Effects of High Frequency Repetitive Transcranial Magnetic Stimulation (HF-rTMS) on Delay Discounting in Major Depressive Disorder: An Open-Label Uncontrolled Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.2.1. Intervention
2.2.2. Outcome Measures
2.3. Data Analysis
3. Results
3.1. Participants Characteristics, Missing Data, and Outliers
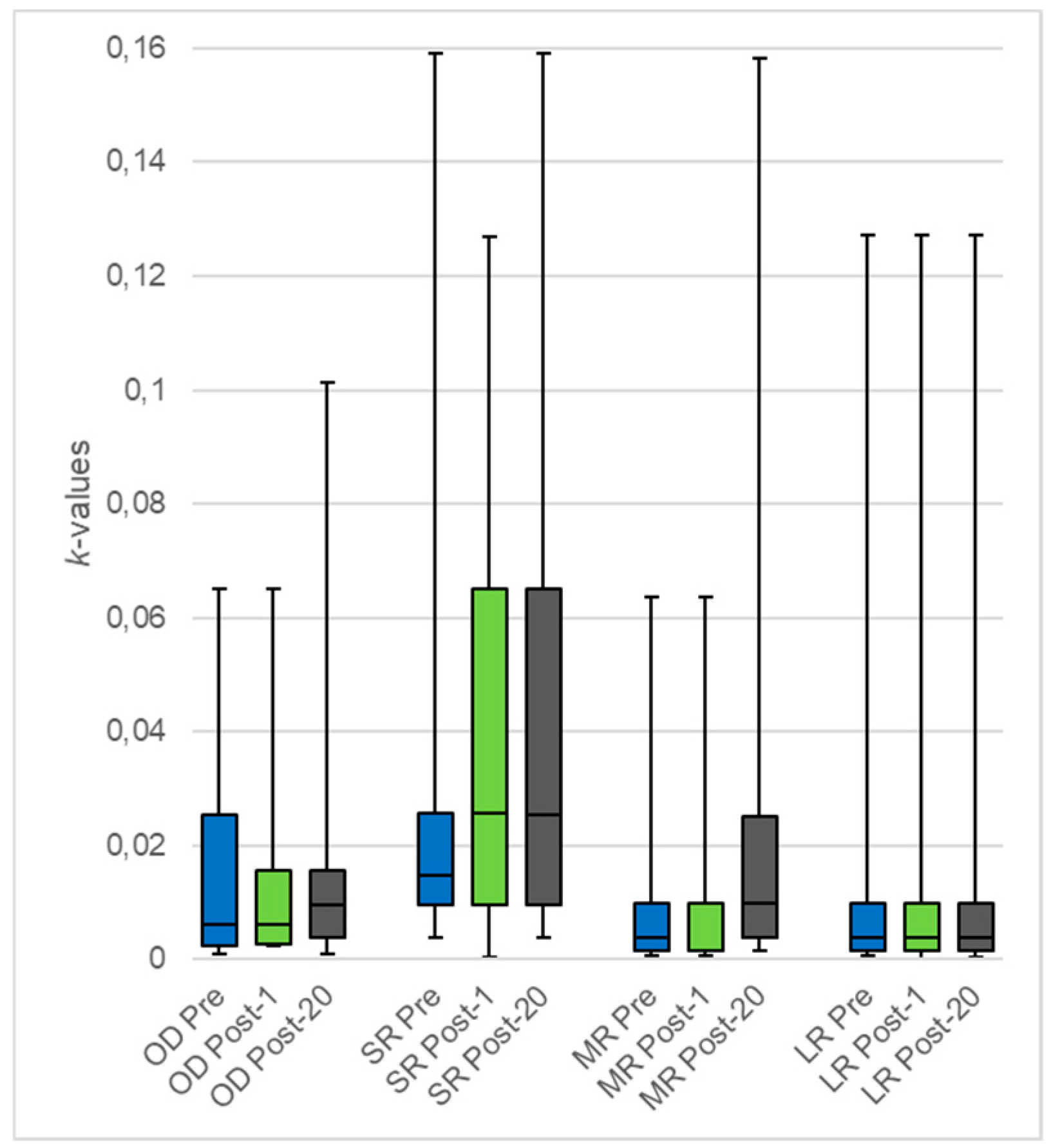
3.2. Impact of 20 rTMS Sessions on DD
3.3. Impact of One rTMS Session on DD
3.4. Impact of rTMS on the BIS-10
3.5. Impact of rTMS on the BFI-Fr
3.6. Impact of rTMS on Depressive Symptoms
3.7. Correlation between DD and Other Psychiatric Measures
3.8. Side Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pizzagalli, D.A.; Iosifescu, D.; Hallett, L.A.; Ratner, K.G.; Fava, M. Reduced Hedonic Capacity in Major Depressive Disorder: Evidence from a Probabilistic Reward Task. J. Psychiatr. Res. 2008, 43, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Pulcu, E.; Trotter, P.D.; Thomas, E.J.; McFarquhar, M.; Juhasz, G.; Sahakian, B.J.; Deakin, J.F.; Zahn, R.; Anderson, I.M.; Elliott, R. Temporal discounting in major depressive disorder. Psychol. Med. 2014, 44, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Szuhany, K.L.; MacKenzie, D.; Otto, M.W. The impact of depressed mood, working memory capacity, and priming on delay discounting. J. Behav. Ther. Exp. Psychiatry 2018, 60, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Oono, H.; Inoue, T.; Boku, S.; Kako, Y.; Kitaichi, Y.; Kusumi, I.; Masui, T.; Nakagawa, S.; Suzuki, K.; et al. Depressive patients are more impulsive and inconsistent in intertemporal choice behavior for monetary gain and loss than healthy subjects--an analysis based on Tsallis’ statistics. Neuro Endocrinol. Lett. 2008, 29, 351–358. [Google Scholar] [PubMed]
- Vinckier, F.; Gourion, D.; Mouchabac, S. Anhedonia predicts poor psychosocial functioning: Results from a large cohort of patients treated for major depressive disorder by general practitioners. Eur. Psychiatry 2017, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lempert, K.M.; Pizzagalli, D.A. Delay Discounting and Future-directed Thinking in Anhedonic Individuals. J. Behav. Ther. Exp. Psychiatry 2010, 41, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Jiang, N.-Z.; Cheung, E.F.; Sun, H.-W.; Chan, R.C. Role of depression severity and impulsivity in the relationship between hopelessness and suicidal ideation in patients with major depressive disorder. J. Affect. Disord. 2015, 183, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Kirby, K.N.; Petry, N.M.; Bickel, W.K. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J. Exp. Psychol. Gen. 1999, 128, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.; Büchel, C. The neural mechanisms of inter-temporal decision-making: understanding variability. Trends Cogn. Sci. 2011, 15, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Manning, J.; Hedden, T.; Wickens, N.; Whitfield-Gabrieli, S.; Prelec, D.; Gabrieli, J.D.E. Personality Influences Temporal Discounting Preferences: Behavioral and Brain Evidence. NeuroImage 2014, 98, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Hadzibeganovic, T.; A Cannas, S.; Makino, T.; Fukui, H.; Kitayama, S. Cultural neuroeconomics of intertemporal choice. Neuro Endocrinol. Lett. 2009, 30, 185–191. [Google Scholar] [PubMed]
- Pine, A.; Shiner, T.; Seymour, B.; Dolan, R.J. Dopamine, Time, and Impulsivity in Humans. J. Neurosci. 2010, 30, 8888–8896. [Google Scholar] [CrossRef] [PubMed]
- Swann, A.C.; Bjork, J.M.; Moeller, F.; Dougherty, D.M. Two models of impulsivity: relationship to personality traits and psychopathology. Boil. Psychiatry 2002, 51, 988–994. [Google Scholar] [CrossRef]
- McClure, S.M.; Laibson, D.I.; Loewenstein, G.; Cohen, J.D. Separate Neural Systems Value Immediate and Delayed Monetary Rewards. Science 2004, 306, 503–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainslie, G. Specious reward: A behavioral theory of impulsiveness and impulse control. Psychol. Bull. 1975, 82, 463–496. [Google Scholar] [CrossRef] [PubMed]
- Frederick, S.; Loewenstein, G.; O’Donoghue, T.; O’Donoghue, T. Time Discounting and Time Preference: A Critical Review. J. Econ. Lit. 2002, 40, 351–401. [Google Scholar] [CrossRef]
- Dalley, J.W.; Everitt, B.J.; Robbins, T.W. Impulsivity, Compulsivity, and Top-Down Cognitive Control. Neuron 2011, 69, 680–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brevet-Aeby, C.; Brunelin, J.; Iceta, S.; Padovan, C.; Poulet, E. Prefrontal cortex and impulsivity: Interest of noninvasive brain stimulation. Neurosci. Biobehav. Rev. 2016, 71, 112–134. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P.; André-Obadia, N.; Antal, A.; Ayache, S.S.; Baeken, C.; Benninger, D.H.; Cantello, R.M.; Cincotta, M.; De Carvalho, M.; De Ridder, D.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef] [PubMed]
- Perera, T.; George, M.S.; Grammer, G.; Janicak, P.G.; Pascual-Leone, A.; Wiercki, T.S. The Clinical TMS Society Consensus Review and Treatment Recommendations for TMS Therapy for Major Depressive Disorder. Brain Stimul. 2016, 9, 336–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefaucheur, J.-P. Methods of therapeutic cortical stimulation. Neurophysiol. Clin. Neurophysiol. 2009, 39, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Esslinger, C.; Schüler, N.; Sauer, C.; Gass, D.; Mier, D.; Braun, U.; Ochs, E.; Schulze, T.G.; Rietschel, M.; Kirsch, P.; et al. Induction and quantification of prefrontal cortical network plasticity using 5 Hz rTMS and fMRI. Hum. Brain Mapp. 2014, 35, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Völlm, B.; Khalifa, N. The Effects of rTMS on Impulsivity in Normal Adults: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2018, 28, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Figner, B.; Knoch, D.; Johnson, E.J.; Krosch, A.R.; Lisanby, S.H.; Fehr, E.; Weber, E.U. Lateral prefrontal cortex and self-control in intertemporal choice. Nat. Neurosci. 2010, 13, 538–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheffer, C.E.; Mennemeier, M.; Landes, R.D.; Bickel, W.K.; Brackman, S.; Dornhoffer, J.; Kimbrell, T.; Brown, G. Neuromodulation of delay discounting, the reflection effect, and cigarette consumption. J. Subst. Abus. Treat. 2013, 45, 206–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheffer, C.E.; Bickel, W.K.; Brandon, T.H.; Franck, C.T.; Deen, D.; Panissidi, L.; Abdali, S.A.; Pittman, J.C.; Lunden, S.E.; Prashad, N.; et al. Preventing relapse to smoking with transcranial magnetic stimulation: Feasibility and potential efficacy. Drug Alcohol Depend. 2018, 182, 8–18. [Google Scholar] [CrossRef]
- Zack, M.; Cho, S.S.; Parlee, J.; Jacobs, M.; Li, C.; Boileau, I.; Strafella, A. Effects of High Frequency Repeated Transcranial Magnetic Stimulation and Continuous Theta Burst Stimulation on Gambling Reinforcement, Delay Discounting, and Stroop Interference in Men with Pathological Gambling. Brain Stimul. 2016, 9, 867–875. [Google Scholar] [CrossRef]
- Montgomery, S.A.; Åsberg, M. A New Depression Scale Designed to be Sensitive to Change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef]
- Ruhé, H.G.; Van Rooijen, G.; Spijker, J.; Peeters, F.P.; Schene, A.H. Staging methods for treatment resistant depression. A systematic review. J. Affect. Disord. 2012, 137, 35–45. [Google Scholar] [CrossRef]
- Barratt, E.S. ANXIETY AND IMPULSIVENESS RELATED TO PSYCHOMOTOR EFFICIENCY. Percept. Mot. Ski. 1959, 9, 191–198. [Google Scholar] [CrossRef]
- Baylé, F.J.; Bourdel, M.C.; Caci, H.; Gorwood, P.; Chignon, J.-M.; Adés, J.; Lôo, H. Structure factorielle de la traduction française de l’échelle d’impulsivité de Barratt (BIS-10). Can. J. Psychiatry 2000, 45, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Benet-Martinez, V.; John, O.P. Los Cinco Grandes across cultures and ethnic groups: Multitrait-multimethod analyses of the Big Five in Spanish and English. J. Pers. Soc. Psychol. 1998, 75, 729–750. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.; Trivedi, M.H.; Ibrahim, H.M.; Carmody, T.J.; Arnow, B.; Klein, D.N.; Markowitz, J.C.; Ninan, P.T.; Kornstein, S.; Manber, R.; et al. The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Boil. Psychiatry 2003, 54, 573–583. [Google Scholar] [CrossRef]
- Angeletos, G.-M.; Laibson, D.; Repetto, A.; Tobacman, J.; Weinberg, S. The Hyperbolic Consumption Model: Calibration, Simulation, and Empirical Evaluation. J. Econ. Perspect. 2001, 15, 47–68. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, B.A.; Lemley, S.M.; Reed, D.D.; Jarmolowicz, D.P. 21- and 27-Item Monetary Choice Questionnaire Automated Scorers. Available online: https://kuscholarworks.ku.edu/handle/1808/15424 (accessed on 19 April 2018).
- Baeken, C.; Vanderhasselt, M.-A.; Remue, J.; Herremans, S.; Vanderbruggen, N.; Zeeuws, D.; Santermans, L.; De Raedt, R. Intensive HF-rTMS treatment in refractory medication-resistant unipolar depressed patients. J. Affect. Disord. 2013, 151, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, J.B.; Ogston, K.M.; Miles, T.S. Age and sex differences in human motor cortex input-output characteristics. J. Physiol. 2003, 546, 605–613. [Google Scholar] [CrossRef]
- Silvanto, J.; Pascual-Leone, A. State-Dependency of Transcranial Magnetic Stimulation. Brain Topogr. 2008, 21, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, F.; Keenan, J.P.; Tormos, J.M.; Topka, H.; Pascual-Leone, A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp. Brain Res. 2000, 133, 425–430. [Google Scholar] [CrossRef]
- Vanderhasselt, M.-A.; Baeken, C.; Hendricks, M.; De Raedt, R. The effects of high frequency rTMS on negative attentional bias are influenced by baseline state anxiety. Neuropsychologia 2011, 49, 1824–1830. [Google Scholar] [CrossRef] [Green Version]
- Pripfl, J.; Neumann, R.; Köhler, U.; Lamm, C. Effects of transcranial direct current stimulation on risky decision making are mediated by ‘hot’ and ‘cold’ decisions, personality, and hemisphere. Eur. J. Neurosci. 2013, 38, 3778–3785. [Google Scholar] [CrossRef]
- Miranda, P.C.; Mekonnen, A.; Salvador, R.; Basser, P.J. Predicting the electric field distribution in the brain for the treatment of glioblastoma. Phys. Med. Boil. 2014, 59, 4137–4147. [Google Scholar] [CrossRef] [PubMed]
- Willner, P.; Scheel-Krüger, J.; Belzung, C. The neurobiology of depression and antidepressant action. Neurosci. Biobehav. Rev. 2013, 37, 2331–2371. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M. Precision psychiatry: A neural circuit taxonomy for depression and anxiety. Lancet Psychiatry 2016, 3, 472–480. [Google Scholar] [CrossRef]
- Nery, F.G.; Stanley, J.A.; Chen, H.-H.; Hatch, J.P.; Nicoletti, M.A.; Monkul, E.S.; Matsuo, K.; Caetano, S.C.; Peluso, M.A.; Najt, P.; et al. Normal metabolite levels in the left dorsolateral prefrontal cortex of unmedicated major depressive disorder patients: A single voxel 1H spectroscopy study. Psychiatry Res. Neuroimaging 2009, 174, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Valero-Cabre, A.; Pascual-Leone, A. Noninvasive Human Brain Stimulation. Annu. Rev. Biomed. Eng. 2007, 9, 527–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drysdale, A.T.; Grosenick, L.; Downar, J.; Dunlop, K.; Mansouri, F.; Meng, Y.; Fetcho, R.N.; Zebley, B.; Oathes, D.J.; Etkin, A.; et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 2017, 23, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.S.; Koshimori, Y.; Aminian, K.; Obeso, I.; Rusjan, P.; Lang, A.E.; Daskalakis, Z.J.; Houle, S.; Strafella, A.P. Investing in the future: Stimulation of the medial prefrontal cortex reduces discounting of delayed rewards. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2015, 40, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Strafella, A.P.; Paus, T.; Barrett, J.; Dagher, A. Repetitive Transcranial Magnetic Stimulation of the Human Prefrontal Cortex Induces Dopamine Release in the Caudate Nucleus. J. Neurosci. 2001, 21, RC157. [Google Scholar] [CrossRef]
- Strafella, A.P.; Paus, T.; Fraraccio, M.; Dagher, A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain 2003, 126, 2609–2615. [Google Scholar] [CrossRef]
- Ko, J.H.; Strafella, A.P. Dopaminergic neurotransmission in the human brain: New lessons from perturbation and imaging. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2012, 18, 149–168. [Google Scholar] [CrossRef]
- Pogarell, O.; Koch, W.; Pöpperl, G.; Tatsch, K.; Jakob, F.; Zwanzger, P.; Mulert, C.; Rupprecht, R.; Möller, H.-J.; Hegerl, U. Striatal dopamine release after prefrontal repetitive transcranial magnetic stimulation in major depression: Preliminary results of a dynamic (123I) IBZM SPECT study. J. Psychiatr. Res. 2006, 40, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Evenden, J. Impulsivity: A discussion of clinical and experimental findings. J. Psychopharmacol. 1999, 13, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Winstanley, C.A.; Eagle, D.M.; Robbins, T.W. Behavioral models of impulsivity in relation to ADHD: Translation between clinical and preclinical studies. Clin. Psychol. Rev. 2006, 26, 379–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellanos, F.X.; Tannock, R.; Castellanos, F. Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nat. Rev. Neurosci. 2002, 3, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, B.; Richards, J.B.; Horn, K.; Karraker, K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behav. Process. 2004, 65, 35–42. [Google Scholar] [CrossRef]
- Miller, J.D.; Lynam, D.R.; Jones, S. Externalizing Behavior Through the Lens of the Five-Factor Model: A Focus on Agreeableness and Conscientiousness. J. Pers. Assess. 2008, 90, 158–164. [Google Scholar] [CrossRef] [PubMed]
| Measure | Subjects (n) | Mean | SD |
|---|---|---|---|
| Age | 20 | 54.40 | 9.85 |
| MCQ (k-value) | |||
| Overall | 17 | 0.017 | 0.021 |
| Small Rewards | 18 | 0.030 | 0.039 |
| Medium Rewards | 18 | 0.012 | 0.019 |
| Large Rewards | 17 | 0.014 | 0.030 |
| MADRS | 11 | 28.64 | 5.33 |
| QIDS-SR16 | 18 | 23.61 | 8.10 |
| BIS-10 | |||
| Overall | 16 | 74.81 | 20.36 |
| Cognitive-impulsivity | 16 | 29.37 | 6.04 |
| Motor-impulsivity | 16 | 21.31 | 6.91 |
| Non-planning-impulsivity | 16 | 24.56 | 9.24 |
| BFI-Fr | |||
| Conscientiousness | 18 | 28.22 | 7.34 |
| Neuroticism | 18 | 29.28 | 9.20 |
| Extraversion | 18 | 18.11 | 5.70 |
| Openness to Experience | 18 | 25.89 | 5.27 |
| Agreeableness | 18 | 41.50 | 6.16 |
| Subjects (n) | Median (Q1;Q3) | p-Value | ||
|---|---|---|---|---|
| Pre-rTMS | Post-20 Sessions | |||
| MCQ (k-value) | ||||
| Overall | 17 | 0.006 (0.002;0.025) | 0.010 (0.004;0.016) | 0.855 |
| Small Rewards | 18 | 0.015 (0.010;0.026) | 0.025 (0.010;0.065) | 0.147 |
| Medium Rewards | 18 | 0.004 (0.002;0.010) | 0.010 (0.004;0.025) | 0.029 |
| Large Rewards | 17 | 0.004 (0.002;0.010) | 0.004 (0.002;0.010) | 0.303 |
| BIS-10 | ||||
| Overall | 16 | 76 (66;90.5) | 73.5 (63.5;87.5) | 0.131 |
| Cognitive-impulsivity | 16 | 31 (26;33) | 29.5 (25;32) | 0.007 * |
| Motor-impulsivity | 16 | 20.5 (17;26.5) | 20 (14.5;26.5) | 0.293 |
| Non-planning-impulsivity | 16 | 26.5 (20.5;30.5) | 25.5 (17.5;29.5) | 0.577 |
| BFI-Fr | ||||
| Conscientiousness | 18 | 26.5 (23;34) | 27.5 (26;35) | 0.341 |
| Neuroticism | 18 | 32 (27;35) | 33 (29;35) | 0.585 |
| Extraversion | 18 | 18.5 (13;24) | 15.5 (13;21) | 0.061 |
| Openness to Experience | 18 | 25.5 (23;29) | 24.5 (21;27) | 0.297 |
| Agreeableness | 18 | 42 (38;45) | 41 (36;44) | 0.267 |
| MADRS | 11 | 27 (24;32) | 21 (15;29) | 0.023 * |
| QIDS-SR16 | 18 | 25.5 (20;28) | 19 (14;24) | 0.024 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teti Mayer, J.; Nicolier, M.; Tio, G.; Mouchabac, S.; Haffen, E.; Bennabi, D. Effects of High Frequency Repetitive Transcranial Magnetic Stimulation (HF-rTMS) on Delay Discounting in Major Depressive Disorder: An Open-Label Uncontrolled Pilot Study. Brain Sci. 2019, 9, 230. https://doi.org/10.3390/brainsci9090230
Teti Mayer J, Nicolier M, Tio G, Mouchabac S, Haffen E, Bennabi D. Effects of High Frequency Repetitive Transcranial Magnetic Stimulation (HF-rTMS) on Delay Discounting in Major Depressive Disorder: An Open-Label Uncontrolled Pilot Study. Brain Sciences. 2019; 9(9):230. https://doi.org/10.3390/brainsci9090230
Chicago/Turabian StyleTeti Mayer, Juliana, Magali Nicolier, Grégory Tio, Stephane Mouchabac, Emmanuel Haffen, and Djamila Bennabi. 2019. "Effects of High Frequency Repetitive Transcranial Magnetic Stimulation (HF-rTMS) on Delay Discounting in Major Depressive Disorder: An Open-Label Uncontrolled Pilot Study" Brain Sciences 9, no. 9: 230. https://doi.org/10.3390/brainsci9090230
APA StyleTeti Mayer, J., Nicolier, M., Tio, G., Mouchabac, S., Haffen, E., & Bennabi, D. (2019). Effects of High Frequency Repetitive Transcranial Magnetic Stimulation (HF-rTMS) on Delay Discounting in Major Depressive Disorder: An Open-Label Uncontrolled Pilot Study. Brain Sciences, 9(9), 230. https://doi.org/10.3390/brainsci9090230





