Botulinum Toxin: An Unconventional Tool for the Treatment of Depression?
Abstract
1. Introduction
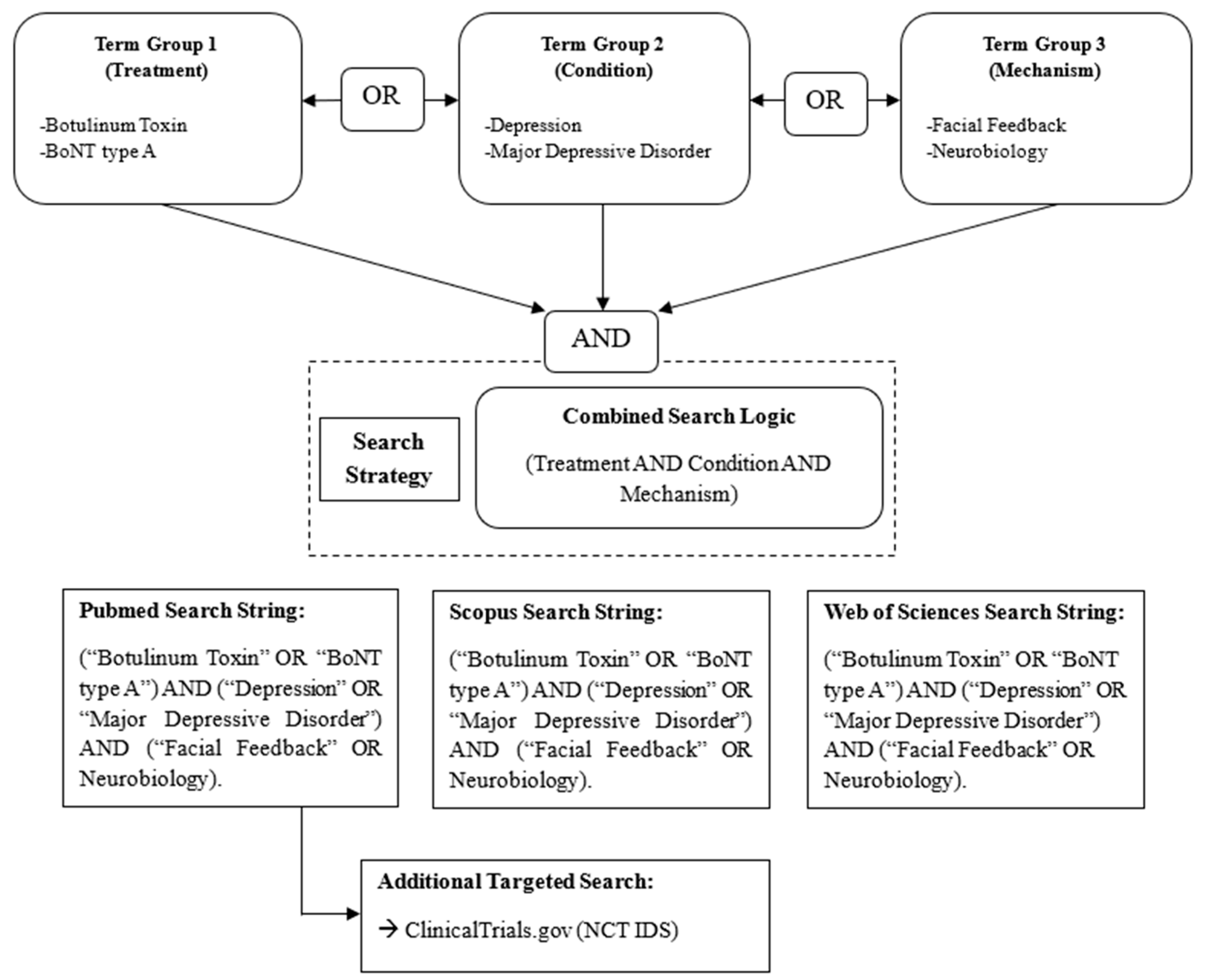
2. Methods
3. Results
3.1. Novel Treatment Options for TRD
3.1.1. Promising Experimental Drugs for TRD
3.1.2. Botulinum Neurotoxin
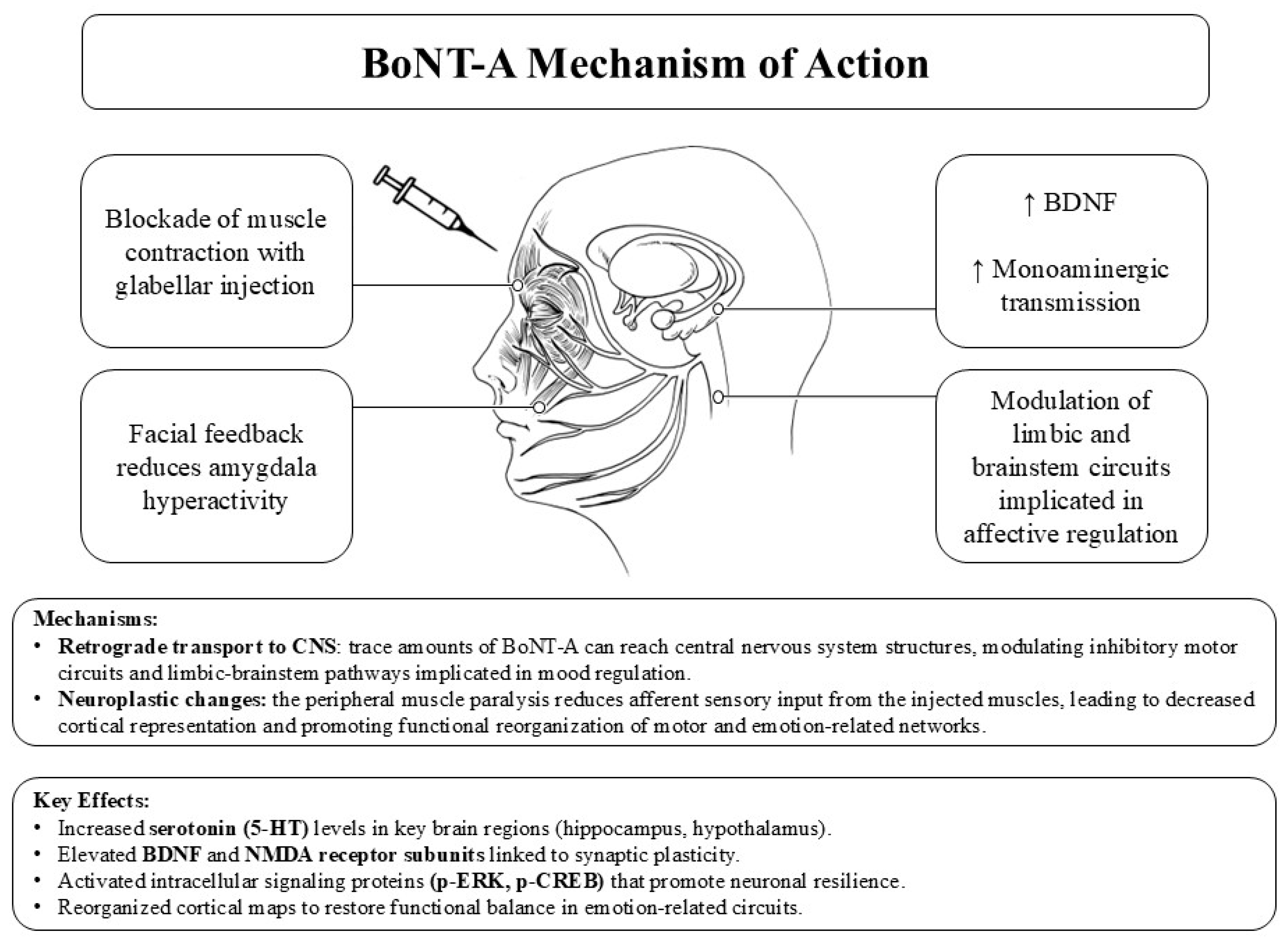
3.2. Neurobiological Mechanism of Action of BoNT-A
3.3. Why Botulinum Toxin May Be Effective in Depression
3.4. Botulin Toxin in the Treatment of MDD: Clinical Evidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Erchinger, V.J.; Ersland, L.; Aukland, S.M.; Abbott, C.C.; Oltedal, L. Magnetic resonance spectroscopy in depressed subjects treated with electroconvulsive therapy—A systematic review of literature. Front. Psychiatry 2021, 12, 608857. [Google Scholar] [CrossRef]
- Monroe, S.M.; Harkness, K.L. Major depression and its recurrences: Life course matters. Annu. Rev. Clin. Psychol. 2022, 18, 329–357. [Google Scholar] [CrossRef]
- GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Primers 2016, 2, 16065. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, Z.; Chen, D.; Peng, Y.; Lu, Z. Prevalence and associated factors of depression and anxiety symptoms among college students: A systematic review and meta-analysis. J. Child Psychol. Psychiatry 2022, 63, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M.; Schatzberg, A.F. Using chronic pain to predict depressive morbidity in the general population. Arch. Gen. Psychiatry 2003, 60, 39–47. [Google Scholar] [CrossRef]
- Liu, W.; Ge, T.; Leng, Y.; Pan, Z.; Fan, J.; Yang, W.; Cui, R. The role of neural plasticity in depression: From hippocampus to prefrontal cortex. Neural Plast. 2017, 2017, 6871089. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Franklin, T.; Iwata, M.; Duman, R.S. Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci. 2016, 17, 497–511. [Google Scholar] [CrossRef]
- Dean, J.; Keshavan, M. The neurobiology of depression: An Integrated View. Asian J. Psychiatry 2017, 27, 101–111. [Google Scholar] [CrossRef]
- Jiang, Y.; Zou, D.; Li, Y.; Gu, S.; Dong, J.; Ma, X.; Xu, S.; Wang, F.; Huang, J.H. Monoamine neurotransmitters control basic emotions and affect major depressive disorders. Pharmaceuticals 2022, 15, 1203. [Google Scholar] [CrossRef]
- Shao, X.; Zhu, G. Associations among monoamine neurotransmitter pathways, personality traits, and major depressive disorder. Front. Psychiatry 2020, 11, 381. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Wang, Y.; Zeng, B.; Zhu, B.; Dai, F. Association between depression and oxidative balance score: National Health and Nutrition Examination Survey (NHANES) 2005–2018. J. Affect. Disord. 2023, 337, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Karabin, T.; Biala, G.; Kruk-Slomka, M. The monoamine theory of depression as a target to effective pharmacotherapy. Curr. Issues Pharm. Med. Sci. 2023, 36, 108–113. [Google Scholar] [CrossRef]
- Schmaal, L.; Veltman, D.J.; van Erp, T.G.M.; Sämann, P.G.; Frodl, T.; Jahanshad, N.; Loehrer, E.; Tiemeier, H.; Hofman, A.; Niessen, W.J.; et al. Subcortical brain alterations in major depressive disorder: Findings from the ENIGMA major depressive disorder working group. Mol. Psychiatry 2016, 21, 806–812. [Google Scholar] [CrossRef]
- Schmaal, L.; Pozzi, E.; Ho, T.C.; Van Velzen, L.S.; Veer, I.M.; Opel, N.; Van Someren, E.J.; Han, L.K.; Aftanas, L.; Aleman, A. ENIGMA MDD: Seven years of global neuroimaging studies of major depression through worldwide data sharing. Transl. Psychiatry 2020, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Van Velzen, L.S.; Kelly, S.; Isaev, D.; Aleman, A.; Aftanas, L.I.; Bauer, J.; Baune, B.T.; Brak, I.V.; Carballedo, A.; Connolly, C.G. White matter disturbances in major depressive disorder: A coordinated analysis across 20 International cohorts in the ENIGMA MDD working group. Mol. Psychiatry 2020, 25, 1511–1525. [Google Scholar] [CrossRef]
- Strawbridge, R.; Young, A.H.; Cleare, A.J. Biomarkers for depression: Recent insights, current challenges and future prospects. Neuropsychiatr. Dis. Treat. 2017, 13, 1245–1262. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zhao, K.; Wei, X.; Carlisle, N.B.; Keller, C.J.; Oathes, D.J.; Fonzo, G.A.; Zhang, Y. Deep graph learning of multimodal brain networks defines treatment-predictive signatures in major depression. Mol. Psychiatry 2025, 30, 3963–3974. [Google Scholar] [CrossRef]
- Castrén, E.; Monteggia, L.M. Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biol. Psychiatry 2021, 90, 128–136. [Google Scholar] [CrossRef]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D Report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef]
- Gaynes, B.N.; Lux, L.; Gartlehner, G.; Asher, G.; Forman-Hoffman, V.; Green, J.; Boland, E.; Weber, R.P.; Randolph, C.; Bann, C.; et al. Defining treatment-resistant depression. Depress. Anxiety 2020, 37, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Byrow, Y. Is treatment-resistant depression a useful concept? BMJ Ment. Health 2016, 19, 1–3. [Google Scholar] [CrossRef]
- Zhdanava, M.; Pilon, D.; Ghelerter, I.; Chow, W.; Joshi, K.; Lefebvre, P.; Sheehan, J.J. The prevalence and national burden of treatment-resistant depression and major depressive disorder in the United States. J. Clin. Psychiatry 2021, 82, 29169. [Google Scholar] [CrossRef]
- Fava, M.; Freeman, M.P.; Flynn, M.; Judge, H.; Hoeppner, B.B.; Cusin, C.; Ionescu, D.F.; Mathew, S.J.; Chang, L.C.; Iosifescu, D.V. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol. Psychiatry 2020, 25, 1592–1603. [Google Scholar] [CrossRef]
- Wiesinger, T.; Kremer, S.; Bschor, T.; Baethge, C. Antidepressants and quality of life in patients with major depressive disorder—Systematic review and meta-analysis of double-blind, placebo-controlled RCTs. Acta Psychiatry Scand. 2023, 147, 545–560. [Google Scholar] [CrossRef]
- Saelens, J.; Gramser, A.; Watzal, V.; Zarate, C.A., Jr.; Lanzenberger, R.; Kraus, C. Relative effectiveness of antidepressant treatments in treatment-resistant depression: A systematic review and network meta-analysis of randomized controlled trials. Neuropsychopharmacology 2025, 50, 913–919. [Google Scholar] [CrossRef]
- Gelenberg, A.J.; Freeman, M.P.; Markowitz, J.C.; Rosenbaum, J.F.; Thase, M.E.; Trivedi, M.H.; Van Rhoads, R.S. American Psychiatric Association practice guideline for the treatment of patients with Major Depressive Disorder. Am. J. Psychiatry 2010, 167, 1–152. [Google Scholar]
- National Institute for Health and Care Excellence (NICE). Depression in Adults: Treatment and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2022. [Google Scholar]
- Marazziti, D. Capitolo 3. Depressione Resistente. In Psicofarmacoterapia Clinica, 7th ed.; Giovanni Fioriti Editore: Rome, Italy, 2025. [Google Scholar]
- Frevert, J. Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type A products. Drugs RD 2015, 15, 1–9. [Google Scholar] [CrossRef]
- Frevert, J.; Dressler, D. Clinical Relevance of Immunoresistance to Botulinum Therapy. In Botulinum Toxin Therapy Manual for Dystonia and Spasticity; InTech: London, UK, 2016. [Google Scholar] [CrossRef]
- Albrecht, P.; Jansen, A.; Lee, J.-I.; Moll, M.; Ringelstein, M.; Rosenthal, D.; Bigalke, H.; Aktas, O.; Hartung, H.-P.; Hefter, H. High prevalence of neutralizing antibodies after long-term botulinum neurotoxin therapy. Neurology 2019, 92, e48–e54. [Google Scholar] [CrossRef] [PubMed]
- Dessy, L.A.; Fallico, N.; Mazzocchi, M.; Scuderi, N. Botulinum toxin for glabellar lines: A review of the efficacy and safety of currently available products. Am. J. Clin. Dermatol. 2011, 12, 377–388. [Google Scholar] [CrossRef]
- Souery, D.; Papakostas, G.I.; Trivedi, M.H. Treatment-resistant depression. J. Clin. Psychiatry 2006, 67, 16. [Google Scholar] [CrossRef]
- Wijeratne, C.; Sachdev, P. Treatment-resistant depression: Critique of current approaches. Aust. N. Z. J. Psychiatry 2008, 42, 751–762. [Google Scholar] [CrossRef]
- Aoki, K.R. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology 2005, 26, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.R.; Guyer, B. Botulinum toxin type A and other botulinum toxin serotypes: A comparative review of biochemical and pharmacological actions. Euro J. Neurol. 2001, 8, 21–29. [Google Scholar] [CrossRef]
- Blasi, J.; Chapman, E.R.; Yamasaki, S.; Binz, T.; Niemann, H.; Jahn, R. Botulinum neurotoxin C1 blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. EMBO J. 1993, 12, 4821–4828. [Google Scholar] [CrossRef] [PubMed]
- Sikorra, S.; Henke, T.; Galli, T.; Binz, T. Substrate recognition mechanism of VAMP/synaptobrevin-cleaving clostridial neurotoxins. J. Biol. Chem. 2008, 283, 21145–21152. [Google Scholar] [CrossRef]
- Carr, W.W.; Jain, N.; Sublett, J.W. Immunogenicity of botulinum toxin formulations: Potential therapeutic implications. Adv. Ther. 2021, 38, 5046–5064. [Google Scholar] [CrossRef]
- Ayoub, N. Botulinum toxin therapy: A comprehensive review on clinical and pharmacological insights. J. Clin. Med. 2025, 14, 2021. [Google Scholar] [CrossRef]
- Mazzocchio, R.; Caleo, M. More than at the neuromuscular synapse: Actions of botulinum neurotoxin A in the central nervous system. Neuroscientist 2015, 21, 44–61. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum neurotoxins: Biology, pharmacology, and toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef]
- Caleo, M.; Restani, L. Direct central nervous system effects of botulinum neurotoxin. Toxicon 2018, 147, 68–72. [Google Scholar] [CrossRef]
- Schulze, J.; Neumann, I.; Magid, M.; Finzi, E.; Sinke, C.; Wollmer, M.A.; Krüger, T.H.C. Botulinum toxin for the management of depression: An updated review of the evidence and meta-analysis. J. Psychiatr. Res. 2021, 135, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Shao, F.; Lenahan, C.; Shao, A.; Li, Y. Efficacy and safety of botulinum toxin vs. placebo in depression: A systematic review and meta-analysis of randomized controlled trials. Front. Psychiatry 2020, 11, 603087. [Google Scholar] [CrossRef]
- Crowley, J.S.; Silverstein, M.L.; Reghunathan, M.; Gosman, A.A. Glabellar botulinum toxin injection improves depression scores: A systematic review and meta-analysis. Plast. Reconstr. Surg. 2022, 150, 211e–220e. [Google Scholar] [CrossRef]
- Arnone, D.; Galadari, H.; Rodgers, C.J.; Östlundh, L.; Aziz, K.A.; Stip, E.; Young, A.H. Efficacy of onabotulinumtoxinA in the treatment of unipolar major depression: Systematic review, meta-analysis and meta-regression analyses of double-blind randomised controlled trials. J. Psychopharmacol. 2021, 35, 910–918. [Google Scholar] [CrossRef]
- Caleo, M.; Spinelli, M.; Colosimo, F.; Matak, I.; Rossetto, O.; Lackovic, Z.; Restani, L. Transynaptic action of botulinum neurotoxin type A at central cholinergic boutons. J. Neurosci. 2018, 38, 10329–10337. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M. Mechanism of action of botulinum neurotoxin: Unexpected consequences. Toxicon 2018, 147, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Makunts, T.; Wollmer, M.A.; Abagyan, R. Postmarketing safety surveillance data reveals antidepressant effects of botulinum toxin across various indications and injection sites. Sci. Rep. 2020, 10, 12851. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Liu, X.; Su, C.-J.; Zhang, Q.-L.; Wang, Z.-H.; Cao, L.-F.; Guo, X.-Y.; Huang, Y.; Luo, W.; et al. Antidepressant-like action of single facial injection of botulinum neurotoxin A is associated with augmented 5-HT Levels and BDNF/ERK/CREB Pathways in Mouse Brain. Neurosci. Bull. 2019, 35, 661–672. [Google Scholar] [CrossRef]
- Ni, L.; Chen, H.; Xu, X.; Sun, D.; Cai, H.; Wang, L.; Tang, Q.; Hao, Y.; Cao, S.; Hu, X. Neurocircuitry underlying the antidepressant effect of retrograde facial botulinum toxin in mice. Cell Biosci. 2023, 13, 30. [Google Scholar] [CrossRef]
- Burgen, A.S.V.; Dickens, F.; Zatman, L.J. The action of botulinum toxin on the neuro-muscular junction. J. Physiol. 1949, 109, 10–24. [Google Scholar] [CrossRef]
- Restani, L.; Novelli, E.; Bottari, D.; Leone, P.; Barone, I.; Galli-Resta, L.; Strettoi, E.; Caleo, M. Botulinum neurotoxin A impairs neurotransmission following retrograde transynaptic transport. Traffic 2012, 13, 1083–1089. [Google Scholar] [CrossRef]
- Ekman, P. The directed facial action task: Emotional responses without appraisal. In Handbook of Emotion Elicitation and Assessment; Series in affective science; Oxford University Press: New York, NY, USA, 2007; pp. 47–53. ISBN 978-0-19-516915-7. [Google Scholar]
- Schwartz, G.E.; Fair, P.L.; Salt, P.; Mandel, M.R.; Klerman, G.L. Facial muscle patterning to affective imagery in depressed and nondepressed subjects. Science 1976, 192, 489–491. [Google Scholar] [CrossRef]
- Rodríguez-Cerdeira, C.; Eckhardt, W. Depression treatment: Is there a role for botulinum toxin type A? Microorganisms 2024, 12, 2615. [Google Scholar] [CrossRef]
- Heckmann, M.; Teichmann, B.; Schröder, U.; Sprengelmeyer, R.; Ceballos-Baumann, A.O. Pharmacologic denervation of frown muscles enhances baseline expression of happiness and decreases baseline expression of anger, sadness, and fear. J. Am. Acad. Dermatol. 2003, 49, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Wollmer, M.A.; Magid, M.; Kruger, T.H.C.; Finzi, E. The use of botulinum toxin for treatment of depression. In Botulinum Toxin Therapy; Whitcup, S.M., Hallett, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 265–278. ISBN 978-3-030-66306-3. [Google Scholar]
- Darwin, C. The Expression of the Emotions in Man and Animals; John Murray: London, UK, 1872; VI, 374p, 7 c. of plates: ill.; 19 cm. [Google Scholar]
- Strack, F.; Martin, L.L.; Stepper, S. Inhibiting and facilitating conditions of the human smile: A nonobtrusive test of the facial feedback hypothesis. J. Personal. Soc. Psychol. 1988, 54, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-G.; Kotha, P.; Chen, Y.-H. Understandings of acupuncture application and mechanisms. Am. J. Transl. Res. 2022, 14, 1469–1481. [Google Scholar]
- Wollmer, M.A.; Kalak, N.; Jung, S.; DeBoer, C.; Magid, M.; Reichenberg, J.S.; Brand, S.; Holsboer-Trachsler, E.; Kruger, T.H.C. Agitation predicts response of depression to botulinum toxin treatment in a randomized controlled trial. Front. Psychiatry 2014, 5, 36. [Google Scholar] [CrossRef]
- Brennan, C. Botulinum toxin type-A (BoNT-A) injections of the corrugator muscles for aesthetics and depression? Plast. Surg. Nurs. 2016, 36, 167–169. [Google Scholar] [CrossRef]
- Finzi, E. The Face of Emotion: How Botox Affects Our Mood and Relationships; Palgrave Macmillan: New York, NY, USA, 2013; ISBN 978-0-230-34185-2. [Google Scholar]
- Finzi, E.; Rosenthal, N.E. Emotional proprioception: Treatment of depression with afferent facial feedback. J. Psychiatr. Res. 2016, 80, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Al Abdulmohsen, T.; Kruger, T.H.C. The contribution of muscular and auditory pathologies to the symptomatology of autism. Med. Hypotheses 2011, 77, 1038–1047. [Google Scholar] [CrossRef]
- Davis, J.I.; Senghas, A.; Brandt, F.; Ochsner, K.N. The effects of BOTOX injections on emotional experience. Emotion 2010, 10, 433–440. [Google Scholar] [CrossRef]
- Lewis, M.B.; Bowler, P.J. Botulinum toxin cosmetic therapy correlates with a more positive mood. J. Cosmet. Dermatol. 2009, 8, 24–26. [Google Scholar] [CrossRef]
- Sommer, B.; Zschocke, I.; Bergfeld, D.; Sattler, G.; Augustin, M. Satisfaction of patients after treatment with botulinum toxin for dynamic facial lines. Dermatol. Surg. 2003, 29, 456. [Google Scholar] [PubMed]
- Sykianakis, D.; Stratigos, A.; Chatziioannou, A.; Christodoulou, C. Botulinum toxin type A treatment is associated with improved social and psychological behavior: A retrospective study. J. Cosmet. Dermatol. 2022, 21, 142–148. [Google Scholar] [CrossRef]
- Baumeister, J.-C.; Papa, G.; Foroni, F. Deeper than Skin Deep—The Effect of Botulinum Toxin-A on Emotion Processing. Toxicon 2016, 118, 86–90. [Google Scholar] [CrossRef]
- Bulnes, L.C.; Mariën, P.; Vandekerckhove, M.; Cleeremans, A. The effects of botulinum toxin on the detection of gradual changes in facial emotion. Sci. Rep. 2019, 9, 11734. [Google Scholar] [CrossRef]
- Havas, D.A.; Glenberg, A.M.; Gutowski, K.A.; Lucarelli, M.J.; Davidson, R.J. Cosmetic use of botulinum toxin-A affects processing of emotional language. Psychol. Sci. 2010, 21, 895–900. [Google Scholar] [CrossRef]
- Hennenlotter, A.; Dresel, C.; Castrop, F.; Ceballos-Baumann, A.O.; Wohlschläger, A.M.; Haslinger, B. The link between facial feedback and neural activity within central circuitries of emotion—New insights from botulinum toxin–induced denervation of frown muscles. Cereb. Cortex 2009, 19, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Neta, M.; Davis, F.C.; Ruberry, E.J.; Dinescu, D.; Heatherton, T.F.; Stotland, M.A.; Whalen, P.J. Botulinum toxin-induced facial muscle paralysis affects amygdala responses to the perception of emotional expressions: Preliminary findings from an A-B-A design. Biol. Mood Anxiety Disord. 2014, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Shin, L.M.; Liberzon, I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010, 35, 169–191. [Google Scholar] [CrossRef]
- Matsuo, K.; Ban, R.; Hama, Y.; Yuzuriha, S. Eyelid opening with trigeminal proprioceptive activation regulates a brainstem arousal mechanism. PLoS ONE 2015, 10, e0134659. [Google Scholar] [CrossRef]
- Finzi, E. Botulinum toxin treatment for depression: A new paradigm for psychiatry. Toxins 2023, 15, 336. [Google Scholar] [CrossRef]
- Wollmer, M.A.; Magid, M.; Kruger, T.H.C.; Finzi, E. Treatment of depression with botulinum toxin. Toxins 2022, 14, 383. [Google Scholar] [CrossRef] [PubMed]
- Myers, B. Corticolimbic regulation of cardiovascular responses to stress. Physiol. Behav. 2017, 172, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Krohn, F.; Novello, M.; van der Giessen, R.S.; De Zeeuw, C.I.; Pel, J.J.M.; Bosman, L.W.J. The integrated brain network that controls respiration. eLife 2023, 12, e83654. [Google Scholar] [CrossRef]
- Šimić, G.; Tkalčić, M.; Vukić, V.; Mulc, D.; Španić, E.; Šagud, M.; Olucha-Bordonau, F.E.; Vukšić, M.; Hof, P.R. Understanding emotions: Origins and roles of the amygdala. Biomolecules 2021, 11, 823. [Google Scholar] [CrossRef]
- Lamotte, G.; Shouman, K.; Benarroch, E.E. Stress and central autonomic network. Auton. Neurosci. 2021, 235, 102870. [Google Scholar] [CrossRef]
- Quadt, L.; Critchley, H.; Nagai, Y. Cognition, emotion, and the central autonomic network. Auton. Neurosci. 2022, 238, 102948. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, T.T.; Yin, G.; Cui, R.; Zhao, G.; Yang, W. Stress-induced functional alterations in amygdala: Implications for neuropsychiatric diseases. Front. Neurosci. 2018, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, W.; Fan, Y.; Li, Y.; Liu, J.; Xu, Y.; Jiang, C.; Tang, Z.; Cao, C.; Liu, T.; et al. The safety and efficacy of botulinum toxin A on the treatment of depression. Brain Behav. 2021, 11, e2333. [Google Scholar] [CrossRef]
- Bang, J.Y.; Zhao, J.; Rahman, M.; St-Cyr, S.; McGowan, P.O.; Kim, J.C. Hippocampus-anterior hypothalamic circuit modulates stress-induced endocrine and behavioral response. Front. Neural Circuits 2022, 16, 894722. [Google Scholar] [CrossRef] [PubMed]
- Keynan, J.N.; Meir-Hasson, Y.; Gilam, G.; Cohen, A.; Jackont, G.; Kinreich, S.; Ikar, L.; Or-Borichev, A.; Etkin, A.; Gyurak, A.; et al. Limbic activity modulation guided by functional magnetic resonance imaging–inspired electroencephalography improves implicit emotion regulation. Biol. Psychiatry 2016, 80, 490–496. [Google Scholar] [CrossRef]
- Barreiros, A.R.; Almeida, I.; Baía, B.C.; Castelo-Branco, M. Amygdala modulation during emotion regulation training with fMRI-cased neurofeedback. Front. Hum. Neurosci. 2019, 13, 89. [Google Scholar] [CrossRef]
- Jentsch, V.L.; Merz, C.J.; Wolf, O.T. Restoring emotional stability: Cortisol effects on the neural network of cognitive emotion regulation. Behav. Brain Res. 2019, 374, 111880. [Google Scholar] [CrossRef] [PubMed]
- Parsaik, A.K.; Mascarenhas, S.S.; Hashmi, A.; Prokop, L.J.; John, V.; Okusaga, O.; Singh, B. Role of botulinum toxin in depression. J. Psychiatr. Pract. 2016, 22, 99. [Google Scholar] [CrossRef] [PubMed]
- Magid, M.; Finzi, E.; Kruger, T.H.C.; Robertson, H.T.; Keeling, B.H.; Jung, S.; Reichenberg, J.S.; Rosenthal, N.E.; Wollmer, M.A. Treating depression with botulinum toxin: A pooled analysis of randomized controlled trials. Pharmacopsychiatry 2015, 48, 205–210. [Google Scholar] [CrossRef]
- Hexsel, D.; Hexsel, C.; Siega, C.; Schilling-Souza, J.; Rotta, F.T.; Rodrigues, T.C. Fields of effects of 2 commercial preparations of botulinum toxin type A at equal labeled unit doses: A double-blind randomized trial. JAMA Dermatol. 2013, 149, 1386–1391. [Google Scholar] [CrossRef]
- Finzi, E.; Wasserman, E. Treatment of depression with botulinum toxin A: A case series. Dermatol. Surg. 2006, 32, 645–649; discussion 649–650. [Google Scholar] [CrossRef]
- Wollmer, M.A.; de Boer, C.; Kalak, N.; Beck, J.; Götz, T.; Schmidt, T.; Hodzic, M.; Bayer, U.; Kollmann, T.; Kollewe, K.; et al. Facing depression with botulinum toxin: A randomized controlled trial. J. Psychiatr. Res. 2012, 46, 574–581. [Google Scholar] [CrossRef]
- Finzi, E.; Rosenthal, N.E. Treatment of depression with onabotulinumtoxinA: A randomized, double-blind, placebo controlled trial. J. Psychiatr. Res. 2014, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Magid, M.; Reichenberg, J.S.; Poth, P.E.; Robertson, H.T.; LaViolette, A.K.; Kruger, T.H.C.; Wollmer, M.A. Treatment of major depressive disorder using botulinum toxin A: A 24-week randomized, double-blind, placebo-controlled study. J. Clin. Psychiatry 2014, 75, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Brin, M.F.; Durgam, S.; Lum, A.; James, L.; Liu, J.; Thase, M.E.; Szegedi, A. OnabotulinumtoxinA for the treatment of major depressive disorder: A phase 2 randomized, double-blind, placebo-controlled trial in adult females. Int. Clin. Psychopharmacol. 2020, 35, 19–28. [Google Scholar] [CrossRef]
- Chugh, S.; Chhabria, A.; Jung, S.; Kruger, T.H.C.; Wollmer, M.A. Botulinum toxin as a treatment for depression in a real-world setting. J. Psychiatr. Pract. 2018, 24, 15–20. [Google Scholar] [CrossRef]
- Shu, H.; Shen, T.; Deng, W.; Cao, J.; Xu, Y.; Liu, J.; Zhou, X.; Luo, W.F. Comparative effectiveness of two different doses of botulinum toxin A for the treatment of mild to moderate depression. J. Affect. Disord. 2024, 350, 824–830. [Google Scholar] [CrossRef]
- Cristel, R.T.; Gandhi, N.D.; Issa, T.Z.; Kola, E.; Demesh, D.; Dayan, S.H. A Randomized, single-blind, crossover study evaluating the impact of onabotulinumtoxinA treatment on mood and appearance during the COVID-19 pandemic. Aesthet. Surg. J. 2021, 41, NP1199–NP1205. [Google Scholar] [CrossRef]
- Klassen, A.F.; Cano, S.J.; Scott, A.; Snell, L.; Pusic, A.L. Measuring patient-reported outcomes in facial aesthetic patients: Development of the FACE-Q. Facial Plast. Surg. 2010, 26, 303–309. [Google Scholar] [CrossRef]
- Lyubomirsky, S.; Lepper, H.S. A measure of subjective happiness: Preliminary reliability and construct validation. Soc. Indic. Res. 1999, 46, 137–155. [Google Scholar] [CrossRef]
- Feng, X.-Y.; Shen, T.-T.; Wu, Q.-C.; Wang, J.; Ni, P.; Liu, J.; Zhou, X.-P.; Hu, H.; Luo, W.-F. A novel approach to treating post-stroke depression: Administration of botulinum toxin A via local facial injection. Front. Neurol. 2024, 15, 1372547. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, T.; Shen, T.; Wu, W.; Cao, J.; Sun, J.; Liu, J.; Zhou, X.; Jiang, C.; Tang, Z.; et al. Botulinum toxin A (BoNT/A) for the treatment of depression: A randomized, double-blind, placebo, controlled trial in China. J. Affect. Disord. 2022, 318, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, F.; Neumann, I.; Krüger, T.H.C.; Wollmer, M.A. Botulinum toxin therapy for psychiatric disorders in clinical practice: A retrospective case study. Toxins 2023, 15, 385. [Google Scholar] [CrossRef]
- Kruger, T.H.C.; Magid, M.; Wollmer, M.A. Can botulinum toxin help patients with borderline personality disorder? Am. J. Psychiatry 2016, 173, 940–941. [Google Scholar] [CrossRef]
- Kruger, T.H.C.; Schulze, J.; Bechinie, A.; Neumann, I.; Jung, S.; Sperling, C.; Engel, J.; Müller, A.; Kneer, J.; Kahl, K.G.; et al. Neuronal effects of glabellar botulinum toxin injections using a valenced inhibition task in borderline personality disorder. Sci. Rep. 2022, 12, 14197. [Google Scholar] [CrossRef]
- Ceolato-Martin, C.; Chevallier-Collins, C.; Clément, J.-P.; Charles, E.; Lacroix, A.; Ranoux, D. OnabotulinumtoxinA in resistant depression: A randomized trial comparing two facial injection sites (OnaDEP Study). Depress. Anxiety 2024, 2024, 1177925. [Google Scholar] [CrossRef]
- Guvenc, U. Botulinum toxin and its effect on depression. EJMI 2023, 7, 477–481. [Google Scholar] [CrossRef]
- Finzi, E.; Kels, L.; Axelowitz, J.; Shaver, B.; Eberlein, C.; Krueger, T.H.; Wollmer, M.A. Botulinum toxin therapy of bipolar depression: A case series. J. Psychiatr. Res. 2018, 104, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A.; Fulkerson, J.M.; El-Mallakh, R.S. Use of botulinum toxin A for depression symptoms in patients with treatment-resistant bipolar illness: A case series. Bipolar Disord. 2023, 25, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, T.; Luo, W. Botulinum neurotoxin therapy for depression: Therapeutic mechanisms and future perspective. Front. Psychiatry 2021, 12, 584416. [Google Scholar] [CrossRef]
- Demchenko, I.; Swiderski, A.; Liu, H.; Jung, H.; Lou, W.; Bhat, V. Botulinum toxin injections for psychiatric disorders: A systematic review of the clinical trial landscape. Toxins 2024, 16, 191. [Google Scholar] [CrossRef]
- Naumann, M.; Jankovic, J. Safety of botulinum toxin type A: A systematic review and meta-analysis. Curr. Med. Res. Opin. 2004, 20, 981–990. [Google Scholar] [CrossRef]
- Connor, K.M.; Cook, J.L.; Davidson, J.R.T. Botulinum toxin treatment of social anxiety disorder with hyperhidrosis: A placebo-controlled double-blind trial. J. Clin. Psychiatry 2006, 67, 30–36. [Google Scholar] [CrossRef]
- Dong, H.; Fan, S.; Luo, Y.; Peng, B. Botulinum toxin relieves anxiety and depression in patients with hemifacial spasm and blepharospasm. Neuropsychiatr. Dis. Treat. 2019, 15, 33–36. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Ji, X.; Liu, M.; Zhou, C. Clinical efficacy of escitalopram combined with botulinum toxin A in patients with generalized anxiety disorder and comorbid headache. Psychopharmacology 2023, 240, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Wollmer, M.A.; Neumann, I.; Jung, S.; Bechinie, A.; Herrmann, J.; Müller, A.; Wohlmuth, P.; Fournier-Kaiser, L.; Sperling, C.; Peters, L.; et al. Clinical effects of glabellar botulinum toxin injections on borderline personality disorder: A randomized controlled trial. J. Psychopharmacol. 2022, 36, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Schulze, J.; Sinke, C.; Neumann, I.; Wollmer, M.A.; Kruger, T.H.C. Effects of glabellar botulinum toxin injections on resting-state functional connectivity in borderline personality disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2024, 274, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Stearns, T.P.; Shad, M.U.; Guzman, G.C. Glabellar botulinum toxin injections in major depressive disorder: A critical review. Prim. Care Companion CNS Disord. 2018, 20, 18r02298. [Google Scholar] [CrossRef]
- Zamanian, A.; Ghanbari Jolfaei, A.; Mehran, G.; Azizian, Z. Efficacy of botox versus placebo for treatment of patients with major depression. Iran. J. Public Health 2017, 46, 982–984. [Google Scholar]
| NCT ID | Agent/Class (Route–Dose–Regimen) | Comparator | Design/Phase/Duration | Population (Key Inclusion/Stratification) | N (Plan) | Primary Endpoint(s) and Timing | Key Secondary/Mechanistic Outcomes | Status and Key Dates |
|---|---|---|---|---|---|---|---|---|
| NCT03748446 | Xenon-O2 (35:65) single inhalation (sub-anesthetic) + TAU | Nitrogen-O2 (35:65) + TAU | Randomized, double-blind crossover; Early Phase 1 | 20 severe depressions: 10 MDD, 10 bipolar depressions (TRD focus) | 20 | Day-1 improvement on HDRS (6-item) and QIDS-C; repeated acute timepoints | None listed | Recruiting; first posted 20 November 2018; last update 18 May 2025 |
| NCT05357040 | Nitrous oxide 25% or 50% (60′ weekly ×4) | Oxygen–air mixture (FiO2 ≈ 0.3) | Phase 2, RCT parallel 1:1; nitrous arm split 25% vs. 50%; double-blind (pt/assessor); 7 wk total | Adults with MDD (incl. TRD); outpatient | 172 | HDRS-21 change over 4 wk | 24-h response/remission; POMS; CAT-MH (dep/anx/suicide); S-STS; dose–response; compliance; VAS well-being; AEs | Recruiting; start 30 June 2021; primary compl. 1 October 2025 |
| NCT05415397 (INFLAMED) | Celecoxib 400 mg/day add-on (12 wk) | Placebo add-on | Phase 3, RCT 1:1, parallel, quadruple-blind | DSM-5 MDD with ImmunoMetabolic Depression (IDS AES ≥ 6) + CRP > 1 mg/L; on SSRI/SNRI | 140 | IDS-SR trajectories (bi-weekly) over 12 wk | IDS response/remission; AES subscore; fatigue, food craving, sleep, anxiety, functioning, pain; pill count; CRP/IL-6/TNF-α/lipids/glucose; AEs | Recruiting; start 28 September 2022; compl. est. July 2025 |
| NCT05558995 | Ketogenic diet (20–30 g carbs; 80–100 g protein; fats allowed) 12 wk; adjunct to SSRIs | None (open-label) | Single-arm feasibility; Phase NA; 2-wk induction + 10-wk maintenance | MDD 18–50, partial SSRI responders, residual anhedonia | 15 | Adherence rate over 12 wk | EEfRT; MADRS; SHAPS; GAD-7; CGI; plasma BDNF and cytokines (TNF-α, IL-1, IL-6, IL-10); extensive safety labs | Recruiting; last update 18 November 2023 |
| NCT05570110 | Enoxolone (11β-HSD2 inhibitor) PO; dose NR | Placebo | Randomized, double-blind; biomarker-stratified; Phase NA | MDD; groups split by baseline SBP (median) and urine aldosterone/cortisol; exploratory HRV, sleep, salt taste, CRP, MRI | NR | Differential clinical response by biomarker strata; biomarker change | BP; aldosterone/cortisol; Na+/K+; HR/HRV; sleep; inflammatory markers; optional MRI/DTI | Recruiting; update 9 April 2024 |
| NCT05570812 | Pregnenolone PO ramp 50→500 mg/day (4 wk) then 500 mg/day (4 wk) | Placebo (identical titration) | Phase 2, randomized, parallel, quadruple-blind | PLWH on ART, 18–85 yrs, CES-D ≥ 20; can stay on ADs | 120 (90 active/30 plc) | Left insular cortex GABA (MRS) Day14 and Day56 (baseline-adjusted) | CES-D; CD14+CD16+ monocytes; responder GABA; AEs; dose mods | Recruiting; start 3 March 2023; primary compl. 30 June 2027 |
| NCT05644301 (INSTA-MD) | Minocycline 100 mg BID × 12 wk or Celecoxib 200 mg BID × 12 wk (add-on to TAU) | Placebo + TAU | Phase 3, randomized, parallel, quadruple-blind; hs-CRP stratified (<3/>3 mg/L); 6 arms | DSM-5 MDD, non-remission to adequate AD; physically healthy | 240 | HDRS-17 change; remission (≤7) at 12 wk | IDS-SR; HDRS response; PSQI; STAI; CORE; MARS; AEs; metabolic markers; cytokines; PBMCs; kynurenine pathway; VEGF, BDNF | Recruiting; start 21 September 2023; compl. est. September 2026 |
| NCT05710887 | Nitrous oxide 50% (45′ single session) + TAU in ED | Oxygen–air mixture + TAU | Phase 2, RCT parallel; double-blind (pt/assessor); ED setting; ≤24 h follow-up | 18–65, acutely suicidal, non-psychotic MDD in ED | 50 | CAT-MH change (suicide/dep/anx) within 24 h | Compliance; rapid (30–60′) and sustained response; correlation with lifetime suicide predictors; AEs | Not yet recruiting; start est. 1 October 2025; primary compl. 1 August 2027 |
| NCT05757791 | Empagliflozin 10 mg × 14 d → 25 mg × 28 d (6 wk) | None (open-label) | Phase 2, single-group | Adults 18–65, MDD (MADRS ≥ 20), ≤2 failed ADs; no prior SGLT2 | 16 | MADRS change baseline→wk6 | C-SSRS; SHAPS | Recruiting; start 17 March 2023; primary compl. est. December 2025 |
| NCT06136546 | Infliximab 5 mg/kg IV (single infusion) | Saline IV | Phase 2, randomized, parallel, triple-blind; 2-wk follow-up | MDD, 25–50 yrs, CRP ≥ 3 mg/L; HAMD-17 ≥ 15; stable/off AD ≥ 4 wk | 100 | Psychomotor speed (Simple RT) and executive function (Choice RT) via TestMyBrain (daily × 2 wk) | HAMD-17; Dimensional Anhedonia Rating Scale; CRP; TNF-α and receptors | Recruiting; start 23 January 2025; primary compl. 31 August 2028 |
| NCT06323785 | Whole-body hyperthermia (water-filtered IR) | Sham hyperthermia | RCT, parallel, quadruple-blind; 6 wk; Phase NA | MDD 18–65; HAMD-17 ≥ 14; German-speaking | 30 | HAMD-17 at 1 wk | BDI; MOS-SF QoL; HAMD-17 at 6 wk | Recruiting; start est. 15 June 2024; primary compl. 1 March 2026 |
| NCT06537921 (CODA) | Minocycline 200 mg/day PO × 8 wk adjunct | None | Single-group, open-label feasibility; 12 wk total | MDD + obesity (BMI ≥ 30) + CRP ≥ 3 mg/L; TRD; MRI-eligible | 35 | Feasibility: enrolment, adherence, completion of biomarkers/MRI/PROs; effect-size estimates | Blood/saliva biomarkers; MRI baseline and wk 8; questionnaires | Recruiting; start 1 October 2024; compl. est. 1 September 2027 |
| NCT06671977 | DMT IV (low and medium bolus+infusion) ± THC comparators | Placebo | Phase 1, randomized crossover; triple-blind; 2 sessions 4 wk apart | Adults 21–65: MDD cohort (moderate–severe, ≥1 inadequate AD) + healthy controls | 60 | Safety/physiology; MEQ30; PSI; VAS anxiety/dep; CEQ; reinforcing effects; tolerability; EEG | Expectancy/blinding indices; blood assays; NEO; AAQ | Recruiting; start 14 March 2025; compl. est. 1 Decembrer 2027 |
| NCT06698666 | Rosuvastatin 10 mg PO daily × 12 wk (± sertraline TAU) | Sertraline (standard care)—details NR | RCT; parallel; convenience sample; Phase NA | Adults 20–45, mild–moderate MDD; MADRS 7–34 | 144 (72/arm) | MADRS change after 12 wk | Safety AEs; serum cholesterol (baseline and wk 12) | Recruiting; start 2022; last update 21 November 2024 |
| Authors and Year of Publication | Type of Study | Study Sample | Type of Intervention | Comparison | Evaluation Tools | Results |
|---|---|---|---|---|---|---|
| Burgen et al. (1949) [54]. | Experimental, observational physiology. | Isolated neuromuscular junction preparations from rats. | Application of botulinum toxin to muscle-nerve preparations. | N.A. | Electrophysiology (muscle contraction amplitude). | Botulinum toxin blocks acetylcholine release at the neuromuscular junction, leading to progressive paralysis. |
| Blasi et al. (1993) [38]. | Experimental, biochemical comparative. | Cultured neurons and recombinant proteins. | Exposure of neurons to BoNT/C1. | Cleaved vs. intact syntaxin. | Protein cleavage assays, neurotransmitter release quantification. | BoNT/C1 specifically cleaves syntaxin (HPC-1), blocking synaptic vesicle exocytosis and thus neurotransmitter release. |
| Sikorra et al. (2008) [39]. | Experimental, biochemical comparative. | Recombinant synaptobrevin/VAMPs. | Exposure to different VAMP-cleaving clostridial. | Comparisons among BoNT serotypes. | Site-directed mutagenesis, proteolytic cleavage assays, structural modeling. | Identification of specific aminoacidic residues critical for VAMP recognition and cleavage by BoNT serotypes, explaining substrate selectivity. |
| Restani et al. (2012) [55]. | Experimental, in vivo observational. | Rodents (mice/rats). | Peripheral BoNT/A injection into retina or nerve terminals. | Injected vs. non-injected controls. | Immunohistochemistry, electrophysiology, synaptic transmission assays. | BoNT/A undergoes retrograde axonal transport and is trans-synaptically transferred, impairing neurotransmission at second-order neurons. |
| Caleo et al. (2018) [49]. | Experimental, in vivo observational. | Rodents (mice). | Peripheral BoNT/A injection. | Injected vs. control groups. | Immunohistochemistry, neuronal activity mapping, synaptic staining. | BoNT/A crosses synapses trans-synaptically, altering neurotransmission in central cholinergic boutons distant from injection site. |
| Li et al. (2019) [52]. | Experimental, in vivo comparative. | Mice. | Single facial injection of BoNT/A vs. saline control. | BoNT/A vs. saline control. | Behavioral tests (forced swim test, tail suspension test), neurochemical assays (5-HT, BDNF, ERK/CREB activation). | BoNT/A-treated mice showed reduced depressive-like behavior, increased serotonin levels, and activation of BDNF/ERK/CREB pathways, suggesting antidepressant potential. |
| Ni et al. (2023) [53]. | Experimental, in vivo comparative. | Mice. | Retrograde facial BoNT/A injection. | BoNT/A vs. controls; chemogenetic silencing vs. intact pathways. | Behavioral tests, neuronal tracing, chemogenetic manipulation, immunohistochemistry. | Mapped neural circuits mediating BoNT/A’s antidepressant effect, identifying serotonergic projections as critical; silencing these pathways abolished behavioral improvement. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambini, M.; Gurrieri, R.; Russomanno, G.; Cecchini, G.; Mucci, F.; Carbone, M.G.; Marazziti, D. Botulinum Toxin: An Unconventional Tool for the Treatment of Depression? Brain Sci. 2025, 15, 971. https://doi.org/10.3390/brainsci15090971
Gambini M, Gurrieri R, Russomanno G, Cecchini G, Mucci F, Carbone MG, Marazziti D. Botulinum Toxin: An Unconventional Tool for the Treatment of Depression? Brain Sciences. 2025; 15(9):971. https://doi.org/10.3390/brainsci15090971
Chicago/Turabian StyleGambini, Matteo, Riccardo Gurrieri, Gerardo Russomanno, Gianmatteo Cecchini, Federico Mucci, Manuel Glauco Carbone, and Donatella Marazziti. 2025. "Botulinum Toxin: An Unconventional Tool for the Treatment of Depression?" Brain Sciences 15, no. 9: 971. https://doi.org/10.3390/brainsci15090971
APA StyleGambini, M., Gurrieri, R., Russomanno, G., Cecchini, G., Mucci, F., Carbone, M. G., & Marazziti, D. (2025). Botulinum Toxin: An Unconventional Tool for the Treatment of Depression? Brain Sciences, 15(9), 971. https://doi.org/10.3390/brainsci15090971








