The Serum Melatonin Levels and Mortality of Patients with Spontaneous Intracerebral Hemorrhage
Abstract
1. Introduction
2. Methods
2.1. Design and Subjects
2.2. Variables Recorded
2.3. Blood Samples and Determination of Serum Melatonin Levels
2.4. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hemphill, J.C., 3rd; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; Macdonald, R.L.; Mitchell, P.H.; et al. American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2015, 46, 2032–2060. [Google Scholar]
- Aronowski, J.; Zhao, X. Molecular pathophysiology of cerebral hemorrhage: Secondary brain injury. Stroke 2011, 42, 1781–1786. [Google Scholar] [CrossRef] [PubMed]
- Aronowski, J.; Hall, C.E. New horizons for primary intracerebral hemorrhage treatment: Experience from preclinical studies. Neurol. Res. 2005, 27, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, H. Exploring neuroprotective drug therapies for intracerebral hemorrhage. J. Pharmacol. Sci. 2010, 114, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Cagnacci, A. Melatonin in relation to physiology in adult humans. J. Pineal Res. 1996, 21, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.; Encel, N. Melatonin and sleep in humans. J. Pineal Res. 1993, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Paredes, S.D.; Manchester, L.C.; Tan, D.X. Reducing oxidative/nitrosative stress: A newly-discovered genre for melatonin. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Wu, C.; Niu, H.J.; Wang, K.; Mo, L.J.; Shao, A.W.; Dixon, B.J.; Zhang, J.M.; Yang, S.X.; Wang, Y.R. Neuroprotective Mechanisms of Melatonin in Hemorrhagic Stroke. Cell. Mol. Neurobiol. 2017, 37, 1173–1185. [Google Scholar] [CrossRef]
- Katsuki, H.; Hijioka, M. Intracerebral Hemorrhage as an Axonal Tract Injury Disorder with Inflammatory Reactions. Biol. Pharm. Bull. 2017, 40, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Martín, M.M.; Abreu-González, P.; Sabatel, R.; Ramos, L.; Argueso, M.; Solé-Violán, J.; Riaño-Ruiz, M.; Jiménez, A.; García-Marín, V. Serum Malondialdehyde Levels and Mortality in Patients with Spontaneous Intracerebral Hemorrhage. World Neurosurg. 2018, 113, e542–e547. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Hemphill, J.C., 3rd; Bonovich, D.C.; Besmertis, L.; Manley, G.T.; Johnston, S.C. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001, 32, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Martín, M.M.; Abreu-González, P.; Pérez-Cejas, A.; Ramos, L.; Argueso, M.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; García-Marín, V. Serum melatonin levels in survivor and non-survivor patients with traumatic brain injury. BMC Neurol. 2017, 17, 138. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Abreu-González, P.; Pérez-Cejas, A.; Ramos, L.; Argueso, M.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; García-Marín, V. Serum melatonin levels are associated with mortality in patients with malignant middle cerebral artery infarction. J. Int. Med. Res. 2018, 46, 3268–3277. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Abreu-González, P.; Ramos, L.; Argueso, M.; Cáceres, J.J.; Solé-Violán, J.; Lorenzo, J.M.; Molina, I.; Jiménez, A. Association between serum malondialdehyde levels and mortality in patients with severe brain trauma injury. J. Neurotrauma 2015, 32, 1–6. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Abreu-González, P.; Ramos, L.; Argueso, M.; Solé-Violán, J.; Riaño-Ruiz, M.; Jiménez, A. Serum malondialdehyde levels in patients with malignant middle cerebral artery infarction are associated with mortality. PLoS ONE 2015, 10, e0125893. [Google Scholar] [CrossRef]
- Ueda, Y.; Masuda, T.; Ishida, A.; Misumi, S.; Shimizu, Y.; Jung, C.G.; Hida, H. Enhanced electrical responsiveness in the cerebral cortex with oral melatonin administration after a small hemorrhage near the internal capsule in rats. J. Neurosci. Res. 2014, 92, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Lekic, T.; Hartman, R.; Rojas, H.; Manaenko, A.; Chen, W.; Ayer, R.; Tang, J.; Zhang, J.H. Protective effect of melatonin upon neuropathology, striatal function, and memory ability after intracerebral hemorrhage in rats. J. Neurotrauma 2010, 27, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Liang, G.B.; Xue, Y.X.; Liu, Y.H. Effects of combination treatment of dexamethasone and melatonin on brain injury in intracerebral hemorrhage model in rats. Brain Res. 2009, 1264, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Rojas, H.; Lekic, T.; Chen, W.; Jadhav, V.; Titova, E.; Martin, R.D.; Tang, J.; Zhang, J. The antioxidant effects of melatonin after intracerebral hemorrhage in rats. Acta Neurochir. Suppl. 2008, 105, 19–21. [Google Scholar] [PubMed]
- Wang, Z.; Zhou, F.; Dou, Y.; Tian, X.; Liu, C.; Li, H.; Shen, H.; Chen, G. Melatonin Alleviates Intracerebral Hemorrhage-Induced Secondary Brain Injury in Rats via Suppressing Apoptosis, Inflammation, Oxidative Stress, DNA Damage, and Mitochondria Injury. Transl. Stroke Res. 2018, 9, 74–91. [Google Scholar] [CrossRef]
- Seifman, M.A.; Gomes, K.; Nguyen, P.N.; Bailey, M.; Rosenfeld, J.V.; Cooper, D.J.; Morganti-Kossmann, M.C. Measurement of serum melatonin in intensive care unit patients: Changes in traumatic brain injury, trauma, and medical conditions. Front. Neurol. 2014, 5, 237. [Google Scholar] [CrossRef] [PubMed]
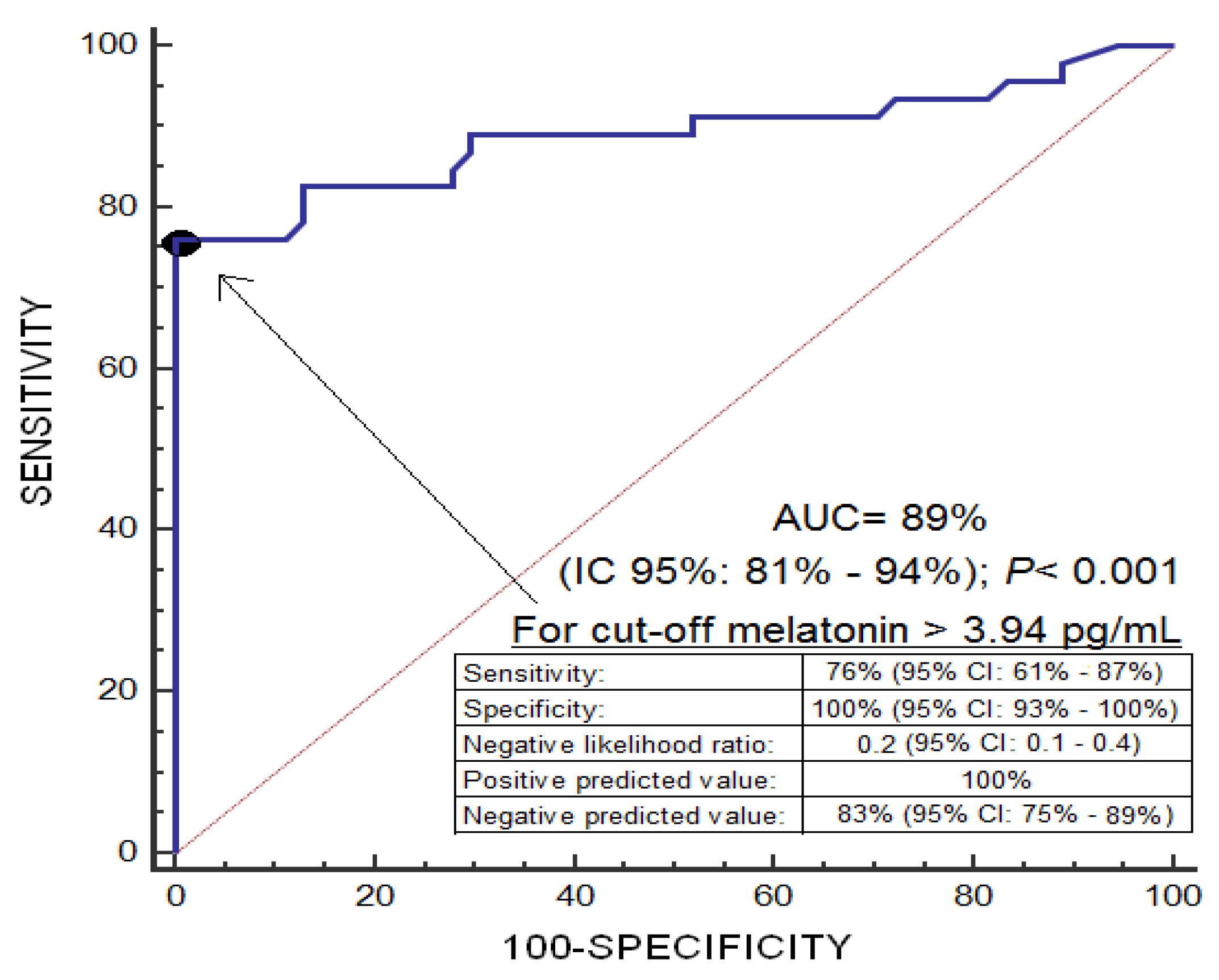
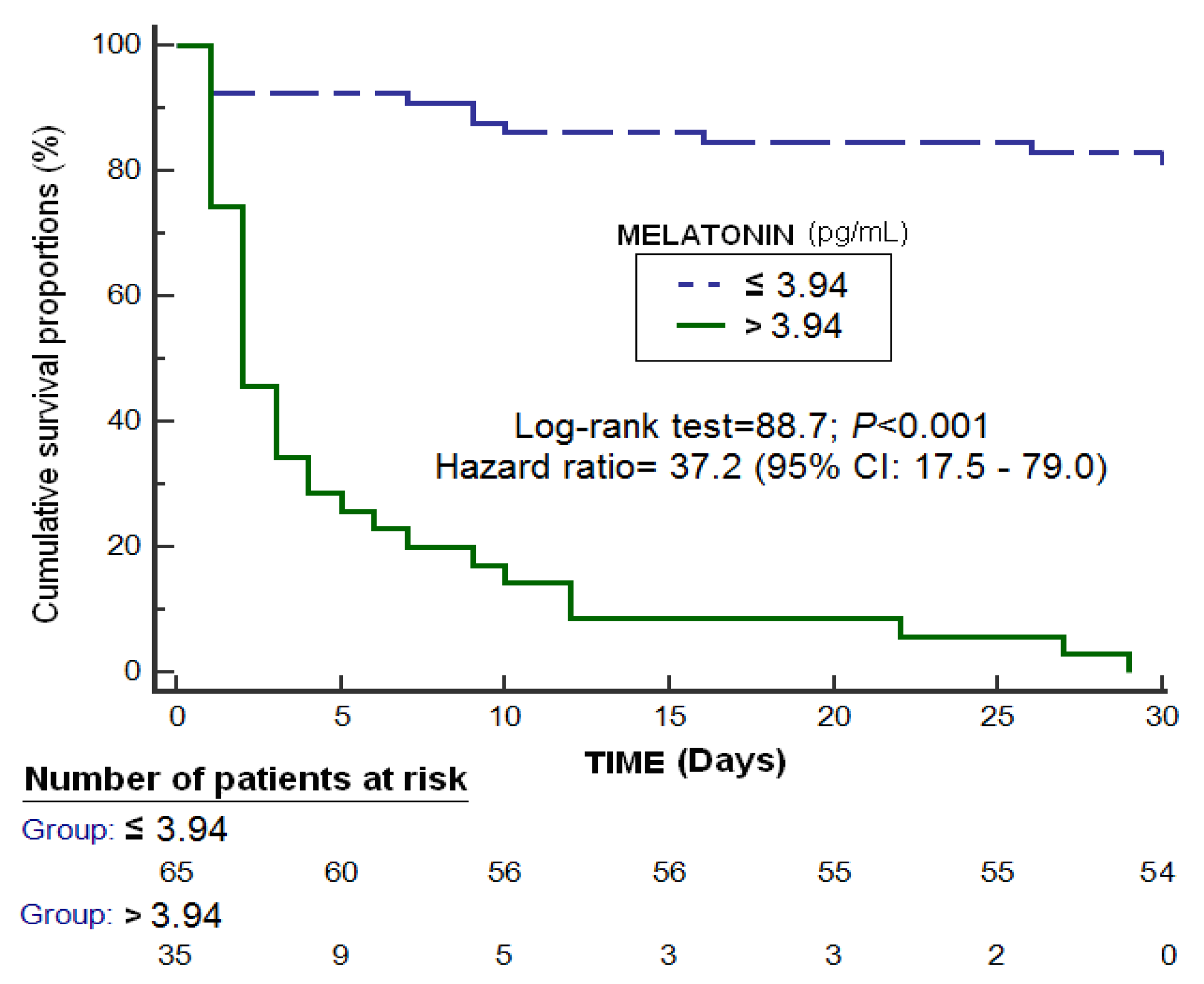
| Non-Surviving (n = 46) | Surviving (n = 54) | p-Value | |
|---|---|---|---|
| Gender female—n (%) | 17 (37.0) | 17 (31.5) | 0.67 |
| Age (years)—median (p 25–75) | 68 (57–74) | 59 (52–67) | 0.006 |
| Site of SIH—n (%) | 0.81 | ||
| Lobar | 33 (71.7) | 41 (75.9) | |
| Basal ganglia | 4 (8.7) | 3 (5.6) | |
| Thalamus | 3 (6.5) | 5 (9.3) | |
| Periventricular | 6 (13.0) | 5 (9.3) | |
| Volume of SIH (cc)—median (p 25–75) | 68 (29–99) | 38 (17–62) | 0.02 |
| Transtentorial herniation—n (%) | 1 (2.2) | 2 (3.7) | 0.99 |
| Midline shift (mm)—median (p 25–75) | 5 (0–11) | 1 (0–7) | 0.005 |
| Hydrocephalus—n (%) | 26 (56.5) | 21 (38.9) | 0.11 |
| Intraventricular hemorrhage—n (%) | 23 (50.0) | 17 (31.5) | 0.07 |
| Cause of SIH—n (%) | 0.07 | ||
| Hypertension | 30 (65.2) | 37 (68.5) | |
| Amyloid angiopathy | 4 (8.7) | 2 (3.7) | |
| Aneurysm | 0 | 3 (5.6) | |
| Arteriovenous malformation | 0 | 5 (9.3) | |
| OAT in therapeutic range | 6 (13.0) | 3 (5.6) | |
| OAT out of therapeutic range | 6 (13.0) | 3 (5.6) | |
| Fibrinolytic treatment | 0 | 1 (1.9) | |
| ICH score—median (p 25–75) | 3 (2–3) | 2 (1–2) | <0.001 |
| GCS—median (p 25–75) | 4 (3–6) | 8 (6–8) | <0.001 |
| APACHE-II score—median (p 25–75) | 24 (20–26) | 18 (14–20) | <0.001 |
| PaO2/FI02 ratio—median (p 25–75) | 289 (215–397) | 270 (189–350) | 0.40 |
| Creatinine (mg/dL)—median (p 25–75) | 0.80 (0.60–1.01) | 0.77 (0.68–0.90) | 0.36 |
| Sodium (mEq/L)—median (p 25–75) | 139 (135–143) | 139 (137–142) | 0.93 |
| Glycemia (g/dL)—median (p 25–75) | 170 (141–216) | 141 (118–190) | 0.01 |
| Lactic acid (mmol/L)—median (p 25–75) | 1.80 (1.30–2.55) | 1.70 (1.00–2.51) | 0.23 |
| INR—median (p 25–75) | 1.14 (1.02–1.87) | 1.10 (1.00–1.31) | 0.34 |
| Fibrinogen (mg/dL)—median (p 25–75) | 382 (350–510) | 390 (280–493) | 0.34 |
| Platelets—median × 103/mm3 (p 25–75) | 198 (159–270) | 193 (145–252) | 0.57 |
| aPTT (s)—median (p 25–75) | 30 (24–34) | 29 (27–32) | 0.68 |
| Early evacuation of SIH—n (%) | 9 (19.6) | 18 (33.3) | 0.18 |
| Melatonin (pg/mL)—median (p 25–75) | 4.78 (3.90–11.10) | 2.62 (2.17–3.08) | <0.001 |
| Variable | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| First model | |||
| Serum melatonin levels (pg/mL) | 8.16 | 2.30–28.59 | 0.001 |
| Glycemia (g/dL) | 0.99 | 0.97–1.01 | 0.23 |
| Early evacuation of SIH (yes vs. non) | 0.11 | 0.01–1.07 | 0.06 |
| ICH score (points) | 2.86 | 0.87–9.38 | 0.08 |
| Midline shift (mm) | 1.27 | 1.05–1.53 | 0.01 |
| Second model | |||
| Serum melatonin levels (pg/mL) | 7.75 | 2.05–29.27 | 0.003 |
| Age (years) | 1.11 | 1.01–1.22 | 0.03 |
| Volume of SIH (cc) | 1.02 | 0.99–1.04 | 0.08 |
| GCS (points) | 0.37 | 0.21–0.66 | 0.001 |
| Intraventricular hemorrhage (yes vs. non) | 1.26 | 0.24–6.67 | 0.79 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorente, L.; Martín, M.M.; Abreu-González, P.; Ramos, L.; Argueso, M.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; García-Marín, V. The Serum Melatonin Levels and Mortality of Patients with Spontaneous Intracerebral Hemorrhage. Brain Sci. 2019, 9, 263. https://doi.org/10.3390/brainsci9100263
Lorente L, Martín MM, Abreu-González P, Ramos L, Argueso M, Solé-Violán J, Cáceres JJ, Jiménez A, García-Marín V. The Serum Melatonin Levels and Mortality of Patients with Spontaneous Intracerebral Hemorrhage. Brain Sciences. 2019; 9(10):263. https://doi.org/10.3390/brainsci9100263
Chicago/Turabian StyleLorente, Leonardo, María M. Martín, Pedro Abreu-González, Luis Ramos, Mónica Argueso, Jordi Solé-Violán, Juan J. Cáceres, Alejandro Jiménez, and Victor García-Marín. 2019. "The Serum Melatonin Levels and Mortality of Patients with Spontaneous Intracerebral Hemorrhage" Brain Sciences 9, no. 10: 263. https://doi.org/10.3390/brainsci9100263
APA StyleLorente, L., Martín, M. M., Abreu-González, P., Ramos, L., Argueso, M., Solé-Violán, J., Cáceres, J. J., Jiménez, A., & García-Marín, V. (2019). The Serum Melatonin Levels and Mortality of Patients with Spontaneous Intracerebral Hemorrhage. Brain Sciences, 9(10), 263. https://doi.org/10.3390/brainsci9100263





