Does Video Gaming Have Impacts on the Brain: Evidence from a Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Quality Assessment
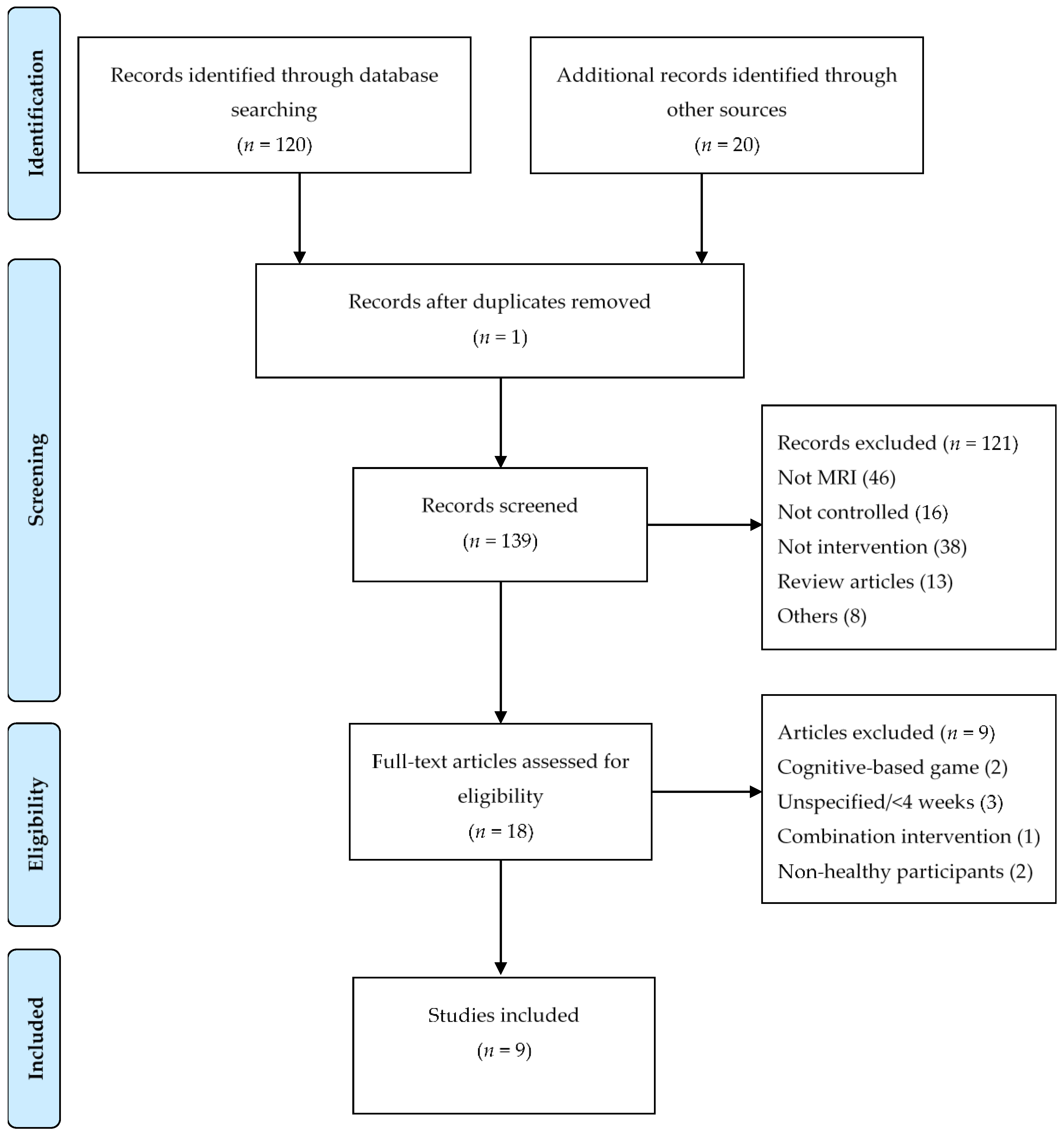
3.2. Inclusion and Exclusion
3.3. Control Group
3.4. Game Title and Genre
3.5. Participants and Sample Size
3.6. Training Period and Intensity
3.7. MRI Analysis and Specifications
4. Discussion
4.1. Participant Age
4.2. Beneficial Effects
4.3. Duration
4.4. Criteria
4.5. Limitations and Recommendations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Section/Topic | # | Checklist Item | Reported on Page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 1, 2 |
| Objectives | 4 | Provide an explicit statement of questions being addressed related to participants, interventions, comparisons, outcomes, and study design (PICOS). | 2 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it is accessible (e.g., Web address), and if available, provide registration information including registration number. | 2 |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 2 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 2 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 2 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and if applicable, included in the meta-analysis). | 3 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 3 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 3 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 2 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | - |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | - |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that might affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | - |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | - |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 3,5 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 5-11 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study, and if available, any outcome level assessment (see item 12). | 5,6 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 4 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | - |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | - |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | - |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 12,13 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 13 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 14 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 14 |
References
- Ryan, R.M.; Rigby, C.S.; Przybylski, A. The Motivational Pull of Video Games: A Self-Determination Theory Approach. Motiv. Emot. 2006, 30, 344–360. [Google Scholar] [CrossRef]
- Gentile, D.A.; Gentile, J.R. Violent Video Games as Exemplary Teachers: A Conceptual Analysis. J. Youth Adolesc. 2008, 37, 127–141. [Google Scholar] [CrossRef]
- McDougall, J.; Duncan, M.J. Children, video games and physical activity: An exploratory study. Int. J. Disabil. Hum. Dev. 2008, 7, 89–94. [Google Scholar] [CrossRef]
- Ni Mhurchu, C.; Maddison, R.; Jiang, Y.; Jull, A.; Prapavessis, H.; Rodgers, A. Couch potatoes to jumping beans: A pilot study of the effect of active video games on physical activity in children. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.C.-S.; Carson, L.; Neal, W.; Baylis, C.; Donley, D.; Yeater, R. Effects of an exercise intervention using Dance Dance Revolution on endothelial function and other risk factors in overweight children. Int. J. Pediatr. Obes. 2009, 4, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Maddison, R.; Foley, L.; Ni Mhurchu, C.; Jiang, Y.; Jull, A.; Prapavessis, H.; Hohepa, M.; Rodgers, A. Effects of active video games on body composition: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 94, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Cole, H.; Griffiths, M.D. Social Interactions in Massively Multiplayer Online Role-Playing Gamers. Cyberpsychol. Behav. 2007, 10, 575–583. [Google Scholar] [CrossRef]
- Gentile, D.A.; Anderson, C.A.; Yukawa, S.; Ihori, N.; Saleem, M.; Ming, L.K.; Shibuya, A.; Liau, A.K.; Khoo, A.; Bushman, B.J.; et al. The Effects of Prosocial Video Games on Prosocial Behaviors: International Evidence from Correlational, Longitudinal, and Experimental Studies. Pers. Soc. Psychol. Bull. 2009, 35, 752–763. [Google Scholar] [CrossRef]
- Greitemeyer, T.; Osswald, S. Effects of prosocial video games on prosocial behavior. J. Pers. Soc. Psychol. 2010, 98, 211. [Google Scholar] [CrossRef]
- Spence, I.; Feng, J. Video Games and Spatial Cognition. Rev. Gen. Psychol. 2010, 14, 92–104. [Google Scholar] [CrossRef]
- Wouters, P.; van Nimwegen, C.; van Oostendorp, H.; van der Spek, E.D. A meta-analysis of the cognitive and motivational effects of serious games. J. Educ. Psychol. 2013, 105, 249. [Google Scholar] [CrossRef]
- Toril, P.; Reales, J.M.; Ballesteros, S. Video game training enhances cognition of older adults: A meta-analytic study. Psychol. Aging 2014, 29, 706. [Google Scholar] [CrossRef] [PubMed]
- Shams, T.A.; Foussias, G.; Zawadzki, J.A.; Marshe, V.S.; Siddiqui, I.; Müller, D.J.; Wong, A.H.C. The Effects of Video Games on Cognition and Brain Structure: Potential Implications for Neuropsychiatric Disorders. Curr. Psychiatry Rep. 2015, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Wilms, I.L.; Petersen, A.; Vangkilde, S. Intensive video gaming improves encoding speed to visual short-term memory in young male adults. Acta Psychol. 2013, 142, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, L.; Sellaro, R.; Stock, A.-K.; Beste, C.; Colzato, L.S. Action Video Gaming and Cognitive Control: Playing First Person Shooter Games Is Associated with Improved Action Cascading but Not Inhibition. PLoS ONE 2015, 10, e0144364. [Google Scholar] [CrossRef] [PubMed]
- Moisala, M.; Salmela, V.; Hietajärvi, L.; Carlson, S.; Vuontela, V.; Lonka, K.; Hakkarainen, K.; Salmela-Aro, K.; Alho, K. Gaming is related to enhanced working memory performance and task-related cortical activity. Brain Res. 2017, 1655, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Nouchi, R.; Taki, Y.; Takeuchi, H.; Hashizume, H.; Akitsuki, Y.; Shigemune, Y.; Sekiguchi, A.; Kotozaki, Y.; Tsukiura, T.; Yomogida, Y.; et al. Brain Training Game Improves Executive Functions and Processing Speed in the Elderly: A Randomized Controlled Trial. PLoS ONE 2012, 7, e29676. [Google Scholar] [CrossRef] [PubMed]
- Nouchi, R.; Taki, Y.; Takeuchi, H.; Hashizume, H.; Nozawa, T.; Kambara, T.; Sekiguchi, A.; Miyauchi, C.M.; Kotozaki, Y.; Nouchi, H.; et al. Brain Training Game Boosts Executive Functions, Working Memory and Processing Speed in the Young Adults: A Randomized Controlled Trial. PLoS ONE 2013, 8, e55518. [Google Scholar] [CrossRef] [PubMed]
- Palaus, M.; Marron, E.M.; Viejo-Sobera, R.; Redolar-Ripoll, D. Neural Basis of Video Gaming: A Systematic Review. Front. Hum. Neurosci. 2017, 11, 248. [Google Scholar] [CrossRef]
- Draganski, B.; Gaser, C.; Busch, V.; Schuierer, G.; Bogdahn, U.; May, A. Changes in grey matter induced by training. Nature 2004, 427, 312. [Google Scholar] [CrossRef]
- Gauthier, L.V.; Taub, E.; Perkins, C.; Ortmann, M.; Mark, V.W.; Uswatte, G. Remodeling the Brain: Plastic Structural Brain Changes Produced by Different Motor Therapies After Stroke. Stroke 2008, 39, 1520. [Google Scholar] [CrossRef] [PubMed]
- Engvig, A.; Fjell, A.M.; Westlye, L.T.; Skaane, N.V.; Dale, A.M.; Holland, D.; Due-Tønnessen, P.; Sundseth, Ø.; Walhovd, K.B. Effects of Cognitive Training on Gray Matter Volumes in Memory Clinic Patients with Subjective Memory Impairment. JAD 2014, 41, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Matura, S.; Fleckenstein, J.; Deichmann, R.; Engeroff, T.; Füzéki, E.; Hattingen, E.; Hellweg, R.; Lienerth, B.; Pilatus, U.; Schwarz, S.; et al. Effects of aerobic exercise on brain metabolism and grey matter volume in older adults: Results of the randomised controlled SMART trial. Transl. Psychiatry 2017, 7, e1172. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, K.; Lüders, A.; Hökelmann, A.; Lessmann, V.; Kaufmann, J.; Brigadski, T.; Müller, P.; Müller, N.G. Dance training is superior to repetitive physical exercise in inducing brain plasticity in the elderly. PLoS ONE 2018, 13, e0196636. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.J.; Bailey, J.A.; Zatorre, R.J.; Penhune, V.B. Early Musical Training and White-Matter Plasticity in the Corpus Callosum: Evidence for a Sensitive Period. J. Neurosci. 2013, 33, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Bonzano, L.; Tacchino, A.; Brichetto, G.; Roccatagliata, L.; Dessypris, A.; Feraco, P.; Lopes De Carvalho, M.L.; Battaglia, M.A.; Mancardi, G.L.; Bove, M. Upper limb motor rehabilitation impacts white matter microstructure in multiple sclerosis. Neuroimage 2014, 90, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.; Hijmans, B.S.; Cerliani, L.; Bangert, M.; Nanetti, L.; Keller, P.E.; Keysers, C. Inter-Individual Differences in Audio-Motor Learning of Piano Melodies and White Matter Fiber Tract Architecture: Inter-Individual Piano Learning Abilities and White Matter Tracts. Hum. Brain Mapp. 2014, 35, 2483–2497. [Google Scholar] [CrossRef] [PubMed]
- Rasova, K.; Prochazkova, M.; Tintera, J.; Ibrahim, I.; Zimova, D.; Stetkarova, I. Motor programme activating therapy influences adaptive brain functions in multiple sclerosis: Clinical and MRI study. Int. J. Rehabilit. Res. 2015, 38, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Huber, E.; Donnelly, P.M.; Rokem, A.; Yeatman, J.D. Rapid and widespread white matter plasticity during an intensive reading intervention. Nat. Commun. 2018, 9, 2260. [Google Scholar] [CrossRef]
- Zatorre, R.J.; Fields, R.D.; Johansen-Berg, H. Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat. Neurosci. 2012, 15, 528. [Google Scholar] [CrossRef]
- Kühn, S.; Romanowski, A.; Schilling, C.; Lorenz, R.; Mörsen, C.; Seiferth, N.; Banaschewski, T.; Barbot, A.; Barker, G.J.; Büchel, C.; et al. The neural basis of video gaming. Transl. Psychiatry 2011, 1, e53. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; Gallinat, J. Amount of lifetime video gaming is positively associated with entorhinal, hippocampal and occipital volume. Mol. Psychiatry 2014, 19, 842. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Ikeda, H.; Kasahara, K.; Kato, R.; Tsubomi, H.; Sugawara, S.K.; Mori, M.; Hanakawa, T.; Sadato, N.; Honda, M.; et al. Larger Right Posterior Parietal Volume in Action Video Game Experts: A Behavioral and Voxel-Based Morphometry (VBM) Study. PLoS ONE 2013, 8, e66998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, G.; Yang, Y.; Qin, W.; Li, X.; Zhang, Q. Higher integrity of the motor and visual pathways in long-term video game players. Front. Hum. Neurosci. 2015, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Ray, N.R.; O’Connell, M.A.; Nashiro, K.; Smith, E.T.; Qin, S.; Basak, C. Evaluating the relationship between white matter integrity, cognition, and varieties of video game learning. RNN 2017, 35, 437–456. [Google Scholar] [CrossRef]
- Granek, J.A.; Gorbet, D.J.; Sergio, L.E. Extensive video-game experience alters cortical networks for complex visuomotor transformations. Cortex 2010, 46, 1165–1177. [Google Scholar] [CrossRef]
- Li, R.W.; Ngo, C.; Nguyen, J.; Levi, D.M. Video-Game Play Induces Plasticity in the Visual System of Adults with Amblyopia. PLoS Biol. 2011, 9, e1001135. [Google Scholar] [CrossRef]
- Gong, D.; He, H.; Liu, D.; Ma, W.; Dong, L.; Luo, C.; Yao, D. Enhanced functional connectivity and increased gray matter volume of insula related to action video game playing. Sci. Rep. 2015, 5, 9763. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, X.-T.; Qi, Z.; Huang, S.; Li, H.-J. Neural Basis of Enhanced Executive Function in Older Video Game Players: An fMRI Study. Front. Aging Neurosci. 2017, 9, 382. [Google Scholar] [CrossRef]
- Haier, R.J.; Karama, S.; Leyba, L.; Jung, R.E. MRI assessment of cortical thickness and functional activity changes in adolescent girls following three months of practice on a visual-spatial task. BMC Res. Notes 2009, 2, 174. [Google Scholar] [CrossRef]
- Martínez, K.; Solana, A.B.; Burgaleta, M.; Hernández-Tamames, J.A.; Álvarez-Linera, J.; Román, F.J.; Alfayate, E.; Privado, J.; Escorial, S.; Quiroga, M.A.; et al. Changes in resting-state functionally connected parietofrontal networks after videogame practice: Videogame Practice and Functional Connectivity. Hum. Brain Mapp. 2013, 34, 3143–3157. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; Gleich, T.; Lorenz, R.C.; Lindenberger, U.; Gallinat, J. Playing Super Mario induces structural brain plasticity: Gray matter changes resulting from training with a commercial video game. Mol. Psychiatry 2014, 19, 265. [Google Scholar] [CrossRef] [PubMed]
- Gleich, T.; Lorenz, R.C.; Gallinat, J.; Kühn, S. Functional changes in the reward circuit in response to gaming-related cues after training with a commercial video game. Neuroimage 2017, 152, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, A.P.; de Vet, H.C.W.; de Bie, R.A.; Kessels, A.G.H.; Boers, M.; Bouter, L.M.; Knipschild, P.G. The Delphi List: A Criteria List for Quality Assessment of Randomized Clinical Trials for Conducting Systematic Reviews Developed by Delphi Consensus. J. Clin. Epidemiol. 1998, 51, 1235–1241. [Google Scholar] [CrossRef]
- Nouchi, R.; Kawashima, R. Improving Cognitive Function from Children to Old Age: A Systematic Review of Recent Smart Ageing Intervention Studies. Adv. Neurosci. 2014, 2014. [Google Scholar] [CrossRef]
- Lee, H.; Voss, M.W.; Prakash, R.S.; Boot, W.R.; Vo, L.T.K.; Basak, C.; VanPatter, M.; Gratton, G.; Fabiani, M.; Kramer, A.F. Videogame training strategy-induced change in brain function during a complex visuomotor task. Behav. Brain Res. 2012, 232, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Roush, R.E. Dance, Dance Revolution: Change in Executive Function Following A Video Dance Intervention in Postmenopausal Women. Ph.D. Thesis, University of Pittsburgh, Pittsburgh, PA, USA, 2013. [Google Scholar]
- Lorenz, R.C.; Gleich, T.; Gallinat, J.; Kühn, S. Video game training and the reward system. Front. Hum. Neurosci. 2015, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- West, G.L.; Zendel, B.R.; Konishi, K.; Benady-Chorney, J.; Bohbot, V.D.; Peretz, I.; Belleville, S. Playing Super Mario 64 increases hippocampal grey matter in older adults. PLoS ONE 2017, 12, e0187779. [Google Scholar] [CrossRef]
- West, G.L.; Konishi, K.; Diarra, M.; Benady-Chorney, J.; Drisdelle, B.L.; Dahmani, L.; Sodums, D.J.; Lepore, F.; Jolicoeur, P.; Bohbot, V.D. Impact of video games on plasticity of the hippocampus. Mol. Psychiatry 2018, 23, 1566. [Google Scholar] [CrossRef] [PubMed]
- DiPietro, J.A. Baby and The Brain: Advances in Child Development. Annu. Rev. Public Health 2000, 21, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Lenroot, R.K.; Giedd, J.N. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosc. Biobehav. Rev. 2006, 30, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, S.-J.; Choudhury, S. Development of the adolescent brain: Implications for executive function and social cognition. J. Child Psychol. Psychiatry 2006, 47, 296–312. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Hidalgo, J.J. Brain structural and functional changes in adolescents with psychiatric disorders. Int. J. Adolesc. Med. Health 2013, 25, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Tamnes, C.K.; Walhovd, K.B.; Torstveit, M.; Sells, V.T.; Fjell, A.M. Performance monitoring in children and adolescents: A review of developmental changes in the error-related negativity and brain maturation. Dev. Cognit. Neurosci. 2013, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, N.; Allen, N.B.; Youssef, G.; Dennison, M.; Yücel, M.; Simmons, J.G.; Whittle, S. Brain development during adolescence: A mixed-longitudinal investigation of cortical thickness, surface area, and volume: Brain Development During Adolescence. Hum. Brain Mapp. 2016, 37, 2027–2038. [Google Scholar] [CrossRef] [PubMed]
- Arain, M.; Haque, M.; Johal, L.; Mathur, P.; Nel, W.; Rais, A.; Sandhu, R.; Sharma, S. Maturation of the adolescent brain. NDT 2013, 9, 449. [Google Scholar]
- Peters, R. Ageing and the brain. Postgrad. Med. J. 2006, 82, 84–88. [Google Scholar] [CrossRef]
- Persson, J.; Lustig, C.; Nelson, J.K.; Reuter-Lorenz, P.A. Age Differences in Deactivation: A Link to Cognitive Control? J. Cognit. Neurosci. 2007, 19, 1021–1032. [Google Scholar] [CrossRef]
- Tulving, E.; Markowitsch, H.J. Episodic and declarative memory: Role of the hippocampus. Hippocampus 1998, 8, 198–204. [Google Scholar] [CrossRef]
- Barker, G.R.I.; Warburton, E.C. When Is the Hippocampus Involved in Recognition Memory? J. Neurosci. 2011, 31, 10721–10731. [Google Scholar] [CrossRef] [PubMed]
- Hayter, A.L.; Langdon, D.W.; Ramnani, N. Cerebellar contributions to working memory. Neuroimage 2007, 36, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Nat. Acad. Sci. 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-I.; Park, M.-H.; Park, B.; Kim, S.-Y.; Kim, Y.O.; Kim, B.-N.; Park, S.; Song, C.-H. Cerebellar Gray Matter Volume, Executive Function, and Insomnia: Gender Differences in Adolescents. Sci. Rep. 2019, 9, 855. [Google Scholar] [CrossRef] [PubMed]
- Garavan, H.; Kelley, D.; Rosen, A.; Rao, S.M.; Stein, E.A. Practice-related functional activation changes in a working memory task. Microsc. Res. Tech. 2000, 51, 54–63. [Google Scholar] [CrossRef]
- Jansma, J.M.; Ramsey, N.F.; Slagter, H.A.; Kahn, R.S. Functional Anatomical Correlates of Controlled and Automatic Processing. J. Cognit. Neurosci. 2001, 13, 730–743. [Google Scholar] [CrossRef]
- Milham, M.P.; Banich, M.T.; Claus, E.D.; Cohen, N.J. Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage 2003, 18, 483–493. [Google Scholar] [CrossRef]
- Landau, S.M.; Schumacher, E.H.; Garavan, H.; Druzgal, T.J.; D’Esposito, M. A functional MRI study of the influence of practice on component processes of working memory. Neuroimage 2004, 22, 211–221. [Google Scholar] [CrossRef]
- Vartanian, O.; Jobidon, M.-E.; Bouak, F.; Nakashima, A.; Smith, I.; Lam, Q.; Cheung, B. Working memory training is associated with lower prefrontal cortex activation in a divergent thinking task. Neuroscience 2013, 236, 186–194. [Google Scholar] [CrossRef]
- Arias-Carrión, O.; Stamelou, M.; Murillo-Rodríguez, E.; Menéndez-González, M.; Pöppel, E. Dopaminergic reward system: A short integrative review. Int. Arch. Med. 2010, 3, 24. [Google Scholar] [CrossRef]
- Demirakca, T.; Cardinale, V.; Dehn, S.; Ruf, M.; Ende, G. The Exercising Brain: Changes in Functional Connectivity Induced by an Integrated Multimodal Cognitive and Whole-Body Coordination Training. Neural Plast. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Chirles, T.J.; Reiter, K.; Weiss, L.R.; Alfini, A.J.; Nielson, K.A.; Smith, J.C. Exercise Training and Functional Connectivity Changes in Mild Cognitive Impairment and Healthy Elders. JAD 2017, 57, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, L.; Adolphs, R. Emotion processing and the amygdala: From a “low road” to “many roads” of evaluating biological significance. Nat. Rev. Neurosci. 2010, 11, 773. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Taki, Y.; Sassa, Y.; Hashizume, H.; Sekiguchi, A.; Fukushima, A.; Kawashima, R. Working Memory Training Using Mental Calculation Impacts Regional Gray Matter of the Frontal and Parietal Regions. PLoS ONE 2011, 6, e23175. [Google Scholar] [CrossRef] [PubMed]
- Metzler-Baddeley, C.; Caeyenberghs, K.; Foley, S.; Jones, D.K. Task complexity and location specific changes of cortical thickness in executive and salience networks after working memory training. Neuroimage 2016, 130, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.M.; Schneider, W. Brain Changes in the Development of Expertise: Neuroanatomical and Neurophysiological Evidence about Skill-Based Adaptations. In The Cambridge Handbook of Expertise and Expert Performance; Ericsson, K.A., Charness, N., Feltovich, P.J., Hoffman, R.R., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 653–682. ISBN 978-0-511-81679-6. [Google Scholar]
- Witte, A.V.; Savli, M.; Holik, A.; Kasper, S.; Lanzenberger, R. Regional sex differences in grey matter volume are associated with sex hormones in the young adult human brain. Neuroimage 2010, 49, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Yang, J. The influence of motor expertise on the brain activity of motor task performance: A meta-analysis of functional magnetic resonance imaging studies. Cogn. Affect Behav. Neurosci. 2015, 15, 381–394. [Google Scholar] [CrossRef]
- Jang, H.; Lee, J.Y.; Lee, K.I.; Park, K.M. Are there differences in brain morphology according to handedness? Brain Behav. 2017, 7, e00730. [Google Scholar] [CrossRef]
- Colloca, L.; Miller, F.G. Role of expectations in health. Curr. Opin. Psychiatry 2011, 24, 149–155. [Google Scholar] [CrossRef]
- Shahar, E.; Shahar, D. Causal diagrams, the placebo effect, and the expectation effect. IJGM 2013, 6, 821. [Google Scholar] [CrossRef]
- Brown, W.A. Expectation, the Placebo Effect and the Response to Treatment. R. I. Med. J. 2015, 98, 19. [Google Scholar]
- Cunningham, J.A.; Kypri, K.; McCambridge, J. Exploratory randomized controlled trial evaluating the impact of a waiting list control design. BMC Med. Res. Methodol. 2013, 13, 150. [Google Scholar] [CrossRef] [PubMed]
- FDA Step 3: Clinical Research. Available online: https://www.fda.gov/ forpatients/approvals/drugs/ ucm405622.htm (accessed on 29 May 2019).
- Locke, H.S.; Braver, T.S. Motivational influences on cognitive control: Behavior, brain activation, and individual differences. Cogn. Affect. Behav. Neurosc. 2008, 8, 99–112. [Google Scholar] [CrossRef]
| Difference | Previous Review | Current Review |
|---|---|---|
| Type of reviewed studies | Experimental and correlational studies | Experimental studies only |
| Neuroimaging technique of reviewed studies | CT, fMRI, MEG, MRI, PET, SPECT, tDCS, EEG, and NIRS | fMRI and MRI only |
| Participants of reviewed studies | Healthy and addicted participant | Healthy participants Only |
| Author | Year | Participant Age | Game Genre | Control | Duration | Beneficial Effect |
|---|---|---|---|---|---|---|
| Gleich et al. [43] | 2017 | 18–36 | 3D adventure | passive | 8 weeks | Increased activity in hippocampus |
| Decreased activity in DLPFC | ||||||
| Haier et al. [40] | 2009 | 12–15 | puzzle | passive | 3 months | Increased GM in several visual–spatial processing area |
| Decreased activity in frontal area | ||||||
| Kuhn et al. [42] | 2014 | 19–29 | 3D adventure | passive | 8 weeks | Increased GM in hippocampal, DLPFC and cerebellum |
| Lee et al. [47] | 2012 | 18–30 | strategy | active | 8–10 weeks | Decreased activity in DLPFC |
| 8–11 weeks | Non-significant activity difference | |||||
| Lorenz et al. [49] | 2015 | 19–27 | 3D adventure | passive | 8 weeks | Preserved activity in ventral striatum |
| Martinez et al. [41] | 2013 | 16–21 | puzzle | passive | 4 weeks | Functional connectivity change in multimodal integration system |
| Functional connectivity change in higher-order executive processing | ||||||
| Roush [48] | 2013 | 50–65 | rhythm dance | active | 24 weeks | Increased activity in visuospatial working memory area |
| Increased activity in emotional and attention area | ||||||
| passive | Similar compared to active control- | |||||
| West et al. [50] | 2017 | 55–75 | 3D adventure | active | 24 weeks | Non-significant GM difference |
| passive | Increased cognitive performance and short-term memory | |||||
| Increased GM in hippocampus and cerebellum | ||||||
| West et al. [51] | 2018 | 18–29 | FPS | active | 8 weeks | Increased GM in hippocampus (spatial learner *) |
| Increased GM in amygdala (response learner *) | ||||||
| Decreased GM in hippocampus (response learner) |
| Author | Year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gleich et al. [43] | 2017 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 6 |
| Haier et al. [40] | 2009 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 5 |
| Kuhn et al. [42] | 2014 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 5 |
| Lee et al. [47] | 2012 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Lorenz et al. [49] | 2015 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 7 |
| Martinez et al. [41] | 2013 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 3 |
| Roush [48] | 2013 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 7 |
| West et al. [50] | 2017 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 9 |
| West et al. [51] | 2018 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 7 |
| Score | 6 | 2 | 9 | 9 | 2 | 0 | 0 | 3 | 4 | 8 | 7 | 5 |
| Author | Year | Inclusion | Exclusion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| i1 | i2 | i3 | e1 | e2 | e3 | e4 | e5 | ||
| Gleich et al. [43] | 2017 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| Haier et al. [40] | 2009 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| Kuhn et al. [42] | 2014 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| Lee et al. [47] | 2012 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 |
| Lorenz et al. [49] | 2015 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 |
| Martinez et al. [41] | 2013 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| Roush [48] | 2013 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| West et al. [50] | 2017 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 |
| West et al. [51] | 2018 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
| total | 8 | 4 | 3 | 8 | 7 | 6 | 5 | 4 | |
| Control | Author | Year |
|---|---|---|
| Active control | Lee et al. [47] | 2012 |
| West et al. [51] | 2018 | |
| Passive control | Gleich et al. [43] | 2017 |
| Haier et al. [40] | 2009 | |
| Kuhn et al. [42] | 2014 | |
| Lorenz et al. [49] | 2015 | |
| Martinez et al. [41] | 2013 | |
| Active–passive control | Roush [48] | 2013 |
| West et al. [50] | 2017 |
| Genre | Author | Year | Title |
|---|---|---|---|
| 3D adventure | Gleich et al. [43] | 2017 | Super Mario 64 DS |
| Kuhn et al. [42] | 2014 | Super Mario 64 | |
| Lorenz et al. [49] | 2015 | Super Mario 64 DS | |
| West et al. [50] | 2017 | Super Mario 64 | |
| FPS | West et al. * [51] | 2018 | Call of Duty |
| Puzzle | Haier et al. [40] | 2009 | Tetris |
| Martinez et al. [41] | 2013 | Professor Layton and The Pandora’s Box | |
| Rhythm dance | Roush [48] | 2013 | Dance Revolution |
| Strategy | Lee et al. [47] | 2012 | Space Fortress |
| Category | Author | Year | Age | Sample Size | Ratio (%) | Detail | |||
|---|---|---|---|---|---|---|---|---|---|
| Lowest | Highest | Range | Female | Male | |||||
| Teenager | Haier et al. [40] | 2009 | 12 | 15 | 3 | 44 | 70.45 | 29.54 | Training (n = 24) Control (n = 20) |
| Young adult | Gleich et al. [43] | 2017 | 18 | 36 | 18 | 26 | 100 | 0 | Training (n = 15) |
| Control (n = 11) | |||||||||
| Kuhn et al. [42] | 2014 | 19 | 29 | 10 | 48 | 70.8 | 29.2 | Training (n = 23) | |
| Control (n = 25) | |||||||||
| Lee et al. [47] | 2012 | 18 | 30 | 12 | 75 | 61.4 | 38.6 | Training A (n = 25) | |
| Training B (n = 25) | |||||||||
| Control (n = 25) | |||||||||
| Lorenz et al. [49] | 2015 | 19 | 27 | 8 | 50 | 72 | 28 | Training (n = 25 | |
| Control (n = 25) | |||||||||
| Martinez et al. [41] | 2013 | 16 | 21 | 5 | 20 | 100 | 0 | Training (n = 10) | |
| Control (n = 10) | |||||||||
| West et al. [51] | 2018 | 18 | 29 | 11 | 43 | 67.4 | 32.5 | Action game (n = 21) | |
| Non-action game (n = 22) | |||||||||
| Older adult | Roush [48] | 2013 | 50 | 65 | 15 | 39 | 100 | 0 | Training (n = 19) |
| Active control (n = 15) | |||||||||
| Passive control (n = 5) | |||||||||
| West et al. [50] | 2017 | 55 | 75 | 20 | 48 | 66.7 | 33.3 | Training (n = 19) | |
| Active control (n = 14) | |||||||||
| Passive control (n = 15) | |||||||||
| Author | Year | Length (Week) | Total Hours | Average Intensity (h/Week) |
|---|---|---|---|---|
| Gleich et al. [43] | 2017 | 8 | 49.5 | 6.2 |
| Haier et al. [40] | 2009 | 12 | 18 | 1.5 |
| Kuhn et al. [42] | 2014 | 8 | 46.88 | 5.86 |
| Lorenz et al. [49] | 2012 | 8 | 28 | 3.5 |
| Lee et al. [47] | 2015 | 8–11 * | 27 | n/a |
| Martinez et al. [41] | 2013 | 4 | 16 | 4 |
| Roush [48] | 2013 | 24 | ns | n/a |
| West et al. [50] | 2017 | 24 | 72 | 3 |
| West et al. [51] | 2018 | 8.4 | 90 | 10.68 |
| MRI Analysis | Author | Year | Contrast | Statistical Tool | Statistical Method | p Value |
|---|---|---|---|---|---|---|
| Resting | Martinez et al. [41] | 2013 | (post- > pre-training) > (post>pre-control) | MATLAB; SPM8 | TFCE uncorrected | <0.005 |
| Structural | Haier et al. * [40] | 2009 | (post>pre-training) > (post>pre-control) | MATLAB 7; SurfStat | FWE corrected | <0.005 |
| Kuhn et al. [42] | 2014 | (post>pre-training) > (post>pre-control) | VBM8; SPM8 | FWE corrected | <0.001 | |
| West et al. [50] | 2017 | (post>pre-training) > (post>pre-control) | Bpipe | Uncorrected | <0.0001 | |
| West et al. [51] | 2018 | (post>pre-training) > (post>pre-control) | Bpipe | Bonferroni corrected | <0.001 | |
| Task | Gleich et al. [43] | 2017 | (post>pre-training) > (post>pre-control) | SPM12 | Monte Carlo corrected | <0.05 |
| Haier et al. * [40] | 2009 | (post>pre-training) > (post>pre-control) | SPM7 | FDR corrected | <0.05 | |
| Lee et al. [47] | 2012 | (post>pre-training) > (post>pre-control) | FSL; FEAT | uncorrected | <0.01 | |
| Lorenz et al. [49] | 2015 | (post>pre-training) > (post>pre-control) | SPM8 | Monte Carlo corrected | <0.05 | |
| Roush + [48] | 2013 | post>pre-training | MATLAB 7; SPM8 | uncorrected | =0.001 |
| Author | Year | Resting State | Structural | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Imaging | TR (s) | TE (ms) | Slice | Imaging | TR (s) | TE (ms) | Slice | ||
| Martinez et al. [41] | 2013 | gradient-echo planar image | 3 | 28.1 | 36 | T1-weighted | 0.92 | 4.2 | 158 |
| Author | Year | Imaging | TR (s) | TE (ms) |
|---|---|---|---|---|
| Kuhn et al. [42] | 2014 | 3D T1 weighted MPRAGE | 2.5 | 4.77 |
| West et al. [50] | 2017 | 3D gradient echo MPRAGE | 2.3 | 2.91 |
| West et al. [51] | 2018 | 3D gradient echo MPRAGE | 2.3 | 2.91 |
| Author | Year | Task | BOLD | Structural | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Imaging | TR (s) | TE (ms) | Slice | Imaging | TR (s) | TE (ms) | Slice | |||
| Gleich et al. [43] | 2017 | win–loss paradigm | T2 echo-planar image | 2 | 30 | 36 | T1-weighted | 2.5 | 4.77 | 176 |
| Haier et al. [40] | 2009 | Tetris | Functional echo planar | 2 | 29 | ns | 5-echo MPRAGE | 2.53 | 1.64; 3.5; 5.36; 7.22; 9.08 | ns |
| Lee et al. [47] | 2012 | game control | fast echo-planar image | 2 | 25 | ns | T1-weighted MPRAGE | 1.8 | 3.87 | 144 |
| Lorenz et al. [49] | 2015 | slot machine paradigm | T2 echo-planar image | 2 | 30 | 36 | T1-weighted MPRAGE | 2.5 | 4.77 | ns |
| Roush [48] | 2013 | digit symbol substitution | fast echo-planar image | 2 | 25 | 34 | diffusion weighted image | ns | ns | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brilliant T., D.; Nouchi, R.; Kawashima, R. Does Video Gaming Have Impacts on the Brain: Evidence from a Systematic Review. Brain Sci. 2019, 9, 251. https://doi.org/10.3390/brainsci9100251
Brilliant T. D, Nouchi R, Kawashima R. Does Video Gaming Have Impacts on the Brain: Evidence from a Systematic Review. Brain Sciences. 2019; 9(10):251. https://doi.org/10.3390/brainsci9100251
Chicago/Turabian StyleBrilliant T., Denilson, Rui Nouchi, and Ryuta Kawashima. 2019. "Does Video Gaming Have Impacts on the Brain: Evidence from a Systematic Review" Brain Sciences 9, no. 10: 251. https://doi.org/10.3390/brainsci9100251
APA StyleBrilliant T., D., Nouchi, R., & Kawashima, R. (2019). Does Video Gaming Have Impacts on the Brain: Evidence from a Systematic Review. Brain Sciences, 9(10), 251. https://doi.org/10.3390/brainsci9100251





