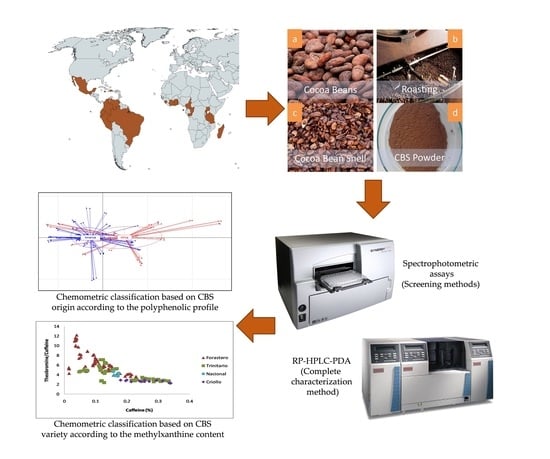
Chemometric Classification of Cocoa Bean Shells Based on Their Polyphenolic Profile Determined by RP-HPLC-PDA Analysis and Spectrophotometric Assays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Standars
2.2. Samples
2.3. Polyphenolic Extracts
2.4. Spectrophotometric Assays
2.4.1. Total Phenolic, Flavonoid, and Tannin Contents
2.4.2. Antioxidant Capacity
2.5. RP-HPLC-PDA Analysis
2.6. Statistical and Chemometric Analyses
3. Results and Discussion
3.1. Polyphenolic Quantification by Spectrophotometric Analyses
3.2. Polyphenolic and Methylxanthine Profile of the CBS Characterized by RP-HPLC-PDA
3.3. Classification of CBS Based on Their Polyphenolic Fingerprints and Spectrophotometric Measurements
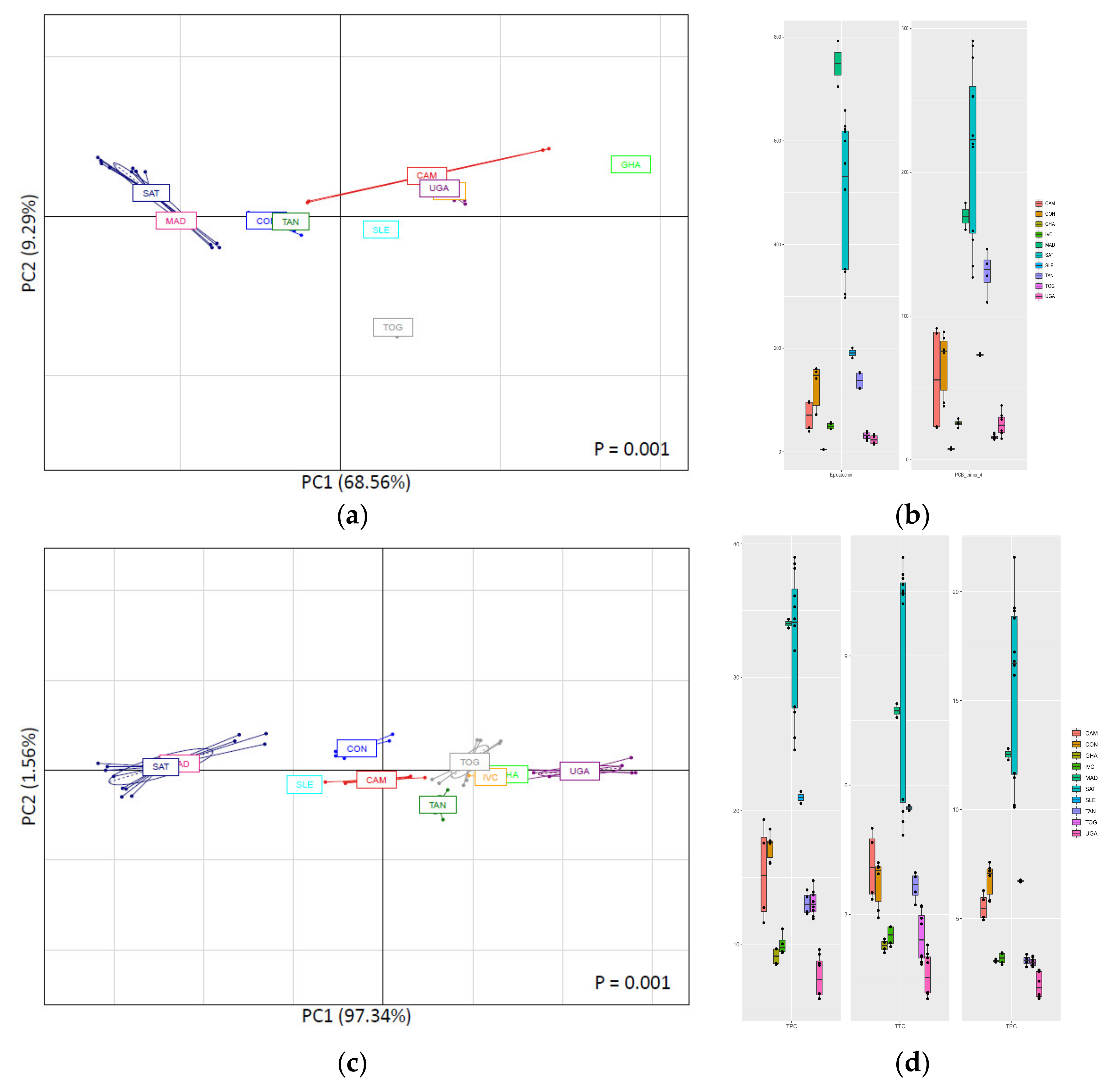
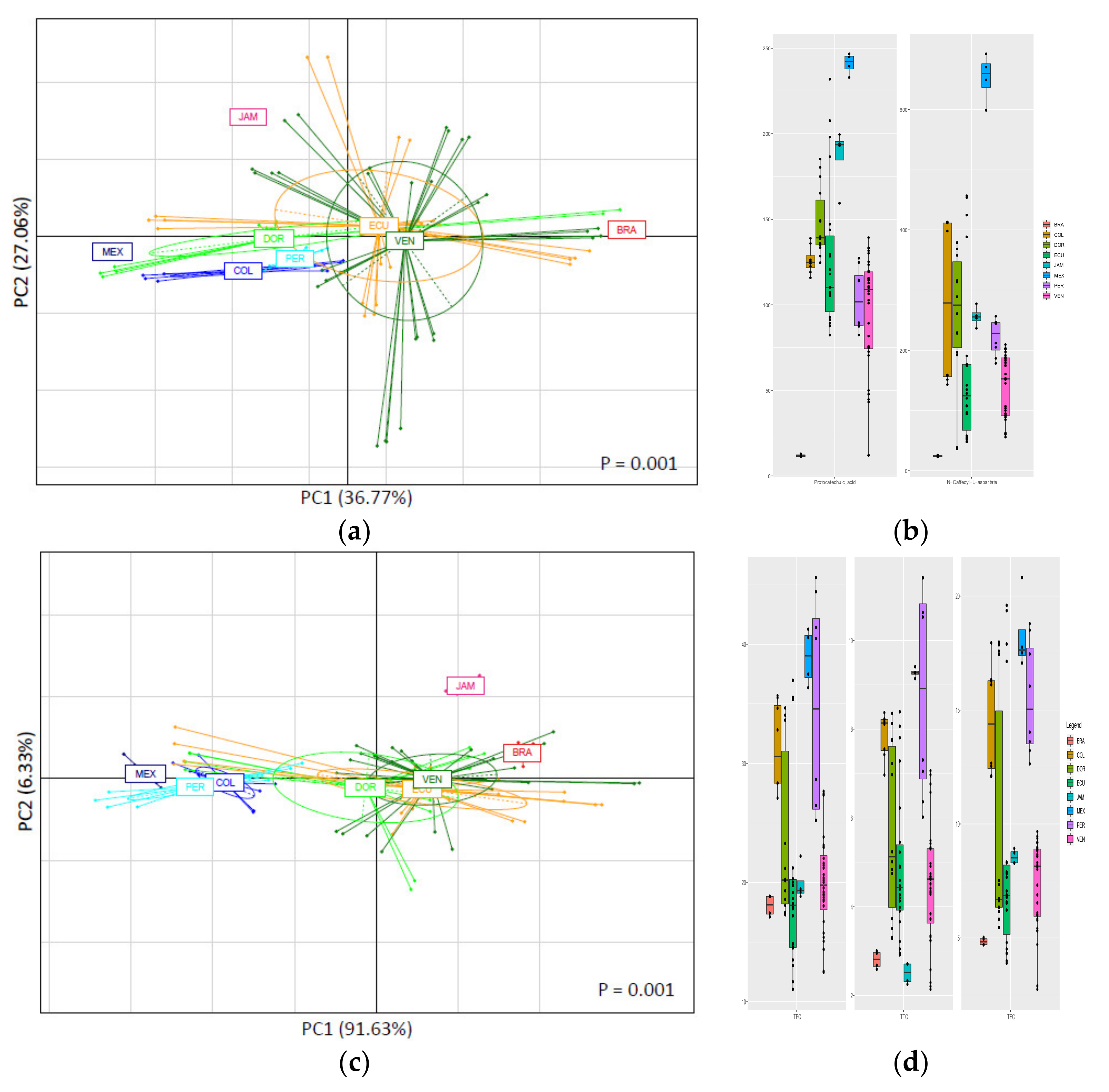
3.3.1. Classification of CBS Samples According to Their Geographical Origin
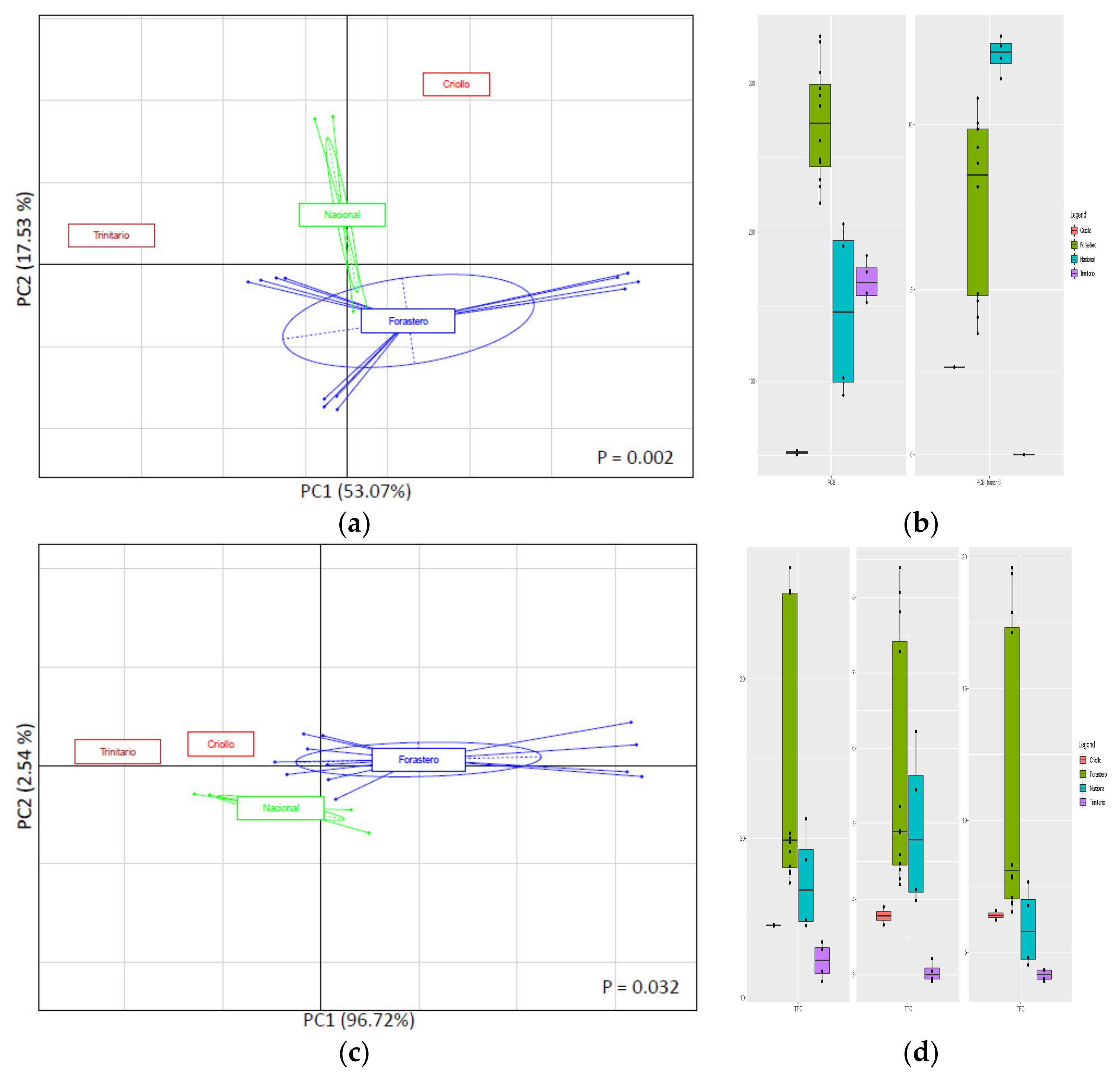
3.3.2. Classification of CBS According to the Cultivar Localized Case Study (Ecuador)
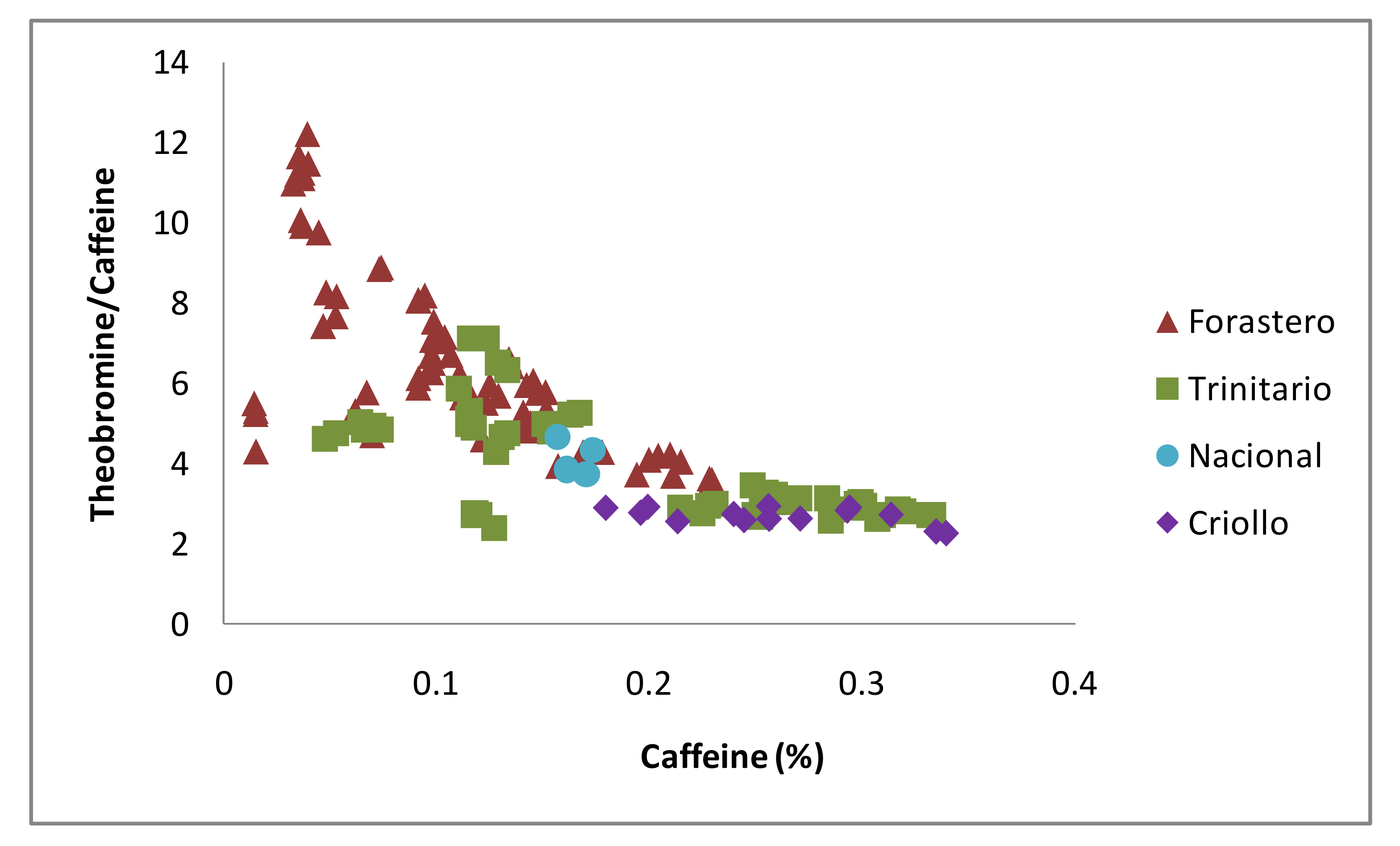
3.4. Classification of CBS Based on Their Methylxanthine Ratio According to Variety
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Zeppa, G.; Stévigny, C. Cocoa Bean Shell—A By-Product with Nutritional Properties and Biofunctional Potential. Nutrients 2020, 12, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heck, C.I.; De Mejia, E.G. Yerba Mate Tea (Ilex paraguariensis): A comprehensive review on chemistry, health implications, and technological considerations. J. Food Sci. 2007, 72, R138–R151. [Google Scholar] [CrossRef]
- Wollgast, J.; Anklam, E. Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000, 33, 423–447. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, e-Library; 2006. [Google Scholar]
- Martín, M.A.; Ramos, S. Cocoa polyphenols in oxidative stress: Potential health implications. J. Funct. Foods 2016, 27, 570–588. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.A.; Goya, L.; Ramos, S. Potential for preventive effects of cocoa and cocoa polyphenols in cancer. Food Chem. Toxicol. 2013, 56, 336–351. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.Á.; Goya, L.; Ramos, S. Antidiabetic actions of cocoa flavanols. Mol. Nutr. Food Res. 2016, 60, 1756–1769. [Google Scholar] [CrossRef] [Green Version]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojo-Poveda, O.; Ribeiro, S.O.; Anton-Sales, C.; Keymeulen, F.; Barbosa-Pereira, L.; Delporte, C.; Zeppa, G.; Stévigny, C. Evaluation of Cocoa Bean Shell Antimicrobial Activity: A Tentative Assay Using a Metabolomic Approach for Active Compound Identification. Planta Med. 2021, 87, 841–849. [Google Scholar]
- Okiyama, D.C.; Navarro, S.L.; Rodrigues, C.E. Cocoa shell and its compounds: Applications in the food industry. Trends Food Sci. Technol. 2017, 63, 103–112. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Orden, D.; Stévigny, C.; Zeppa, G.; Bertolino, M. Physical Properties and Consumer Evaluation of Cocoa Bean Shell-Functionalized Biscuits Adapted for Diabetic Consumers by the Replacement of Sucrose with Tagatose. Foods 2020, 9, 814. [Google Scholar] [CrossRef]
- Cantele, C.; Rojo-Poveda, O.; Bertolino, M.; Ghirardello, D.; Cardenia, V.; Barbosa-Pereira, L.; Zeppa, G. In vitro bioaccessibility and functional properties of phenolic compounds from enriched beverages based on cocoa bean shell. Foods 2020, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Mateus-Reguengo, L.; Bertolino, M.; Stévigny, C.; Zeppa, G. Effects of particle size and extraction methods on cocoa bean shell functional beverage. Nutrients 2019, 11, 867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa-Pereira, L.; Rojo-Poveda, O.; Ferrocino, I.; Giordano, M.; Zeppa, G. Assessment of volatile fingerprint by HS-SPME/GC-qMS and E-nose for the classification of cocoa bean shells using chemometrics. Food Res. Int. 2019, 123, 684–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C.A. Food authentication: Techniques, trends & emerging approaches. TrAC Trends Anal. Chem. 2016, 85, 123–132. [Google Scholar]
- Acierno, V.; Alewijn, M.; Zomer, P.; van Ruth, S.M. Making cocoa origin traceable: Fingerprints of chocolates using flow infusion-electro spray ionization-mass spectrometry. Food Control 2018, 85, 245–252. [Google Scholar] [CrossRef]
- Carrillo, L.C.; Londoño-Londoño, J.; Gil, A. Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia. Food Res. Int. 2014, 60, 273–280. [Google Scholar] [CrossRef]
- Mandrile, L.; Barbosa-Pereira, L.; Sorensen, K.M.; Giovannozzi, A.M.; Zeppa, G.; Engelsen, S.B.; Rossi, A.M. Authentication of cocoa bean shells by near-and mid-infrared spectroscopy and inductively coupled plasma-optical emission spectroscopy. Food Chem. 2019, 292, 47–57. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Guglielmetti, A.; Zeppa, G. Pulsed electric field assisted extraction of bioactive compounds from cocoa bean shell and coffee silverskin. Food Bioprocess Technol. 2018, 11, 818–835. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Belviso, S.; Ferrocino, I.; Rojo-Poveda, O.; Zeppa, G. Characterization and Classification of Cocoa Bean Shells from Different Regions of Venezuela Using HPLC-PDA-MS/MS and Spectrophotometric Techniques Coupled to Chemometric Analysis. Foods 2021, 10, 1791. [Google Scholar] [CrossRef] [PubMed]
- Oracz, J.; Zyzelewicz, D.; Nebesny, E. The content of polyphenolic compounds in cocoa beans (Theobroma cacao L.), depending on variety, growing region, and processing operations: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1176–1192. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.d.l.L.; Fernández-Ochoa, Á.; Leyva-Jiménez, F.J.; Guerrero-Muñoz, N.; Villegas-Aguilar, M.d.C.; Pimentel-Moral, S.; Ramos-Escudero, F.; Segura-Carretero, A. LC-MS and Spectrophotometric Approaches for Evaluation of Bioactive Compounds from Peru Cocoa By-Products for Commercial Applications. Molecules 2020, 25, 3177. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, C.; Morales Sillero, A.; Fernández-Bolaños, J.; Bermúdez Oria, A.; Azpeitia Morales, A.; Rodríguez-Gutiérrez, G. Cocoa bean husk: Industrial source of antioxidant phenolic extract. J. Sci. Food Agric. 2019, 99, 325–333. [Google Scholar] [CrossRef]
- Mazzutti, S.; Rodrigues, L.G.G.; Mezzomo, N.; Venturi, V.; Ferreira, S.R.S. Integrated green-based processes using supercritical CO2 and pressurized ethanol applied to recover antioxidant compouds from cocoa (Theobroma cacao) bean hulls. J. Supercrit. Fluids 2018, 135, 52–59. [Google Scholar] [CrossRef]
- Pavlović, N.; Jakovljević, M.; Miškulin, M.; Molnar, M.; Ačkar, Đ.; Jokić, S. Green extraction techniques of bioactive components from cocoa shell. Croat. J. Food Sci. Technol. 2019, 11, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Hernández, C.; Viera-Alcaide, I.; Morales-Sillero, A.M.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Bioactive compounds in Mexican genotypes of cocoa cotyledon and husk. Food Chem. 2018, 240, 831–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedan, V.; Weber, C.; Do, T.; Fischer, N.; Reich, E.; Rohn, S. HPTLC fingerprint profile analysis of cocoa proanthocyanidins depending on origin and genotype. Food Chem. 2018, 267, 277–287. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; van Camp, J.; Alasalvar, C.; Ismail, A.B. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Gültekin-Özgüven, M.; Berktaş, I.; Özçelik, B. Change in stability of procyanidins, antioxidant capacity and in-vitro bioaccessibility during processing of cocoa powder from cocoa beans. LWT-Food Sci. Technol. 2016, 72, 559–565. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; El khattabi, C.; Youl, E.N.H.; Bertolino, M.; Delporte, C.; Pochet, S.; Stévigny, C. Polyphenolic and Methylxanthine Bioaccessibility of Cocoa Bean Shell Functional Biscuits: Metabolomics Approach and Intestinal Permeability through Caco-2 Cell Models. Antioxidants 2020, 9, 1164. [Google Scholar] [CrossRef] [PubMed]
- Okiyama, D.C.; Soares, I.D.; Cuevas, M.S.; Crevelin, E.J.; Moraes, L.A.; Melo, M.P.; Oliveira, A.L.; Rodrigues, C.E. Pressurized liquid extraction of flavanols and alkaloids from cocoa bean shell using ethanol as solvent. Food Res. Int. 2018, 114, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Barnaba, C.; Nardin, T.; Pierotti, A.; Malacarne, M.; Larcher, R. Targeted and untargeted characterisation of free and glycosylated simple phenols in cocoa beans using high resolution-tandem mass spectrometry (Q-Orbitrap). J. Chromatogr. A 2017, 1480, 41–49. [Google Scholar] [CrossRef]
- Brunetto, M.a.d.R.; Gutiérrez, L.; Delgado, Y.; Gallignani, M.; Zambrano, A.; Gómez, Á.; Ramos, G.; Romero, C. Determination of theobromine, theophylline and caffeine in cocoa samples by a high-performance liquid chromatographic method with on-line sample cleanup in a switching-column system. Food Chem. 2007, 100, 459–467. [Google Scholar] [CrossRef]
- Damm, I.; Enger, E.; Chrubasik-Hausmann, S.; Schieber, A.; Zimmermann, B.F. Fast and comprehensive analysis of secondary metabolites in cocoa products using ultra high-performance liquid chromatography directly after pressurized liquid extraction. J. Sep. Sci. 2016, 39, 3113–3122. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Jaramillo, Y.; Bedoya, C.; Llano, S.M.; Gallego, V.; Quijano, J.; Londono-Londono, J. Chemometric approaches for postharvest quality tracing of cocoa: An efficient method to distinguish plant material origin. Heliyon 2019, 5, e01650. [Google Scholar] [CrossRef] [Green Version]
- Barišić, V.; Flanjak, I.; Križić, I.; Jozinović, A.; Šubarić, D.; Babić, J.; Miličević, B.; Ačkar, Đ. Impact of high-voltage electric discharge treatment on cocoa shell phenolic components and methylxanthines. J. Food Process Eng. 2019, e13057. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Gaspari, A.; Graziani, G.; Sandini, A.; Ritieni, A. Fast analysis of polyphenols and alkaloids in cocoa-based products by ultra-high performance liquid chromatography and Orbitrap high resolution mass spectrometry (UHPLC-Q-Orbitrap-MS/MS). Food Res. Int. 2018, 111, 229–236. [Google Scholar] [CrossRef]
- Jokić, S.; Pavlović, N.; Jozinović, A.; Ačkar, Đ.; Babić, J.; Šubarić, D. High-Voltage Electric Discharge Extraction of Bioactive Compounds from the Cocoa Bean Shell. Chem. Biochem. Eng. Q. 2019, 33, 271–280. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Casals, I.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventós, R.M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; de Mejia, E.G. Cocoa Shell Aqueous Phenolic Extract Preserves Mitochondrial Function and Insulin Sensitivity by Attenuating Inflammation between Macrophages and Adipocytes In Vitro. Mol. Nutr. Food Res. 2019, 63, 1801413. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Borges, G.; Nagai, C.; Jackson, M.C.; Yokota, T.; Crozier, A.; Ashihara, H. Profiles of phenolic compounds and purine alkaloids during the development of seeds of Theobroma cacao cv. Trinitario. J. Agric. Food Chem. 2013, 61, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Lechtenberg, M.; Henschel, K.; Liefländer-Wulf, U.; Quandt, B.; Hensel, A. Fast determination of N-phenylpropenoyl-L-amino acids (NPA) in cocoa samples from different origins by ultra-performance liquid chromatography and capillary electrophoresis. Food Chem. 2012, 135, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Megías-Pérez, R.; Grimbs, S.; D’Souza, R.N.; Bernaert, H.; Kuhnert, N. Profiling, quantification and classification of cocoa beans based on chemometric analysis of carbohydrates using hydrophilic interaction liquid chromatography coupled to mass spectrometry. Food Chem. 2018, 258, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Pereira, L.; Rojo-Poveda, O.; Ferrocino, I.; Giordano, M.; Zeppa, G. Analytical dataset on volatile compounds of cocoa bean shells from different cultivars and geographical origins. Data in brief 2019, 25, 104268. [Google Scholar] [CrossRef] [PubMed]
- Davrieux, F.; Assemat, S.; Boulanger, R.; Cros, E. Determination of cocoa purine content by near infrared spectroscopy. In Proceedings of the 14th International Cocoa Research Conference, Accra, Ghana, 13–18 October 2003. [Google Scholar]

| Sample Code | Variety | Country | Continent |
|---|---|---|---|
| BRA | Trinitario | Brazil | America |
| CAM1 | Forastero | Cameroon | Africa |
| CAM2 | Trinitario | Cameroon | Africa |
| COL1 | Forastero | Colombia | America |
| COL2 | Trinitario | Colombia | America |
| CON1 | Forastero | Congo | Africa |
| CON2 | Forastero | Congo | Africa |
| DOR1 | Trinitario | Dominican Republic | America |
| DOR2 | Forastero | Dominican Republic | America |
| DOR3 | Trinitario | Dominican Republic | America |
| DOR4 | Trinitario | Dominican Republic | America |
| ECU1 | Forastero | Ecuador | America |
| ECU2 | Trinitario | Ecuador | America |
| ECU3 | Forastero | Ecuador | America |
| ECU4 | Nacional | Ecuador | America |
| ECU5 | Nacional | Ecuador | America |
| ECU6 | Forastero | Ecuador | America |
| ECU7 | Criollo | Ecuador | America |
| GHA | Forastero | Ghana | Africa |
| IVC | Forastero | Ivory Coast | Africa |
| JAM | Trinitario | Jamaica | America |
| MAD | Forastero | Madagascar | Africa |
| MEX | Trinitario | Mexico | America |
| PER1 | Forastero | Peru | America |
| PER2 | Trinitario | Peru | America |
| SAT1 | Forastero | São Tomé | Africa |
| SAT2 | Forastero | São Tomé | Africa |
| SAT3 | Forastero | São Tomé | Africa |
| SLE | Forastero | Sierra Leone | Africa |
| TAN | Forastero | Tanzania | Africa |
| TOG1 | Forastero | Togo | Africa |
| TOG2 | Forastero | Togo | Africa |
| UGA1 | Forastero | Uganda | Africa |
| UGA2 | Forastero | Uganda | Africa |
| VEN1 | Trinitario | Venezuela | America |
| VEN2 | Trinitario | Venezuela | America |
| VEN3 | Trinitario | Venezuela | America |
| VEN4 | Trinitario | Venezuela | America |
| VEN5 | Criollo | Venezuela | America |
| VEN6 | Trinitario | Venezuela | America |
| VEN7 | Criollo | Venezuela | America |
| VEN8 | Criollo | Venezuela | America |
| VEN9 | Criollo | Venezuela | America |
| VEN10 | Criollo | Venezuela | America |
| Peak | Rt (min) | λmax | Compound | Concentration Range (mg kg−1 of CBS) |
|---|---|---|---|---|
| 1 | 4.73 | 293 | Protocatechuic acid | 11.89‒241.01 |
| 2 | 6.4 | 272 | Theobromine | 764.88‒9028.28 |
| 3 | 6.8 | 279 | N-Coumaroyl-L-aspartate_1 | 1.41‒24.55 |
| 4 | 8.24 | 320 | N-Caffeoyl-L-aspartate | 5.86‒652.58 |
| 5 | 8.63 | 278 | Catechin | 12.72‒180.37 |
| 6 | 9.5 | 279 | Catechin-3-O-glucoside | 6.40‒101.49 |
| 7 | 9.7 | 280 | Procyanidin B-type trimer_1 | 0.90‒130.89 |
| 8 | 10.6 | 306 | N-Coumaroyl-L-aspartate_2 | 4.46‒184.30 |
| 9 | 11.08 | 278 | Procyanidin B-type trimer_2 | 0.00‒163.15 |
| 10 | 11.1 | 285 | Procyanidin B-type | 6.84‒672.55 |
| 11 | 11.5 | 278 | Epicatechin | 4.47‒748.79 |
| 12 | 11.87 | 286 | N-Coumaroyl-L-glutamate | 1.10‒12.58 |
| 13 | 12.2 | 272 | Caffeine | 150.81‒3370.77 |
| 14 | 12.78 | 279 | Procyanidin B-type trimer_3 | 1.51‒97.04 |
| 15 | 13 | 278 | Procyanidin B-type trimer_4 | 7.62‒264.48 |
| 16 | 13.22 | 281 | Procyanidin B-type trimer_5 | 6.52‒280.29 |
| 17 | 13.86 | 280 | Procyanidin A-type hexoside_1 | 8.51‒560.65 |
| 18 | 14.6 | 279 | Procyanidin B-type trimer_6 | 0.00‒28.68 |
| 19 | 14.9 | 280 | Procyanidin A-type pentoside_1 | 1.27‒198.93 |
| 20 | 15.82 | 279 | Procyanidin B-type trimer_7 | 2.49‒104.37 |
| 21 | 16.03 | 279 | Procyanidin A-type trimer arabinoside | 2.44‒114.09 |
| 22 | 17.2 | 278 | Procyanidin A-type hexoside_2 | 2.49‒73.34 |
| 23 | 17.5 | 283 | N-Coumaroyl-L-tyrosine | 1.99‒48.70 |
| 24 | 17.7 | 278 | Procyanidin A-type pentoside_2 | 0.00‒40.02 |
| 25 | 18.2 | 355 | Quercetin-3-O-glucoside | 3.47‒47.59 |
| 26 | 19.4 | 355 | Quercetin-3-O-arabinoside | 3.76‒86.53 |
| 27 | 21.83 | 365 | Quercetin | 1.07‒22.37 |
| Sample Code | ∑ Phenolic Acids | ∑ Flavan-3- ols | ∑ Catechin- 3-O- glycosides | ∑ PCB | ∑ PCB Trimers | ∑ PCA Glycosides | ∑ Flavonols | ∑ Flavonol-3- O-glycosides | ∑ N-phenyl -propenoyl-L- aminoacids | ∑ Total Polyphenols | ∑ Total Methylxantines (g/kg CBS) | TPC (mg GAE/g CBS) | TFC (mg CE/g CBS) | TTC (mg CE/g CBS) | RSA (μmol TE/g CBS) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BRA | 11.9 ± 0.8 | 58 ± 5 | 31 ± 3 | 9.8 ± 0.8 | 195 ± 10 | 103 ± 4 | 305 ± 03 | 39 ± 3 | 41 ± 1 | 493 ± 13 | 4.1 ± 0.2 | 18.1 ± 0.9 | 4.9 ± 0.2 | 2.8 ± 0.2 | 60 ± 3 |
| CAM1 | 112 ± 3 | 116 ± 2 | 32 ± 1 | 68 ± 8 | 181 ± 4 | 225 ± 5 | 3.4 ± 0.2 | 30.6 ± 0.1 | 105 ± 2 | 874 ± 11 | 7.3 ± 0.1 | 18 ± 1 | 6.1 ± 0.3 | 4.8 ± 0.2 | 78 ± 5 |
| CAM2 | 28 ± 2 | 57 ± 5 | 16.8 ± 0.7 | 16.1 ± 0.4 | 46 ± 2 | 32 ± 2 | 1.1 ± 0.1 | 12.2 ± 0.5 | 29 ± 1 | 238 ± 6 | 3.2 ± 0.3 | 12.2 ± 0.8 | 5.0 ± 0.1 | 3.4 ± 0.1 | 54 ± 1 |
| COL1 | 122 ± 5 | 344 ± 22 | 26 ± 1 | 210 ± 21 | 363 ± 18 | 339 ± 6 | 6.6 ± 0.5 | 56 ± 4 | 220 ± 8 | 1686 ± 37 | 7.3 ± 0.4 | 28.0 ± 0.6 | 12.4 ± 0.3 | 7.7 ± 0.6 | 119 ± 6 |
| COL2 | 130 ± 8 | 859 ± 12 | 41 ± 4 | 289 ± 29 | 650 ± 34 | 497 ± 26 | 9 ± 1 | 89 ± 5 | 543 ± 12 | 3108 ± 56 | 10.8 ± 0.1 | 35 ± 1 | 16.7 ± 0.9 | 8.1 ± 0.4 | 144 ± 9 |
| CON1 | 138 ± 8 | 86.0 ± 0.6 | 16.2 ± 0.6 | 274 ± 30 | 150 ± 3 | 128 ± 4 | 3.3 ± 0.4 | 47 ± 2 | 197 ± 10 | 1040 ± 33 | 8.4 ± 0.4 | 16.1 ± 0.1 | 5.81 ± 0.03 | 3.0 ± 0.1 | 64.24 ± 0.02 |
| CON2 | 141 ± 9 | 180 ± 9 | 67 ± 4 | 141 ± 16 | 238 ± 9 | 271 ± 14 | 5.5 ± 0.3 | 41 ± 4 | 161 ± 9 | 1244 ± 29 | 8.4 ± 0.2 | 17.9 ± 0.5 | 7.3 ± 0.3 | 4.1 ± 0.1 | 76 ± 2 |
| DOR1 | 176 ± 9 | 588 ± 45 | 71 ± 4 | 584 ± 21 | 601 ± 15 | 425 ± 17 | 10.3 ± 0.7 | 94 ± 4 | 584 ± 15 | 3134 ± 57 | 12.3 ± 0.1 | 34.0 ± 0.5 | 17.7 ± 0.2 | 8.0 ± 0.3 | 146 ± 3 |
| DOR2 | 136 ± 10 | 245 ± 11 | 39 ± 3 | 275 ± 21 | 305 ± 9 | 296 ± 15 | 3.1 ± 0.2 | 72 ± 4 | 459 ± 27 | 1831 ± 41 | 9.3 ± 0.4 | 21 ± 2 | 6.9 ± 0.6 | 5.0 ± 0.2 | 79 ± 4 |
| DOR3 | 131 ± 3 | 227 ± 23 | 13.8 ± 0.9 | 78 ± 11 | 252 ± 10 | 184 ± 9 | 1.4 ± 0.1 | 19.3 ± 0.7 | 68 ± 2 | 975 ± 29 | 2.9 ± 0.2 | 17.8 ± 0.5 | 5.6 ± 0.3 | 7.1 ± 0.2 | 78.46 ± 0.03 |
| DOR4 | 141 ± 6 | 288 ± 18 | 48 ± 2 | 236 ± 26 | 276 ±10 | 310 ± 11 | 7.0 ± 0.3 | 62 ± 3 | 395 ± 24 | 1762 ± 43 | 11.1 ± 0.2 | 18 ± 1 | 6.6 ± 0.3 | 3.5 ± 0.2 | 74 ± 5 |
| ECU1 | 99 ± 9 | 250 ± 20 | 25 ± 2 | 310 ± 23 | 216 ± 9 | 302 ± 31 | 2.6 ± 0.1 | 35 ± 2 | 155 ± 8 | 1394 ± 46 | 6.8 ± 0.5 | 19 ± 2 | 6.8 ± 0.2 | 4.8 ± 0.4 | 71 ± 4 |
| ECU2 | 122 ± 9 | 214 ± 8 | 12.2 ± 0.7 | 167 ± 14 | 162 ± 9 | 122 ± 4 | 3.1 ± 0.3 | 23.8 ± 0.6 | 93 ± 4 | 919 ± 21 | 7.2 ± 0.3 | 12 ± 1 | 4.1 ± 0.2 | 3.0 ± 0.1 | 53 ± 2 |
| ECU3 | 206 ± 19 | 480 ± 13 | 48 ± 1 | 233 ± 12 | 476 ± 13 | 496 ± 22 | 9.9 ± 0.9 | 112 ± 2 | 667 ± 34 | 2728 ± 50 | 10.7 ± 0.3 | 35.8 ± 0.8 | 19 ± 1 | 7.9 ± 0.5 | 150 ± 6 |
| ECU4 | 145 ± 2 | 414 ± 14 | 22 ± 1 | 198 ± 10 | 248 ± 5 | 306 ± 12 | 6.9 ± 0.4 | 56.6 ± 0.7 | 213 ± 7 | 1610 ± 23 | 9.1 ± 0.2 | 20 ± 2 | 7.2 ± 0.6 | 5.8 ± 0.5 | 84 ± 8 |
| ECU5 | 113 ± 8 | 287 ± 1 | 27.2 ± 0.1 | 96 ± 8 | 214 ± 14 | 294 ± 8 | 3.0 ± 0.3 | 37 ± 2 | 160 ± 2 | 1231 ± 20 | 8.0 ± 0.1 | 14.7 ± 0.2 | 4.7 ± 0.2 | 4.1 ± 0.1 | 59.9 ± 0.3 |
| ECU6 | 88 ± 5 | 462 ± 8 | 15.6 ± 0.2 | 277 ± 28 | 298 ± 14 | 371 ± 18 | 6.1 ± 0.6 | 36 ± 2 | 264 ± 10 | 1818 ± 39 | 8.1 ± 0.3 | 18.8 ± 0.8 | 8.1 ± 0.3 | 4.5 ± 0.3 | 78 ± 4 |
| ECU7 | 108 ± 3 | 437 ± 13 | 37 ± 3 | 52 ± 2 | 284 ± 7 | 292 ± 12 | 2.8 ± 0.2 | 65.4 ± 0.8 | 277 ± 6 | 1556 ± 20 | 10.8 ± 0.8 | 14.6 ± 0.1 | 6.4 ± 0.3 | 3.8 ± 0.2 | 62.4 ± 0.9 |
| GHA | 20.9 ± 0.8 | 17.2 ± 0.6 | 6.8 ± 0.1 | 6.8 ± 0.4 | 26 ± 1 | 16.3 ± 0.6 | 1.29 ± 0.03 | 7.2 ± 0.4 | 17.1 ± 0.7 | 120 ± 2 | 0.9 ± 0.1 | 9.1 ± 0.6 | 3.1 ± 0.1 | 2.3 ± 0.1 | 39 ± 1 |
| IVC | 54 ± 4 | 77 ± 7 | 21 ± 1 | 46 ± 5 | 74 ± 3 | 68 ± 2 | 1.1 ± 0.1 | 13.4 ± 0.8 | 50 ± 2 | 405 ± 10 | 4.2 ± 0.3 | 10.0 ± 0.8 | 3.2 ± 0.3 | 2.5 ± 0.2 | 44 ± 4 |
| JAM | 187 ± 18 | 245 ± 16 | 58 ± 2 | 57 ± 6 | 455 ± 17 | 287 ± 16 | 6.9 ± 0.9 | 76 ± 1 | 450 ± 17 | 1822 ± 39 | 11.9 ± 0.2 | 20 ± 2 | 8.6 ± 0.3 | 3.7 ± 0.2 | 82 ± 7 |
| MAD | 87 ± 4 | 888 ± 63 | 31.5 ± 0.6 | 478 ± 19 | 518 ± 20 | 412 ± 9 | 6.9 ± 0.1 | 50 ± 2 | 371 ± 4 | 2843 ± 70 | 10.55 ± 0.02 | 3.0 ± 0.5 | 12.5 ± 0.4 | 7.7 ± 0.2 | 139 ± 1 |
| MEX | 241 ± 6 | 523 ± 13 | 89 ± 4 | 261 ± 35 | 538 ± 23 | 658 ± 38 | 22.4 ± 0.6 | 93 ± 4 | 810 ± 40 | 3235 ± 71 | 9.8 ± 0.3 | 39 ± 2 | 18 ± 2 | 9.3 ± 0.1 | 172 ± 5 |
| PER1 | 120 ± 7 | 413 ± 30 | 27 ± 2 | 138 ± 2 | 272 ± 10 | 417 ± 20 | 7.5 ± 0.3 | 55 ± 1 | 311 ± 16 | 1759 ± 41 | 8.3 ± 0. | 26 ± 2 | 13.4 ± 0.6 | 6.7 ± 0.6 | 116 ± 8 |
| PER2 | 87 ± 3 | 385 ± 42 | 33 ± 2 | 341 ± 26 | 340 ± 14 | 351 ± 8 | 6.3 ± 0.5 | 51.9 ± 0.7 | 404 ± 10 | 1999 ± 53 | 9.7 ± 0.7 | 43 ± 2 | 18 ± 1 | 11.0 ± 0.5 | 182 ± 6 |
| SAT1 | 120 ± 4 | 352 ± 29 | 42 ± 1 | 640 ± 41 | 484 ± 20 | 387 ± 21 | 4.7 ± 0.3 | 38 ± 2 | 203 ± 13 | 2271 ± 59 | 8.9 ± 0.3 | 26 ± 2 | 10.9 ± 0.8 | 5.3 ± 0.4 | 103 ± 9 |
| SAT2 | 198 ± 11 | 751 ± 20 | 101 ± 3 | 191 ± 20 | 654 ± 36 | 833 ± 21 | 13.9 ± 0.5 | 122 ± 6 | 539 ± 31 | 3403 ± 61 | 10.1 ± 0.2 | 34 ± 1 | 16.7 ± 0.4 | 10.9 ± 0.3 | 155 ± 8 |
| SAT3 | 164 ± 16 | 656 ± 46 | 79 ± 3 | 246 ± 30 | 566 ± 32 | 682 ± 61 | 8.0 ± 0.9 | 62 ± 2 | 406 ± 11 | 2869 ± 90 | 10.0 ± 0.3 | 38 ± 1 | 20 ± 1 | 10.4 ± 0.1 | 165 ± 6 |
| SLE | 14.4 ± 0.7 | 225 ± 14 | 29 ± 2 | 136 ± 12 | 199 ± 11 | 129 ± 10 | 2.0 ± 0.1 | 28 ± 1 | 61 ± 1 | 823 ± 24 | 4.7 ± 0.3 | 21.0 ± 0.6 | 6.7 ± 0.1 | 5.5 ± 0.1 | 73 ± 1 |
| TAN | 88 ± 7 | 173 ± 18 | 17.9 ± 0.7 | 253 ± 18 | 263 ± 17 | 199 ± 16 | 2.9 ± 0.2 | 32 ± 2 | 93 ± 4 | 1123 ± 35 | 8.0 ± 0.5 | 13.1 ± 0.9 | 3.1 ± 0.2 | 3.6 ± 0.3 | 55 ± 4 |
| TOG1 | 14 ± 1 | 43 ± 3 | 27 ± 2 | 136 ± 12 | 154 ± 7 | 73 ± 3 | 1.1 ± 0.1 | 24 ± 1 | 61 ± 2 | 534 ± 15 | 4.8 ± 0.3 | 12.7 ± 0.9 | 3.0 ± 0.1 | 2.0 ± 0.1 | 45 ± 2 |
| TOG2 | 16 ± 1 | 58 ± 2 | 28 ± 2 | 146 ± 14 | 123 ± 5 | 85 ± 3 | 1.4 ± 0.1 | 24 ± 2 | 45 ± 2 | 526 ± 16 | 4.1 ± 0.1 | 14 ± 1 | 3.0 ± 0.3 | 3.0 ± 0.2 | 49 ± 3 |
| UGA1 | 57 ± 3 | 45 ± 3 | 6.4 ± 0.3 | 129 ± 5 | 76 ± 5 | 45 ± 3 | 2.0 ± 0.1 | 16.3 ± 0.7 | 61 ± 2 | 438 ± 9 | 4.5 ± 0.4 | 9.0 ± 0.6 | 2.5 ± 0.2 | 2.1 ± 0.2 | 37 ± 3 |
| UGA2 | 108 ± 8 | 67 ± 5 | 13 ± 1 | 38 ± 5 | 97 ± 4 | 48 ± 4 | 2.4 ± 0.2 | 15.8 ± 0.5 | 58 ± 2 | 449 ± 12 | 7.8 ± 0.3 | 6.1 ± 0.2 | 1.4 ± 0.1 | 1.1 ± 0.1 | 28 ± 2 |
| VEN1 | 119 ± 5 | 314 ± 26 | 16.8 ± 0.7 | 408 ± 53 | 330 ± 14 | 259 ± 10 | 3.2 ± 0.3 | 28 ± 1 | 104 ± 4 | 1583 ± 62 | 7.3 ± 0.4 | 22 ± 1 | 7.7 ± 0.8 | 6.9 ± 0.2 | 98 ± 6 |
| VEN2 | 73 ± 2 | 195 ± 19 | 14 ± 1 | 673 ± 52 | 617 ± 36 | 179 ± 6 | 5.9 ± 0.8 | 42 ± 2 | 217 ± 16 | 1136 ± 68 | 8.8 ± 0.4 | 27.5 ± 0.1 | 8.7 ± 0.3 | 4.9 ± 0.4 | 99 ± 1 |
| VEN3 | 108 ± 4 | 412 ± 31 | 52 ± 5 | 78 ± 4 | 377 ± 12 | 467 ± 16 | 3.4 ± 0.3 | 76 ± 4 | 319 ± 11 | 2015 ± 40 | 11.2 ± 0.6 | 20 ± 2 | 8.9 ± 0.6 | 5.1 ± 0.3 | 87 ± 4 |
| VEN4 | 46 ± 3 | 171 ± 5 | 20 ± 1 | 83 ± 3 | 126 ± 3 | 98 ± 4 | 2.6 ± 0.1 | 21.5 ± 0.7 | 133 ± 9 | 1892 ± 13 | 4.4 ± 0.0 | 20 ± 1 | 8.9 ± 0.7 | 4.5 ± 0.1 | 82 ± 4 |
| VEN5 | 121 ± 9 | 164 ± 12 | 25 ± 1 | 55 ± 3 | 220 ± 7 | 127 ± 3 | 3.3 ± 0.3 | 33.0 ± 0.9 | 188 ± 6 | 702 ± 18 | 8.3 ± 0.5 | 15 ± 1 | 5.9 ± 0.3 | 3.5 ± 0.3 | 66 ± 4 |
| VEN6 | 124 ± 9 | 256 ± 16 | 30 ± 2 | 182 ± 10 | 375 ± 17 | 252 ± 16 | 6.1 ± 0.5 | 49 ± 2 | 281 ± 17 | 937 ± 36 | 9.9 ± 0.7 | 22 ± 2 | 9.1 ± 0.2 | 4.5 ± 0.2 | 86 ± 8 |
| VEN7 | 104 ± 4 | 319 ± 5 | 34.8 ± 0.5 | 106 ± 9 | 342 ± 8 | 267 ± 3 | 1.66 ± 0.02 | 27 ± 1 | 322 ± 5 | 1555 ± 15 | 11.11 ± 0.05 | 14.9 ± 0.7 | 5.6 ± 0.4 | 2.4 ± 0.2 | 55.6 ± 0.5 |
| VEN8 | 136 ± 4 | 347 ± 7 | 50 ± 3 | 54 ± 2 | 286 ± 10 | 329 ± 21 | 2.94 ± 0.04 | 58 ± 1 | 322 ± 5 | 1523 ± 25 | 11.4 ± 0.2 | 18.5 ± 0.7 | 6.2 ± 0.5 | 3.3 ± 0.1 | 68 ± 2 |
| VEN9 | 86 ± 5 | 215 ± 7 | 25 ± 2 | 68 ± 5 | 208 ± 8 | 230 ± 14 | 3.5 ± 0.4 | 24 ± 1 | 178 ± 4 | 1584 ± 20 | 9.6 ± 0.3 | 12.5 ± 0.1 | 2.8 ± 0.1 | 2.17 ± 0.05 | 47 ± 3 |
| VEN10 | 12.05 ± 0.02 | 306 ± 2 | 32.9 ± 0.6 | 104 ± 12 | 220 ± 4 | 209 ± 11 | 2.4 ± 0.1 | 36 ± 1 | 214 ± 7 | 1036 ± 18 | 7.2 ± 0.2 | 19.4 ± 0.9 | 5.0 ± 0.4 | 5.4 ± 0.1 | 65 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojo-Poveda, O.; Zeppa, G.; Ferrocino, I.; Stévigny, C.; Barbosa-Pereira, L. Chemometric Classification of Cocoa Bean Shells Based on Their Polyphenolic Profile Determined by RP-HPLC-PDA Analysis and Spectrophotometric Assays. Antioxidants 2021, 10, 1533. https://doi.org/10.3390/antiox10101533
Rojo-Poveda O, Zeppa G, Ferrocino I, Stévigny C, Barbosa-Pereira L. Chemometric Classification of Cocoa Bean Shells Based on Their Polyphenolic Profile Determined by RP-HPLC-PDA Analysis and Spectrophotometric Assays. Antioxidants. 2021; 10(10):1533. https://doi.org/10.3390/antiox10101533
Chicago/Turabian StyleRojo-Poveda, Olga, Giuseppe Zeppa, Ilario Ferrocino, Caroline Stévigny, and Letricia Barbosa-Pereira. 2021. "Chemometric Classification of Cocoa Bean Shells Based on Their Polyphenolic Profile Determined by RP-HPLC-PDA Analysis and Spectrophotometric Assays" Antioxidants 10, no. 10: 1533. https://doi.org/10.3390/antiox10101533
APA StyleRojo-Poveda, O., Zeppa, G., Ferrocino, I., Stévigny, C., & Barbosa-Pereira, L. (2021). Chemometric Classification of Cocoa Bean Shells Based on Their Polyphenolic Profile Determined by RP-HPLC-PDA Analysis and Spectrophotometric Assays. Antioxidants, 10(10), 1533. https://doi.org/10.3390/antiox10101533