White Matter Hyperintensity Regression: Comparison of Brain Atrophy and Cognitive Profiles with Progression and Stable Groups
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MRI Acquisition
2.3. White Matter Hyperintensity Calculations
2.4. Longitudinal Change Calculations
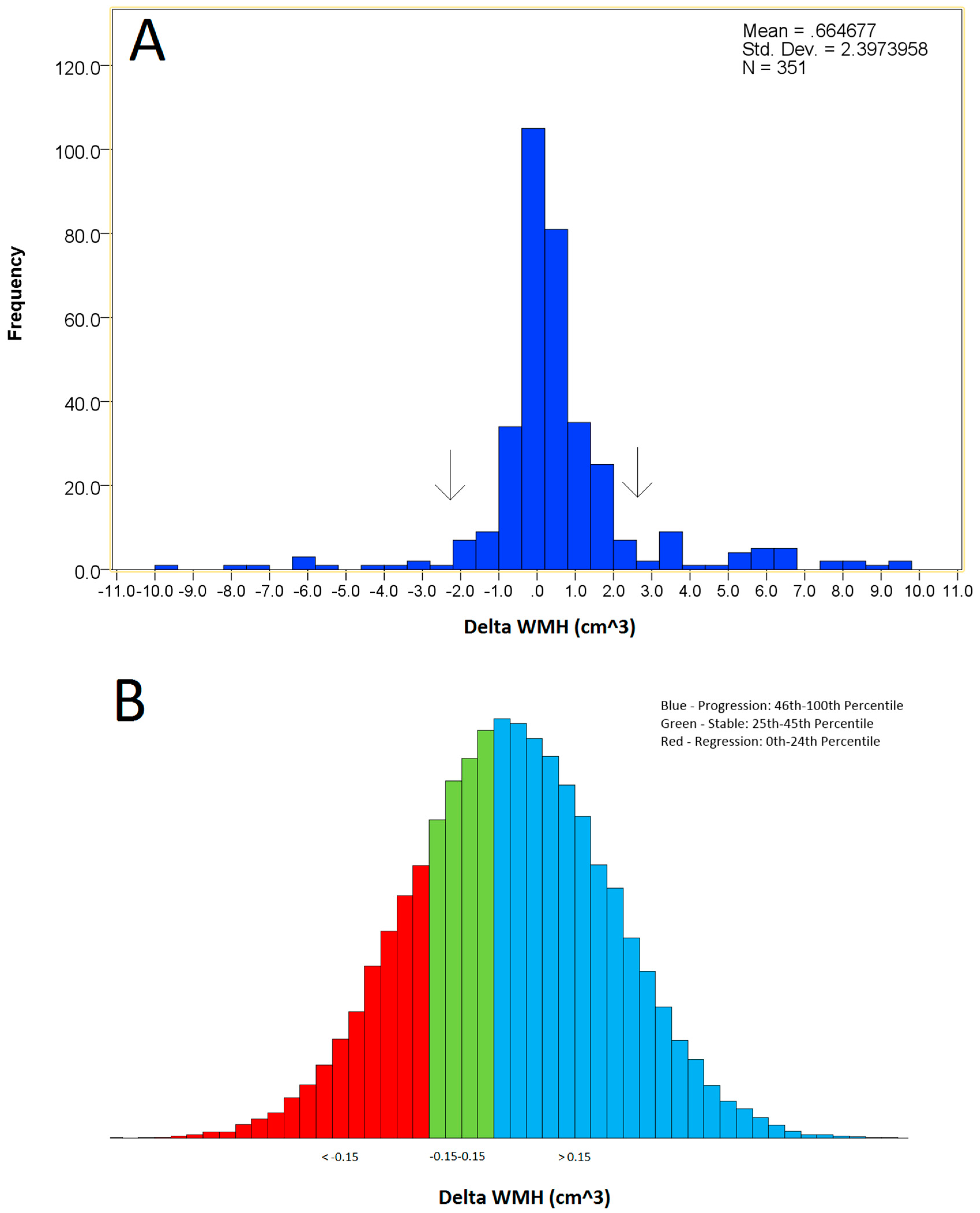
2.5. WMH Categorization
2.6. Atrophy Composite Calculation
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pantoni, L.; Garcia, J.H. The significance of cerebral white matter abnormalities 100 years after binswanger’s report: A review. Stroke 1995, 26, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Fazekas, F.; Kapeller, P.; Schmidt, H.; Hartung, H.-P. Mri white matter hyperintensities three-year follow-up of the austrian stroke prevention study. Neurology 1999, 53, 132. [Google Scholar] [CrossRef] [PubMed]
- Veldink, J.H.; Scheltens, P.; Jonker, C.; Launer, L.J. Progression of cerebral white matter hyperintensities on mri is related to diastolic blood pressure. Neurology 1998, 51, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, Z.; Fleischman, D.A.; Arfanakis, K.; Leurgans, S.E.; Barnes, L.L.; Bennett, D.A. Association of white matter hyperintensities and gray matter volume with cognition in older individuals without cognitive impairment. Brain Struct. Funct. 2016, 221, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, E.; Geerlings, M.I.; Biessels, G.J.; Nederkoorn, P.J.; Kloppenborg, R.P. White matter hyperintensities and cognition in mild cognitive impairment and alzheimer’s disease: A domain-specific meta-analysis. J. Alzheimers Dis. 2018, 63, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.; Wen, W.; Chen, X.; Brodaty, H. Progression of white matter hyperintensities in elderly individuals over 3 years. Neurology 2007, 68, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Maillard, P.; Fletcher, E.; Lockhart, S.N.; Roach, A.E.; Reed, B.; Mungas, D.; DeCarli, C.; Carmichael, O.T. White matter hyperintensities and their penumbra lie along a continuum of injury in the aging brain. Stroke 2014, 45, 1721–1726. [Google Scholar] [CrossRef]
- Van Leijsen, E.M.; Bergkamp, M.I.; van Uden, I.W.; Cooijmans, S.; Ghafoorian, M.; van der Holst, H.M.; Norris, D.G.; Kessels, R.P.; Platel, B.; Tuladhar, A.M.; et al. Cognitive consequences of regression of cerebral small vessel disease. Eur. Stroke J. 2019, 4, 85–89. [Google Scholar] [CrossRef]
- Van Leijsen Esther, M.C.; de Leeuw, F.-E.; Tuladhar Anil, M. Disease progression and regression in sporadic small vessel disease–insights from neuroimaging. Clin. Sci. 2017, 131, 1191–1206. [Google Scholar] [CrossRef]
- Schwarz, C.; Fletcher, E.; DeCarli, C.; Carmichael, O. Fully-automated white matter hyperintensity detection with anatomical prior knowledge and without flair. Inf. Process. Med. Imaging 2009, 21, 239–251. [Google Scholar]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; Van Der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef]
- Fischl, B.; Salat, D.H.; Van Der Kouwe, A.J.; Makris, N.; Ségonne, F.; Quinn, B.T.; Dale, A.M. Sequence-independent segmentation of magnetic resonance images. Neuroimage 2004, 23, S69–S84. [Google Scholar] [CrossRef] [PubMed]
- Crane, P.K.; Carle, A.; Gibbons, L.E.; Insel, P.; Mackin, R.S.; Gross, A.; Jones, R.N.; Mukherjee, S.; Curtis, S.M.; Harvey, D. Development and assessment of a composite score for memory in the alzheimer’s disease neuroimaging initiative (adni). Brain Imaging Behav. 2012, 6, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, L.E.; Carle, A.C.; Mackin, R.S.; Harvey, D.; Mukherjee, S.; Insel, P.; Curtis, S.M.; Mungas, D.; Crane, P.K.; Initiative, A.S.D.N. A composite score for executive functioning, validated in alzheimer’s disease neuroimaging initiative (adni) participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012, 6, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bernstein, M.A.; Fox, N.C.; Thompson, P.; Alexander, G.; Harvey, D.; Borowski, B.; Britson, P.J.; LWhitwell, J.; Ward, C.; et al. The alzheimer’s disease neuroimaging initiative (adni): Mri methods. J. Magn. Reson. Imaging 2008, 27, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.; Aisen, P.; Beckett, L.; Donohue, M.; Gamst, A.; Harvey, D.; Jack, C.; Jagust, W.; Shaw, L.; Toga, A. Alzheimer’s disease neuroimaging initiative (adni) clinical characterization. Neurology 2010, 74, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Van Leijsen, E.M.C.; van Uden, I.W.M.; Ghafoorian, M.; Bergkamp, M.I.; Lohner, V.; Kooijmans, E.C.M.; van der Holst, H.M.; Tuladhar, A.M.; Norris, D.G.; van Dijk, E.J.; et al. Nonlinear temporal dynamics of cerebral small vessel disease: The RUN DMC study. Neurology 2017, 89, 1569–1577. [Google Scholar] [CrossRef]
- DeCarli, C.; Maillard, P.; Fletcher, E. Four Tissue Segmentation in Adni II. Alzheimer’s Disease Neuroimaging Initiative. Department of Neurology and Neurology and Center for Neuroscience, University of California at Davis. Available online: https://www.alz.washington.edu/WEB/adni_proto.pdf (accessed on 18 December 2013).
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.; Woolrich, M.W.; Smith, S.M. Fsl. Neuroimage 2012, 62, 782–790. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E. Advances in functional and structural mr image analysis and implementation as fsl. Neuroimage 2004, 23, S208–S219. [Google Scholar] [CrossRef]
- Coutu, J.-P.; Goldblatt, A.; Rosas, H.D.; Salat, D.H. White matter changes are associated with ventricular expansion in aging, mild cognitive impairment, and alzheimer’s disease. J. Alzheimers Dis. 2016, 49, 329–342. [Google Scholar] [CrossRef]
- Ramirez, J.; McNeely, A.A.; Berezuk, C.; Gao, F.; Black, S.E. Dynamic progression of white matter hyperintensities in alzheimer’s disease and normal aging: Results from the sunnybrook dementia study. Front. Aging Neurosci. 2016, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- DeCarli, C.; Murphy, D.; Tranh, M.; Grady, C.; Haxby, J.; Gillette, J.; Salerno, J.; Gonzales-Aviles, A.; Honvitz, B.; Rapoport, S. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology 1995, 45, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Cees De Groot, J.; De Leeuw, F.E.; Oudkerk, M.; Van Gijn, J.; Hofman, A.; Jolles, J.; Breteler, M.M. Cerebral white matter lesions and cognitive function: The rotterdam scan study. Ann. Neurol. 2000, 47, 145–151. [Google Scholar] [CrossRef]
- Silbert, L.C.; Nelson, C.; Howieson, D.B.; Moore, M.M.; Kaye, J.A. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology 2008, 71, 108–113. [Google Scholar] [CrossRef]
- Longstreth, W.; Arnold, A.M.; Beauchamp, N.J.; Manolio, T.A.; Lefkowitz, D.; Jungreis, C.; Hirsch, C.H.; O’leary, D.H.; Furberg, C.D. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: The cardiovascular health study. Stroke 2005, 36, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Chappell, F.M.; Valdés Hernández, M.D.C.; Makin, S.D.J.; Staals, J.; Shuler, K.; Thrippleton, M.J.; Armitage, P.A.; Muñoz-Maniega, S.; Heye, A.K.; et al. White matter hyperintensity reduction and outcomes after minor stroke. Neurology 2017, 89, 1003–1010. [Google Scholar] [CrossRef]
- Cho, A.H.; Kim, H.-R.; Kim, W.; Yang, D.W. White matter hyperintensity in ischemic stroke patients: It may regress over time. J. Stroke 2015, 17, 60–66. [Google Scholar] [CrossRef]
- Lammie, G.; Brannan, F.; Wardlaw, J. Incomplete lacunar infarction (type i b lacunes). Acta Neuropathol. 1998, 96, 163–171. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Valdés Hernández, M.C.; Muñoz-Maniega, S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J. Am. Heart Assoc. 2015, 4, 001140. [Google Scholar] [CrossRef]
- Yao, M.; Jouvent, E.; During, M.; Godin, O.; Herve, D.; Guichard, J.P.; Zhu, Y.C.; Gschwendtner, A.; Opherk, C.; Dichgans, M.; et al. Extensive white matter hyperintensities may increase brain volume in cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke 2012, 43, 3252–3257. [Google Scholar] [CrossRef] [PubMed]
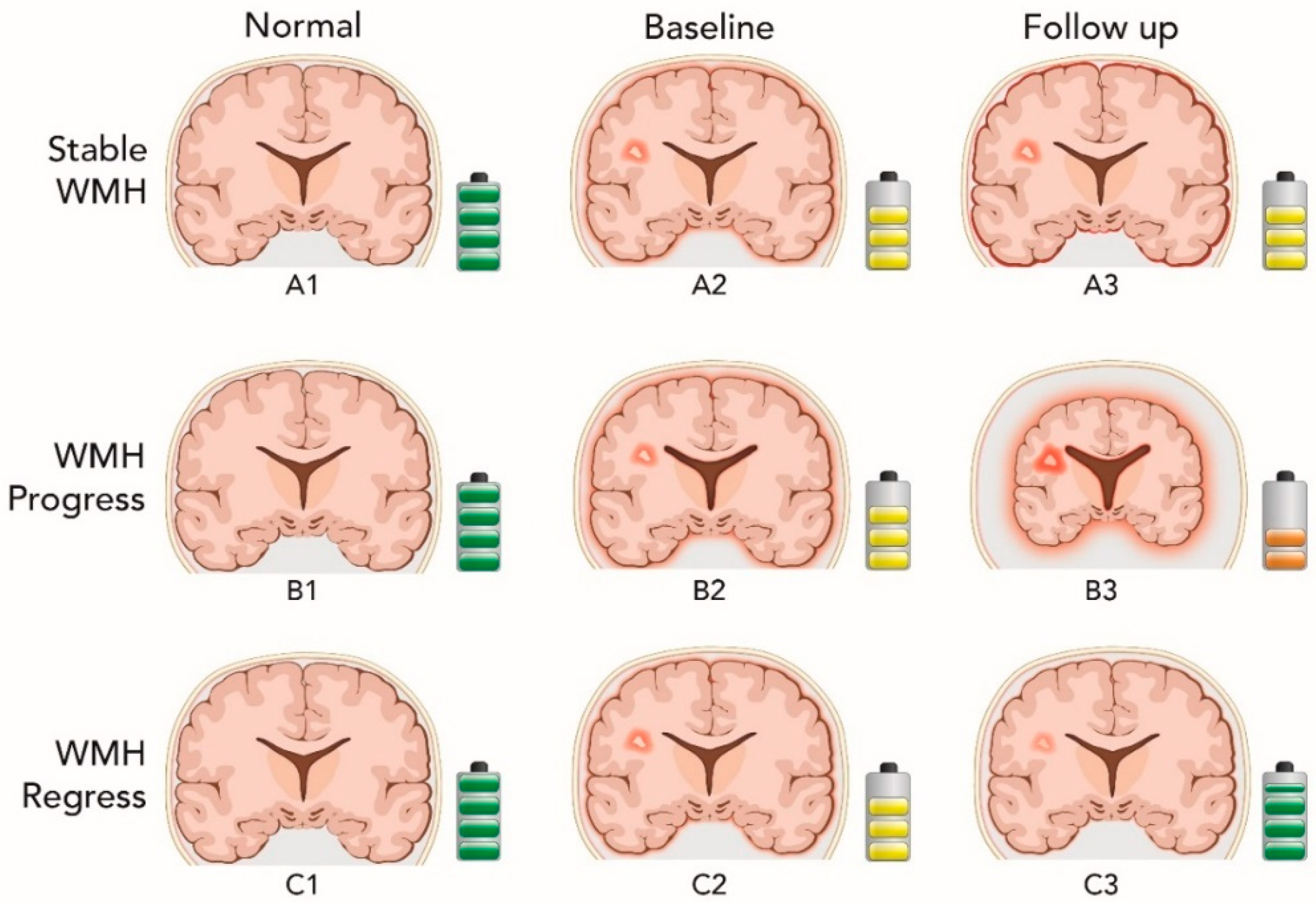
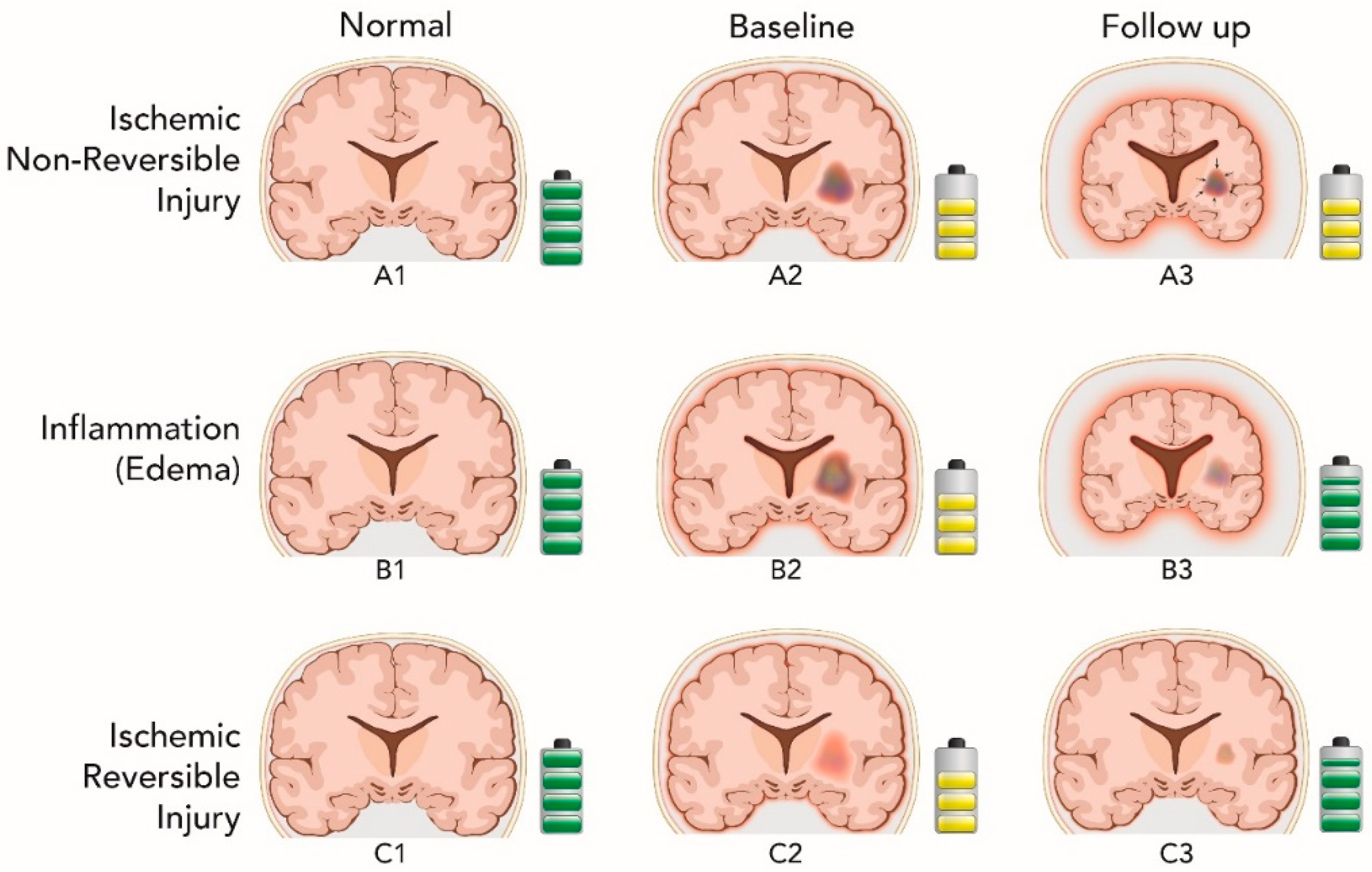
| Possible Etiology for WMH | Potential Cause of Regression | Expected Association with Cerebral Atrophy | Expected Association with Cognitive Performance |
|---|---|---|---|
| Irreversible ischemic injury | Gliotic contraction and microscopic encephalomalacia | Increased atrophy | No change in cognitive performance |
| Inflammation associated with irreversible ischemic injury | Resolution of inflammation and edema with restoration of normal function in the penumbra | Increased atrophy secondary to reduced inflammatory edema | Improvement in cognitive performance |
| Reversible ischemic injury | Healing process | Decreased atrophy | Improvement in cognitive performance |
| Criteria | Progressors (n = 190) | Regressors (n = 93) | Stable (n = 68) | Significance | n; (Progressors, Regressors, Stable) |
|---|---|---|---|---|---|
| Age; (mean, SD) | 72.2 (6.9) | 72.0 (7.3) | 70.3 (7.2) | 0.163 | 190, 93, 68 |
| Education; (mean, SD) | 16.4 (2.6) | 16.7 (1.6) | 16.7 (2.4) | 0.654 | 190, 93, 68 |
| Female; (n, %) | 98 (51.6) | 40 (43.0) | 34 (50.0) | 0.393 | 190, 93, 68 |
| Currently Married; (n, %) | 140 (73.7) | 72 (77.4) | 50 (73.5) | 0.764 | 190, 93, 68 |
| Cognitively Normal; (n, %) | 71 (37.4) | 38 (40.9) | 32 (47.1) | 0.371 | 190, 93, 68 |
| MCI (n, %) | 119 (62.6) | 55 (59.1) | 36 (52.9) | 0.371 | 190, 93, 68 |
| Baseline WMH; (mean, SD) | 6.9 (10.3) | 8.2 (10.6) | 1.9 (2.0) | - | 190, 93, 68 |
| Follow Up WMH; (mean, SD) | 8.7 (11.6) | 6.9 (9.5) | 1.9 (2.0) | - | 190, 93, 68 |
| Δ Memory; (mean, SD) | −0.07 (0.35) | 0.02 (0.32) | 0.05 (0.32) | - | 184, 89, 65 |
| Δ EF; (mean, SD) | −0.06 (0.59) | 0.00 (0.62) | 0.04 (0.56) | - | 182, 90, 65 |
| Δ Atrophy Comp; (mean, SD) | 0.19 (1.40) | −0.17 (1.36) | −0.52 (1.08) | - | 179, 90, 63 |
| Δ Brain Volume; (mean, SD) | −0.07 (0.90) | 0.15 (1.0) | 0.18 (0.78) | - | 187, 93, 68 |
| Δ Ventricular Volume; (mean, SD) | 0.12 (0.94) | −0.08 (0.69) | −0.34 (0.65) | - | 182, 90, 63 |
| ANCOVA | Post-Hoc Comparisons | p-Value | p-Value (FDR-Corrected) |
|---|---|---|---|
| Dependent Variable | |||
| Δ Memory | 0.017 * | 0.028 * | |
| Progression/Regression | 0.024 * | 0.036 * | |
| Progression/Stable | 0.019 * | 0.036 * | |
| Regression/Stable | 0.766 | 0.766 | |
| Δ EF | 0.492 | 0.492 | |
| Progression/Regression | 0.398 | 0.398 | |
| Progression/Stable | 0.293 | 0.293 | |
| Regression/Stable | 0.790 | 0.790 | |
| Δ Atrophy Composite | 0.001 ‡ | 0.005 ** | |
| Progression/Regression | 0.027 * | 0.041 * | |
| Progression/Stable | 0.001‡ | 0.003 ** | |
| Regression/Stable | 0.172 | 0.172 | |
| Δ Ventricular Volume | 0.011 * | 0.028 * | |
| Progression/Regression | 0.036 * | 0.054 | |
| Progression/Stable | 0.007 ** | 0.021 * | |
| Regression/Stable | 0.443 | 0.433 | |
| Δ Brain Volume | 0.061 | 0.076 | |
| Progression/Regression | 0.054 | 0.090 | |
| Progression/Stable | 0.060 | 0.090 | |
| Regression/Stable | 0.887 | 0.887 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Janabi, O.M.; Bauer, C.E.; Goldstein, L.B.; Murphy, R.R.; Bahrani, A.A.; Smith, C.D.; Wilcock, D.M.; Gold, B.T.; Jicha, G.A. White Matter Hyperintensity Regression: Comparison of Brain Atrophy and Cognitive Profiles with Progression and Stable Groups. Brain Sci. 2019, 9, 170. https://doi.org/10.3390/brainsci9070170
Al-Janabi OM, Bauer CE, Goldstein LB, Murphy RR, Bahrani AA, Smith CD, Wilcock DM, Gold BT, Jicha GA. White Matter Hyperintensity Regression: Comparison of Brain Atrophy and Cognitive Profiles with Progression and Stable Groups. Brain Sciences. 2019; 9(7):170. https://doi.org/10.3390/brainsci9070170
Chicago/Turabian StyleAl-Janabi, Omar M., Christopher E. Bauer, Larry B. Goldstein, Richard R. Murphy, Ahmed A. Bahrani, Charles D. Smith, Donna M. Wilcock, Brian T. Gold, and Gregory A. Jicha. 2019. "White Matter Hyperintensity Regression: Comparison of Brain Atrophy and Cognitive Profiles with Progression and Stable Groups" Brain Sciences 9, no. 7: 170. https://doi.org/10.3390/brainsci9070170
APA StyleAl-Janabi, O. M., Bauer, C. E., Goldstein, L. B., Murphy, R. R., Bahrani, A. A., Smith, C. D., Wilcock, D. M., Gold, B. T., & Jicha, G. A. (2019). White Matter Hyperintensity Regression: Comparison of Brain Atrophy and Cognitive Profiles with Progression and Stable Groups. Brain Sciences, 9(7), 170. https://doi.org/10.3390/brainsci9070170





