 2006, 20, 2639–2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, P.G.; Stice, S.S. Development and differentiation of neural rosettes derived from human embryonic stem cells. Stem Cell Rev. 2006, 2, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Grabiec, M.; Hribkova, H.; Varecha, M.; Stritecka, D.; Hampl, A.; Dvorak, P.; Sun, Y.M. Stage-specific roles of FGF2 signaling in human neural development. Stem Cell Res. 2016, 17, 330–341. [Google Scholar] [CrossRef] [Green Version]
- Banda, E.; McKinsey, A.; Germain, N.; Carter, J.; Anderson, N.C.; Grabel, L. Cell polarity and neurogenesis in embryonic stem cell-derived neural rosettes. Stem Cell Dev. 2015, 24, 1022–1033. [Google Scholar] [CrossRef]
- Rice, D.; Barone, S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000, 108 (Suppl. S3), 511–533. [Google Scholar]
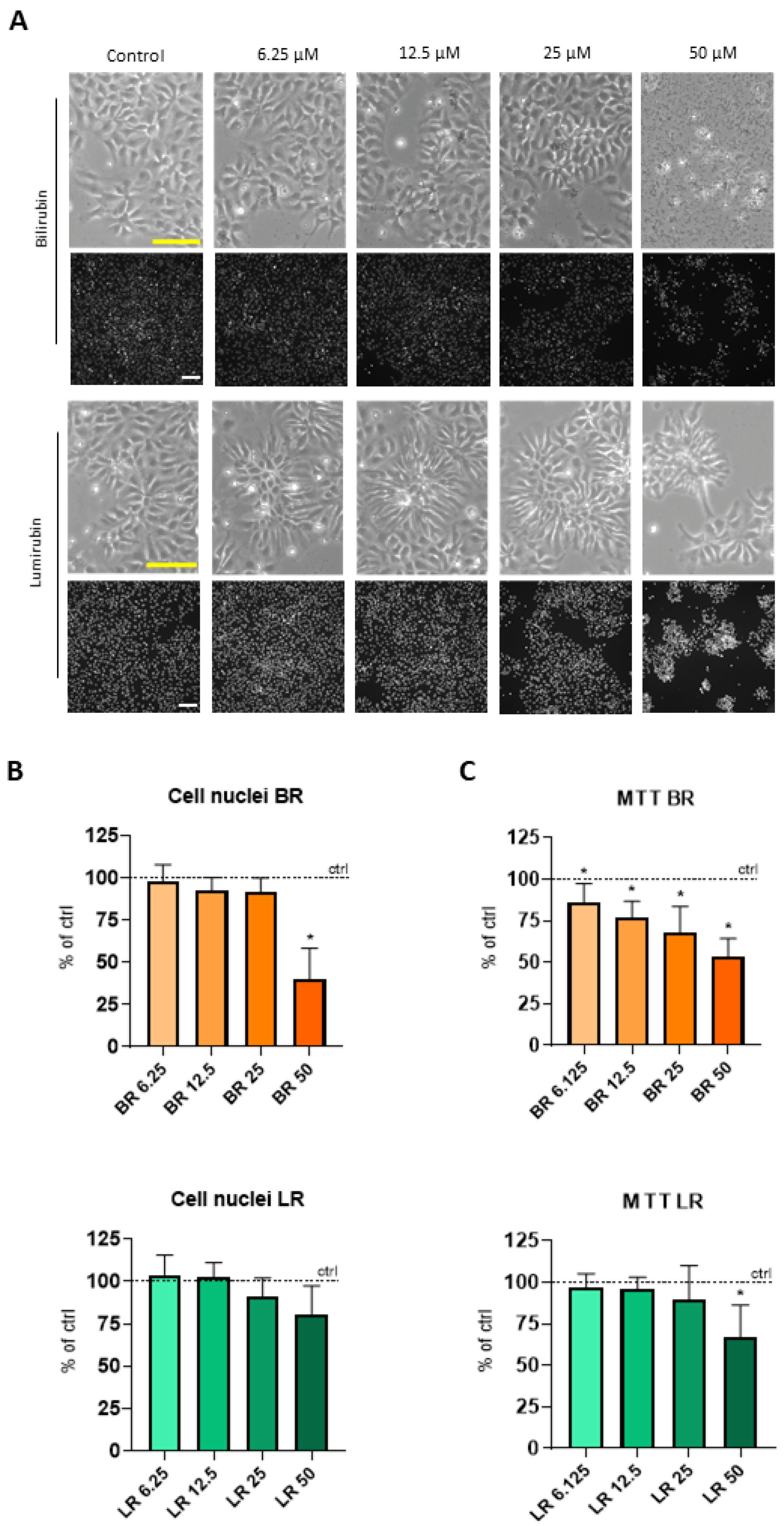
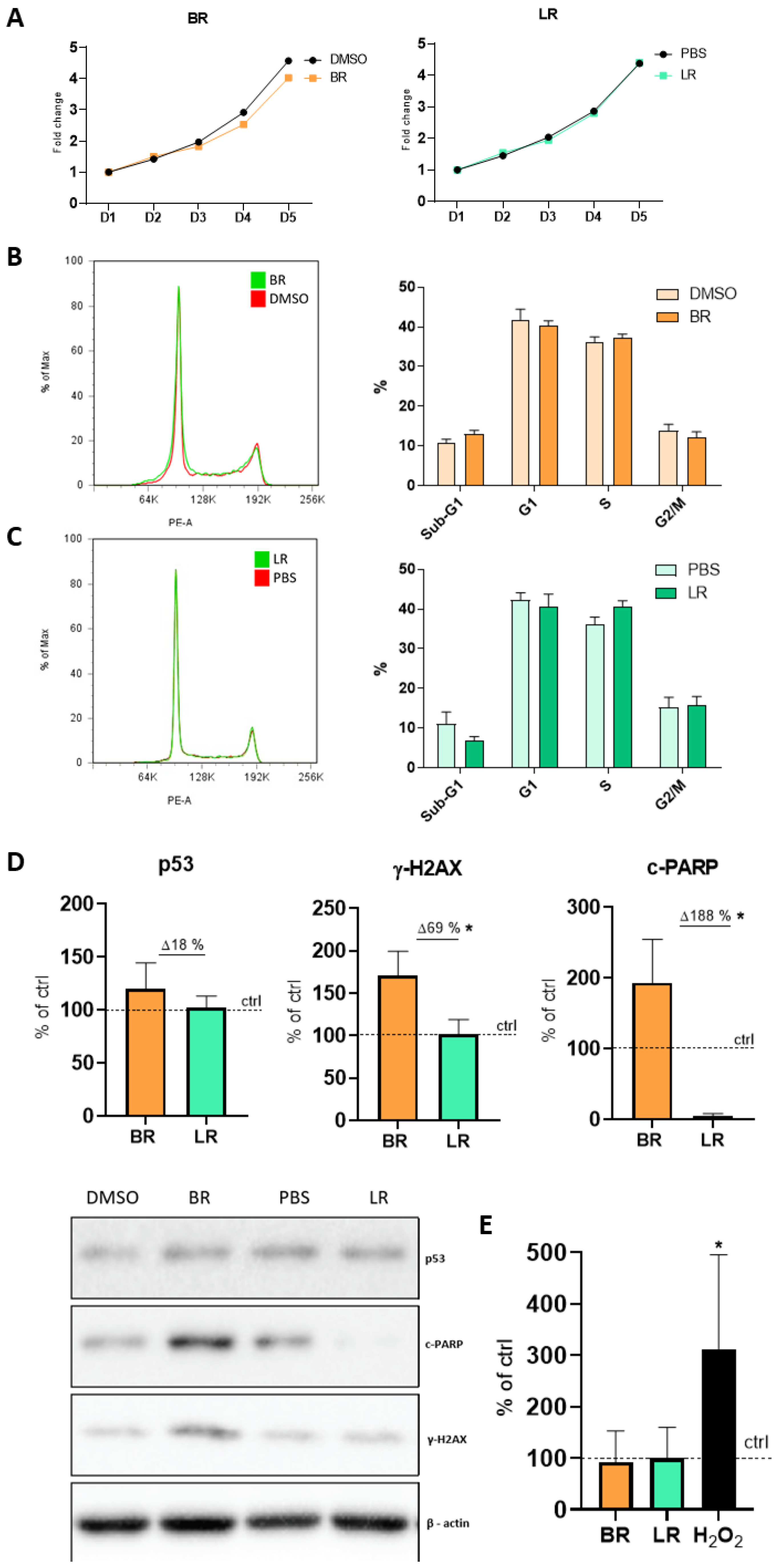
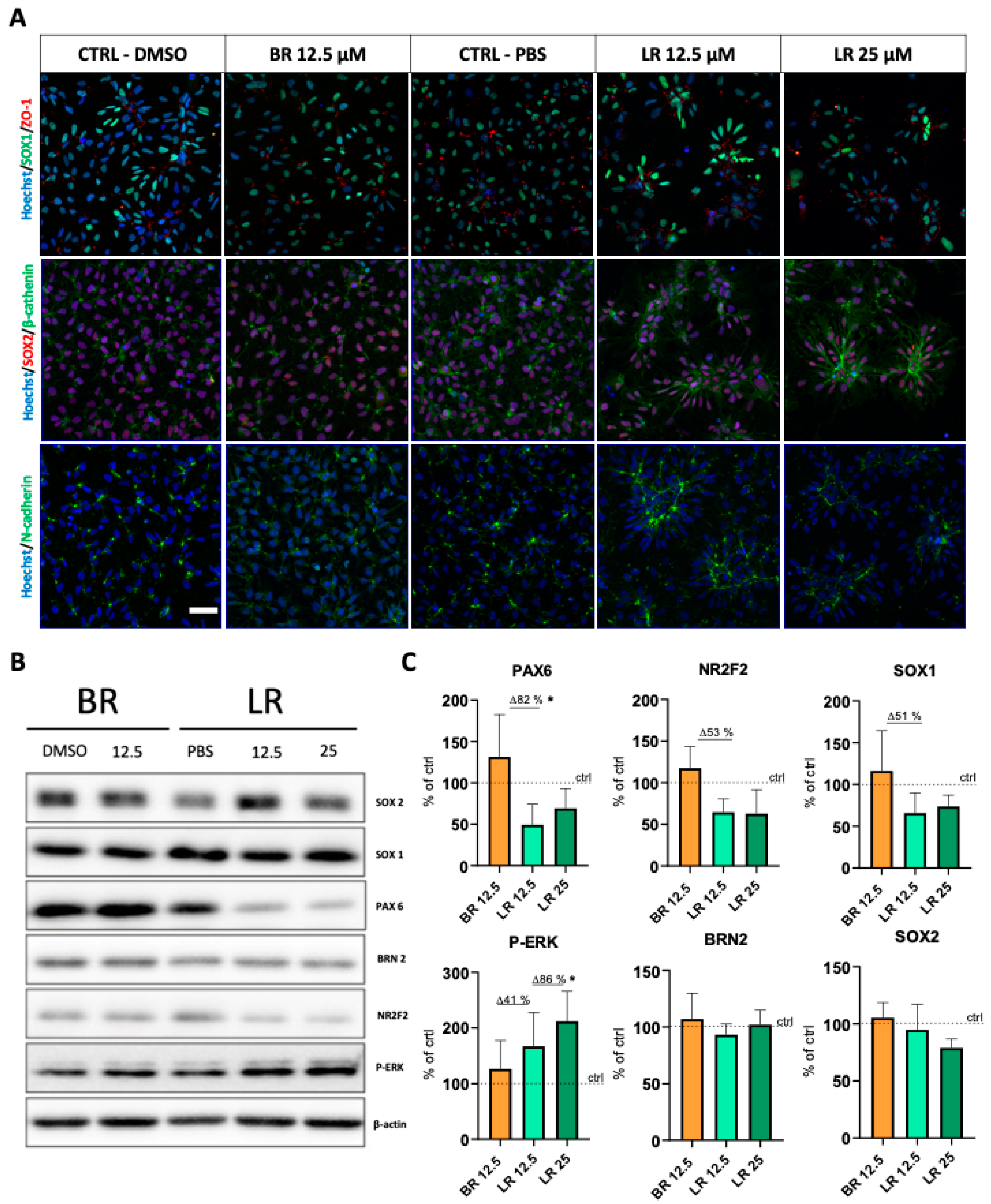
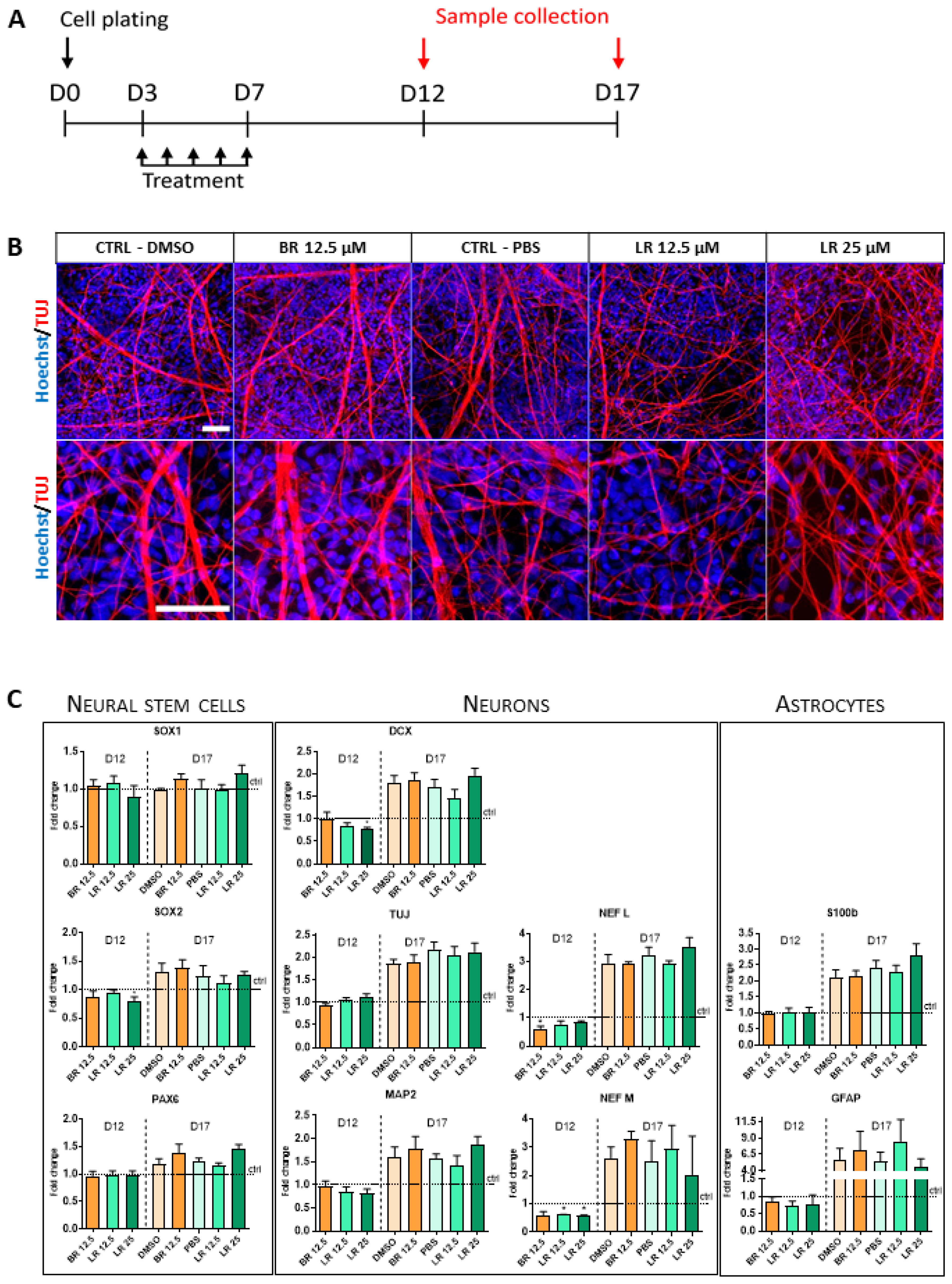
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capková, N.; Pospíšilová, V.; Fedorová, V.; Raška, J.; Pospíšilová, K.; Dal Ben, M.; Dvořák, A.; Viktorová, J.; Bohačiaková, D.; Vítek, L. The Effects of Bilirubin and Lumirubin on the Differentiation of Human Pluripotent Cell-Derived Neural Stem Cells. Antioxidants 2021, 10, 1532. https://doi.org/10.3390/antiox10101532
Capková N, Pospíšilová V, Fedorová V, Raška J, Pospíšilová K, Dal Ben M, Dvořák A, Viktorová J, Bohačiaková D, Vítek L. The Effects of Bilirubin and Lumirubin on the Differentiation of Human Pluripotent Cell-Derived Neural Stem Cells. Antioxidants. 2021; 10(10):1532. https://doi.org/10.3390/antiox10101532
Chicago/Turabian StyleCapková, Nikola, Veronika Pospíšilová, Veronika Fedorová, Jan Raška, Kateřina Pospíšilová, Matteo Dal Ben, Aleš Dvořák, Jitka Viktorová, Dáša Bohačiaková, and Libor Vítek. 2021. "The Effects of Bilirubin and Lumirubin on the Differentiation of Human Pluripotent Cell-Derived Neural Stem Cells" Antioxidants 10, no. 10: 1532. https://doi.org/10.3390/antiox10101532
APA StyleCapková, N., Pospíšilová, V., Fedorová, V., Raška, J., Pospíšilová, K., Dal Ben, M., Dvořák, A., Viktorová, J., Bohačiaková, D., & Vítek, L. (2021). The Effects of Bilirubin and Lumirubin on the Differentiation of Human Pluripotent Cell-Derived Neural Stem Cells. Antioxidants, 10(10), 1532. https://doi.org/10.3390/antiox10101532








