Extent of Resection in Newly Diagnosed Glioblastoma: Impact of a Specialized Neuro-Oncology Care Center
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Imaging Protocol and EOR Volumetry
2.3. Statistical Analysis
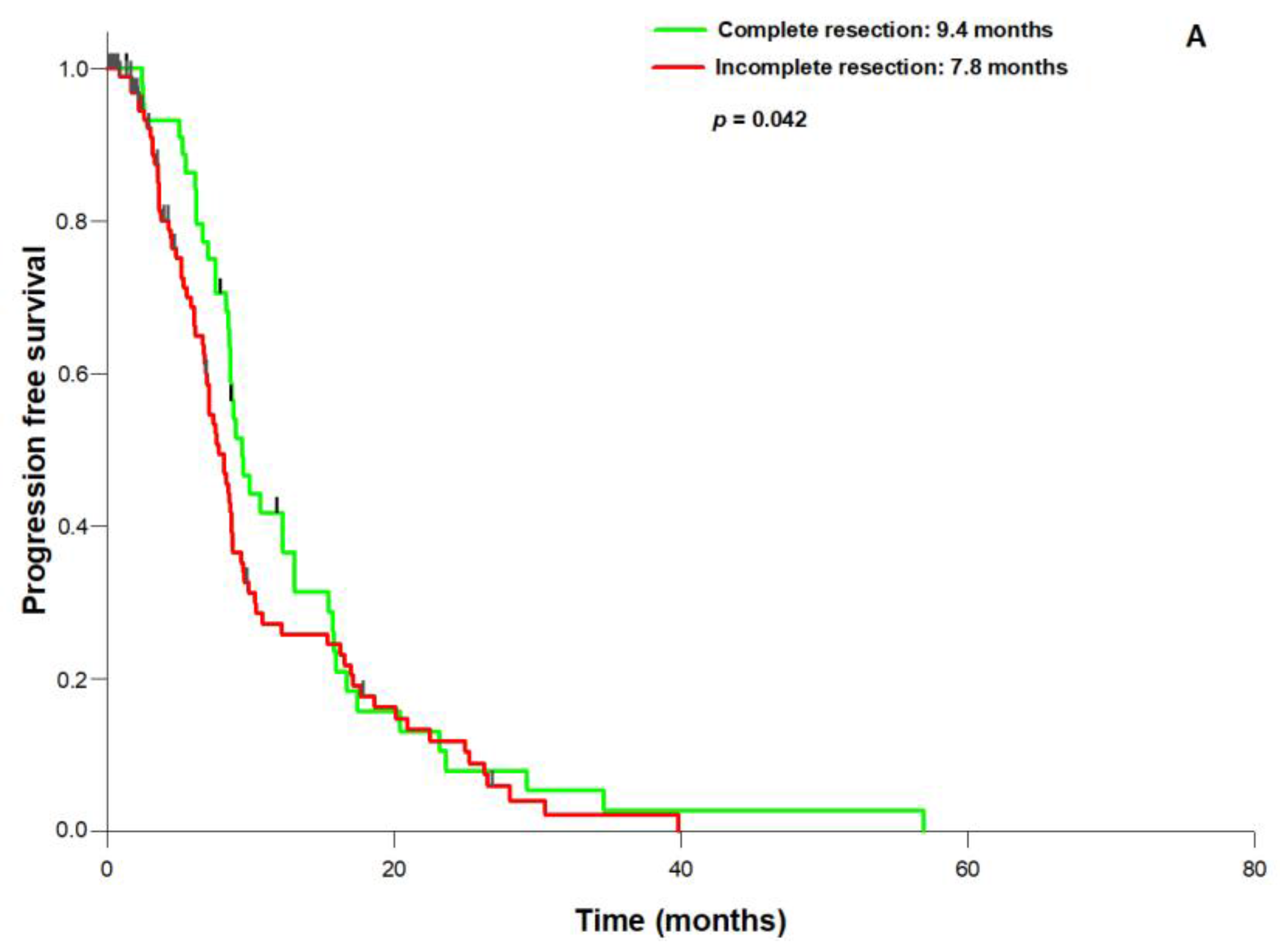
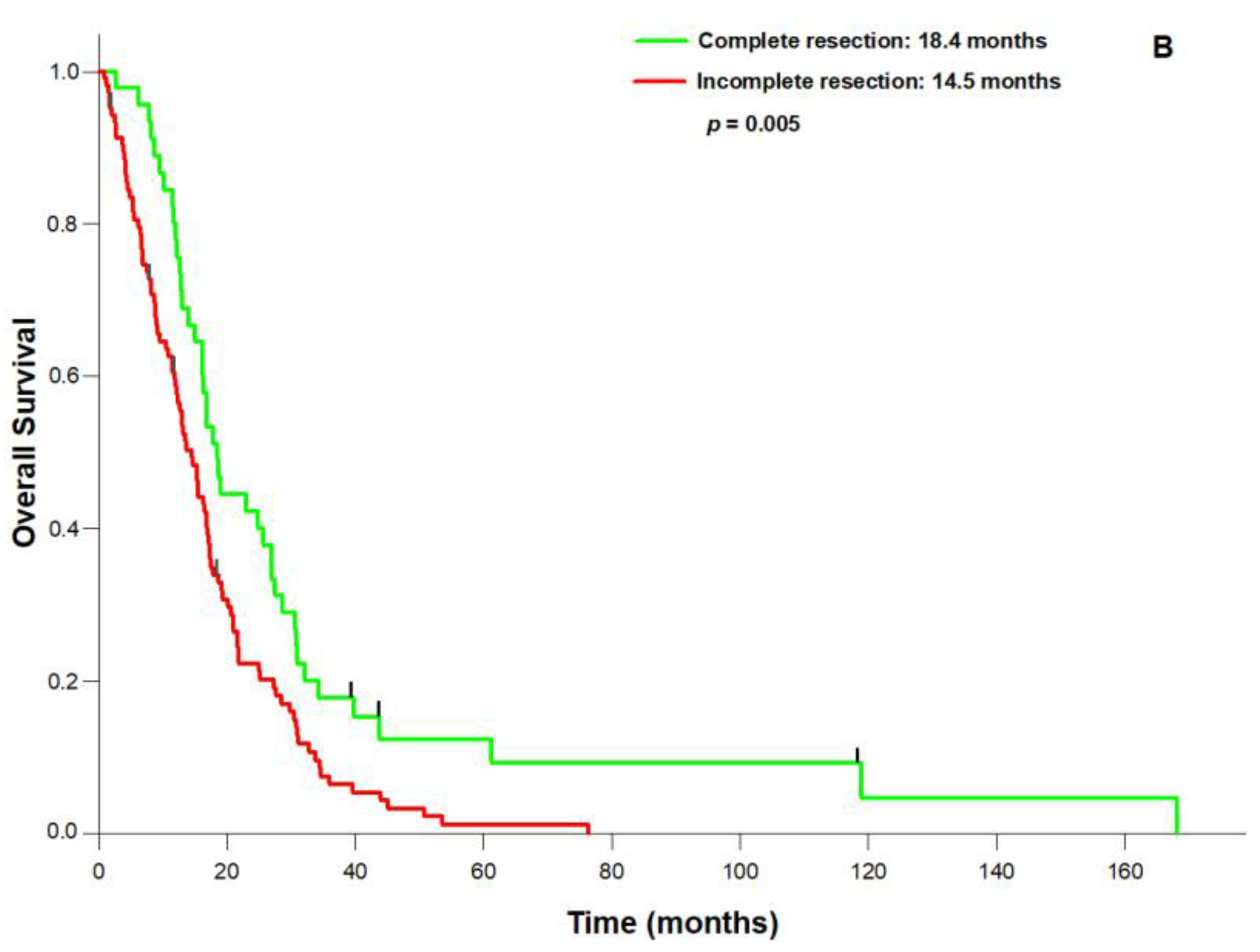
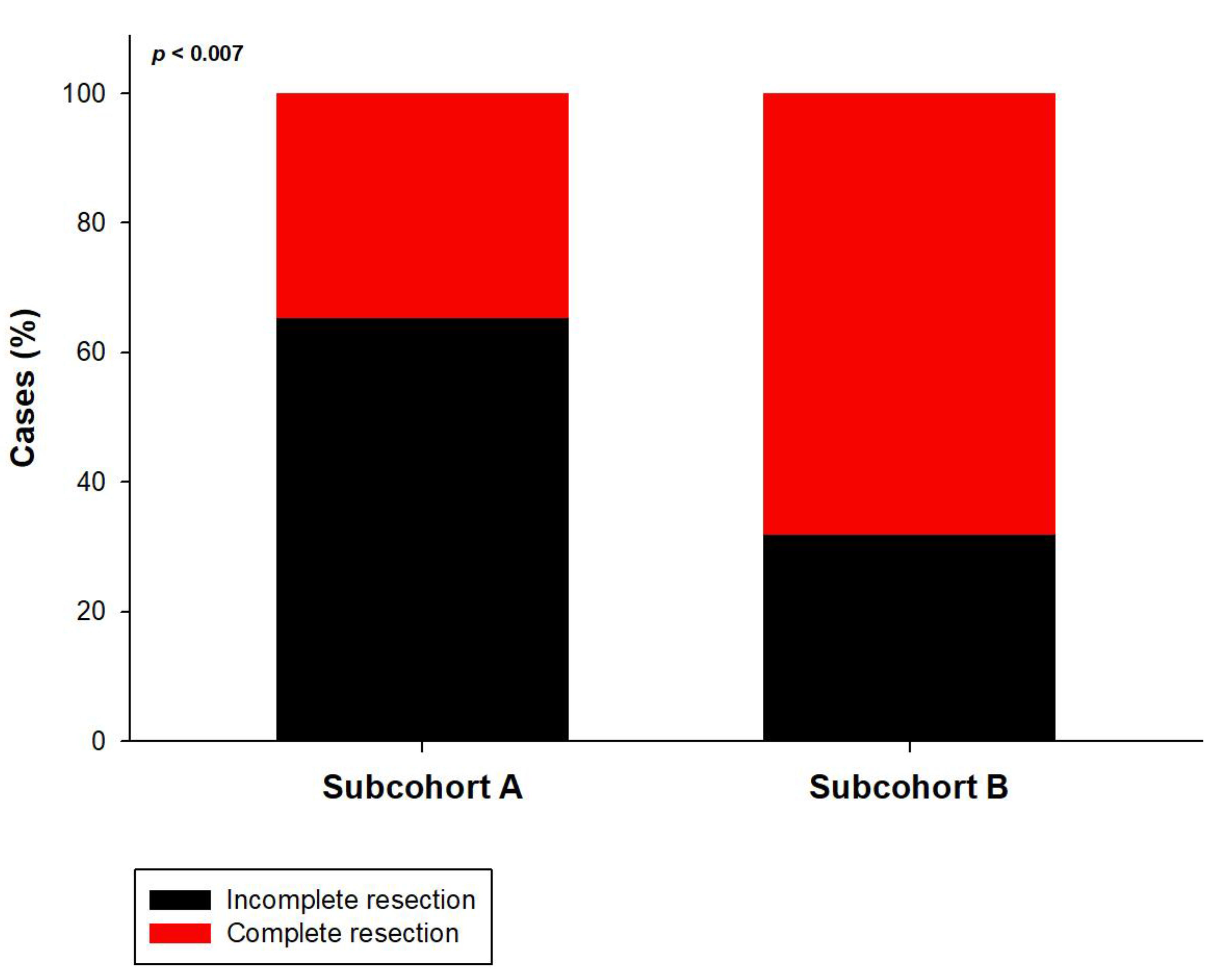
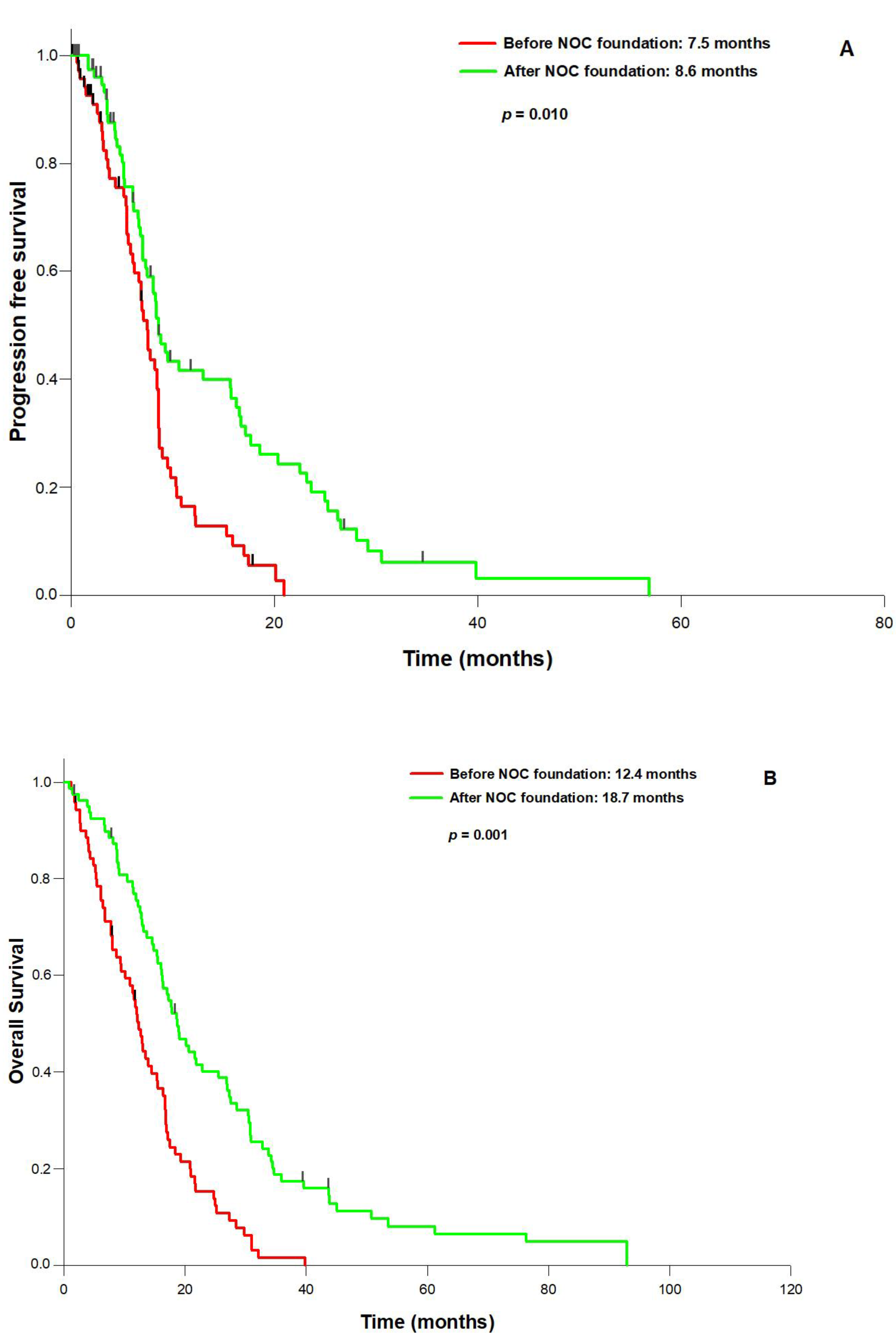
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ohgaki, H.; Kleihues, P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005, 109, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; DeAngelis, L.M. Glioblastoma and other malignant gliomas: A clinical review. JAMA 2013, 310, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the extent of resection with survival in glioblastoma: A systematic review and meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, K.L.; Jusue-Torres, I.; Navarro-Ramirez, R.; Raza, S.M.; Pascual-Gallego, M.; Ibrahim, A.; Hernandez-Hermann, M.; Gomez, L.; Ye, X.; Weingart, J.D.; et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014, 16, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Polley, M.Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kreth, F.W.; Thon, N.; Simon, M.; Westphal, M.; Schackert, G.; Nikkhah, G.; Hentschel, B.; Reifenberger, G.; Pietsch, T.; Weller, M.; et al. Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann. Oncol. 2013, 24, 3117–3123. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.L.; van der Hoorn, A.; Larkin, T.J.; Boonzaier, N.R.; Matys, T.; Price, S.J. Extent of resection of peritumoral diffusion tensor imaging-detected abnormality as a predictor of survival in adult glioblastoma patients. J. Neurosurg. 2017, 126, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Eyupoglu, I.Y.; Hore, N.; Merkel, A.; Buslei, R.; Buchfelder, M.; Savaskan, N. Supra-complete surgery via dual intraoperative visualization approach (diva) prolongs patient survival in glioblastoma. Oncotarget 2016, 7, 25755–25768. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Suki, D.; Hess, K.; Sawaya, R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J. Neurosurg. 2015. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Jakola, A.S.; Nerland, U.S.; Weber, C.; Solheim, O. The risk of getting worse: Surgically acquired deficits, perioperative complications, and functional outcomes after primary resection of glioblastoma. World Neurosurg. 2011, 76, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Jakola, A.S.; Gulati, S.; Weber, C.; Unsgard, G.; Solheim, O. Postoperative deterioration in health related quality of life as predictor for survival in patients with glioblastoma: A prospective study. PLoS ONE 2011, 6, e28592. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.A.; Bhavsar, A.; Asif, H.; Karabatsou, K.; Leggate, J.R.; Sofat, A.; Kamaly-Asl, I.D. Treatment by specialist surgical neurooncologists improves survival times for patients with malignant glioma. J. Neurosurg. 2015, 122, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, C.A.; Weingart, S.N.; Swanson, S.; Mamon, H.J.; Shulman, L.N. Models of Multidisciplinary Cancer Care: Physician and Patient Perceptions in a Comprehensive Cancer Center. J. Oncol. Pract. Am. Soc. Clin. Oncol. 2010, 6, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Lamborn, K.R.; Chang, S.M.; Prados, M.D. Prognostic Factors for Survival of Patients with Glioblastoma: Recursive Partitioning Analysis. Neuro Oncol. 2004, 6, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Bianco, J.; Bastiancich, C.; Jankovski, A.; des Rieux, A.; Preat, V.; Danhier, F. On glioblastoma and the search for a cure: Where do we stand? Cell. Mol. Life Sci. 2017, 74, 2451–2466. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.J.; Ali, S.; Qadir, M.G.; De La Fuente, M.I.; Ivan, M.E.; Komotar, R.J. The role of bevacizumab in the treatment of glioblastoma. J. Neurooncol. 2017, 133, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, F.; Liu, Z.; Tang, H.; Wu, H.; Gong, Q.; Chen, J. Immune checkpoint in glioblastoma: Promising and challenging. Front. Pharmacol. 2017, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Eyupoglu, I.Y.; Buchfelder, M.; Savaskan, N.E. Surgical resection of malignant gliomas-role in optimizing patient outcome. Nat. Rev. Neurol. 2013, 9, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Gramatzki, D.; Dehler, S.; Rushing, E.J.; Zaugg, K.; Hofer, S.; Yonekawa, Y.; Bertalanffy, H.; Valavanis, A.; Korol, D.; Rohrmann, S.; et al. Glioblastoma in the canton of zurich, switzerland revisited: 2005 to 2009. Cancer 2016, 122, 2206–2215. [Google Scholar] [CrossRef] [PubMed]
- Woehrer, A.; Bauchet, L.; Barnholtz-Sloan, J.S. Glioblastoma survival: Has it improved? Evidence from population-based studies. Curr. Opin. Neurol. 2014, 27, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Nava, F.; Tramacere, I.; Fittipaldo, A.; Bruzzone, M.G.; Dimeco, F.; Fariselli, L.; Finocchiaro, G.; Pollo, B.; Salmaggi, A.; Silvani, A.; et al. Survival effect of first- and second-line treatments for patients with primary glioblastoma: A cohort study from a prospective registry, 1997–2010. Neuro Oncol. 2014, 16, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Koshy, M.; Villano, J.L.; Dolecek, T.A.; Howard, A.; Mahmood, U.; Chmura, S.J.; Weichselbaum, R.R.; McCarthy, B.J. Improved survival time trends for glioblastoma using the seer 17 population-based registries. J. Neurooncol. 2012, 107, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Leroy, H.A.; Vermandel, M.; Lejeune, J.P.; Mordon, S.; Reyns, N. Fluorescence guided resection and glioblastoma in 2015: A review. Lasers Surg. Med. 2015, 47, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Schucht, P.; Seidel, K.; Beck, J.; Murek, M.; Jilch, A.; Wiest, R.; Fung, C.; Raabe, A. Intraoperative Monopolar Mapping During 5-Ala-Guided Resections of Glioblastomas Adjacent to Motor Eloquent Areas: Evaluation of Resection Rates and Neurological Outcome. Neurosurg. Focus 2014, 37, E16. [Google Scholar] [CrossRef] [PubMed]
- Solheim, O.; Gulati, S.; Jakola, A.S. Glioblastoma resection: In search of a threshold between worthwhile and futile. Neuro Oncol. 2014, 16, 610–611. [Google Scholar] [CrossRef] [PubMed]
| Parameter | (Number (%)) |
|---|---|
| n | 149 |
| Sex (f/m) | 67/82 (44.9/55.1) |
| Age (years, mean) | 61.8 (range: 26.7–87.8) |
| Preoperative KPS (%, median) | 80 (range: 20–100) |
| Preoperative MRC-NPS (points, median) | 2 (range: 1–5) |
| MGMT promoter status | Methylated: 50 (33.6) |
| Unmethylated: 68 (45.6) | |
| Unknown: 31 (20.8) | |
| IDH1 status | Wild type: 144 (96.6) |
| Mutated: 5 (3.4) | |
| Postsurgical treatment | |
| Stupp | 106 (71.1) |
| Radiation only | 15 (10.1) |
| Chemotherapy only | 16 (10.7) |
| No treatment | 12 (8.1) |
| Parameter | Complete Resection (Number (%)) | Incomplete Resection (Number (%)) | p |
|---|---|---|---|
| n | 74 (49.7) | 75 (50.3) | |
| Sex (f/m) | 34/40 (46.0/54.0) | 33/42 (44.0/56.0) | 0.941 |
| Age (years, mean) | 61.7 (range: 32.9–80.1) | 61.8 (range: 26.7–87.8) | 0.939 |
| Preoperative KPS (%, median) | 90 (range: 60–100) | 80 (range: 20–100) | 0.073 |
| Preoperative MRC-NPS (points, median) | 2 (range: 1–3) | 2 (range: 1–5) | 0.219 |
| MGMT: | 0.746 | ||
| Methylated | 24 (32.4) | 26 (34.7) | |
| Unmethylated | 34 (45.9) | 34 (45.3) | |
| Unknown | 16 (21.6) | 15 (20.0) | |
| IDH1 status: | 0.988 | ||
| Wildtype | 71 (97.3) | 73(97.3) | |
| Mutated | 3 (2.7) | 2 (2.7) | |
| Postsurgical treatment: | 0.782 | ||
| Stupp | 55 (74.3) | 51 (68.0) | |
| Radiation only | 6 (8.1) | 9 (12.0) | |
| Chemotherapy only | 8 (10.8) | 8 (10.7) | |
| No treatment | 5 (6.8) | 7(9.3) |
| Parameter | Hazard Ratio | 95% CI | p | |
|---|---|---|---|---|
| Age | 1.032 | 1.014 | 1.050 | 0.001 |
| NOC foundation | 0.697 | 0.482 | 1.008 | 0.015 |
| Preoperative KPS (>/<70%) | 0.971 | 0.957 | 0.988 | 0.001 |
| MGMT | 1.47 | 1.150 | 1.891 | 0.002 |
| Resection status: (complete vs. incomplete) | 0.981 | 0.964 | 0.998 | 0.032 |
| Parameter | Group A (Number (%)) | Group B (Number (%)) | p |
|---|---|---|---|
| n | 49 (32.9) | 100 (67.1) | |
| Sex (f/m) | 21/28 (42.9/57.1) | 46/54 (46.0/54.0) | 0.717 |
| Age (years, mean) | 62.4 (range: 32.1–81.0) | 61.5 (range: 26.7–87.8) | 0.647 |
| Preoperative KPS (%, median) | 80 (range: 40–100) | 80 (range: 20–100) | 0.658 |
| Preoperative MRC-NPS (points, median) | 2 (range: 1–5) | 2 (range: 1–4) | 0.68 |
| MGMT: | 0.431 | ||
| Methylated | 18 (36.7) | 32 (32.0) | |
| Unmethylated | 20 (40.9) | 48 (48.0) | |
| Unknown | 11 (22.4) | 20 (20.0) | |
| IDH1 status: | 0.889 | ||
| Wildtype | 47 (95.9) | 97 (97.0) | |
| Mutated | 2 (4.1) | 3 (3.0) | |
| Postsurgical treatment: | 0.195 | ||
| Stupp | 30 (61.2) | 76 (76.0) | |
| Radiation only | 8 (16.3) | 7 (7.0) | |
| Chemotherapy only | 4 (8.2) | 12 (12.0) | |
| No treatment | 5 (10.2) | 7 (7.0) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haj, A.; Doenitz, C.; Schebesch, K.-M.; Ehrensberger, D.; Hau, P.; Putnik, K.; Riemenschneider, M.J.; Wendl, C.; Gerken, M.; Pukrop, T.; et al. Extent of Resection in Newly Diagnosed Glioblastoma: Impact of a Specialized Neuro-Oncology Care Center. Brain Sci. 2018, 8, 5. https://doi.org/10.3390/brainsci8010005
Haj A, Doenitz C, Schebesch K-M, Ehrensberger D, Hau P, Putnik K, Riemenschneider MJ, Wendl C, Gerken M, Pukrop T, et al. Extent of Resection in Newly Diagnosed Glioblastoma: Impact of a Specialized Neuro-Oncology Care Center. Brain Sciences. 2018; 8(1):5. https://doi.org/10.3390/brainsci8010005
Chicago/Turabian StyleHaj, Amer, Christian Doenitz, Karl-Michael Schebesch, Denise Ehrensberger, Peter Hau, Kurt Putnik, Markus J. Riemenschneider, Christina Wendl, Michael Gerken, Tobias Pukrop, and et al. 2018. "Extent of Resection in Newly Diagnosed Glioblastoma: Impact of a Specialized Neuro-Oncology Care Center" Brain Sciences 8, no. 1: 5. https://doi.org/10.3390/brainsci8010005
APA StyleHaj, A., Doenitz, C., Schebesch, K.-M., Ehrensberger, D., Hau, P., Putnik, K., Riemenschneider, M. J., Wendl, C., Gerken, M., Pukrop, T., Brawanski, A., & Proescholdt, M. A. (2018). Extent of Resection in Newly Diagnosed Glioblastoma: Impact of a Specialized Neuro-Oncology Care Center. Brain Sciences, 8(1), 5. https://doi.org/10.3390/brainsci8010005





