Resolution of Lipopolysaccharide-Induced Inflammation Followed by DNA Hypomethylation and Increased Tetrahydrobiopterin Biosynthesis in Mouse Hippocampus
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. LPS-Induced Inflammatory Process
2.3. Locomotor Activity
2.4. Determination of Neopterin Levels by High-Performance Liquid Chromatography (HPLC)
2.5. Determination of Monoamine Levels by HPLC
2.6. Measurement of Reactive Oxygen Species (ROS)
2.7. Measurement of Thiobarbituric Acid-Reactive Substances (TBA-RSs)
2.8. Determination of NO Levels
2.9. Total RNA Extraction
2.10. cDNA Synthesis
2.11. Gene Expression by Quantitative Real-Time PCR (qPCR)
2.12. Genomic DNA Extraction
2.13. DNA Methylation in the Promoter Gene
2.14. Protein Determination
2.15. Statistical Analysis
3. Results
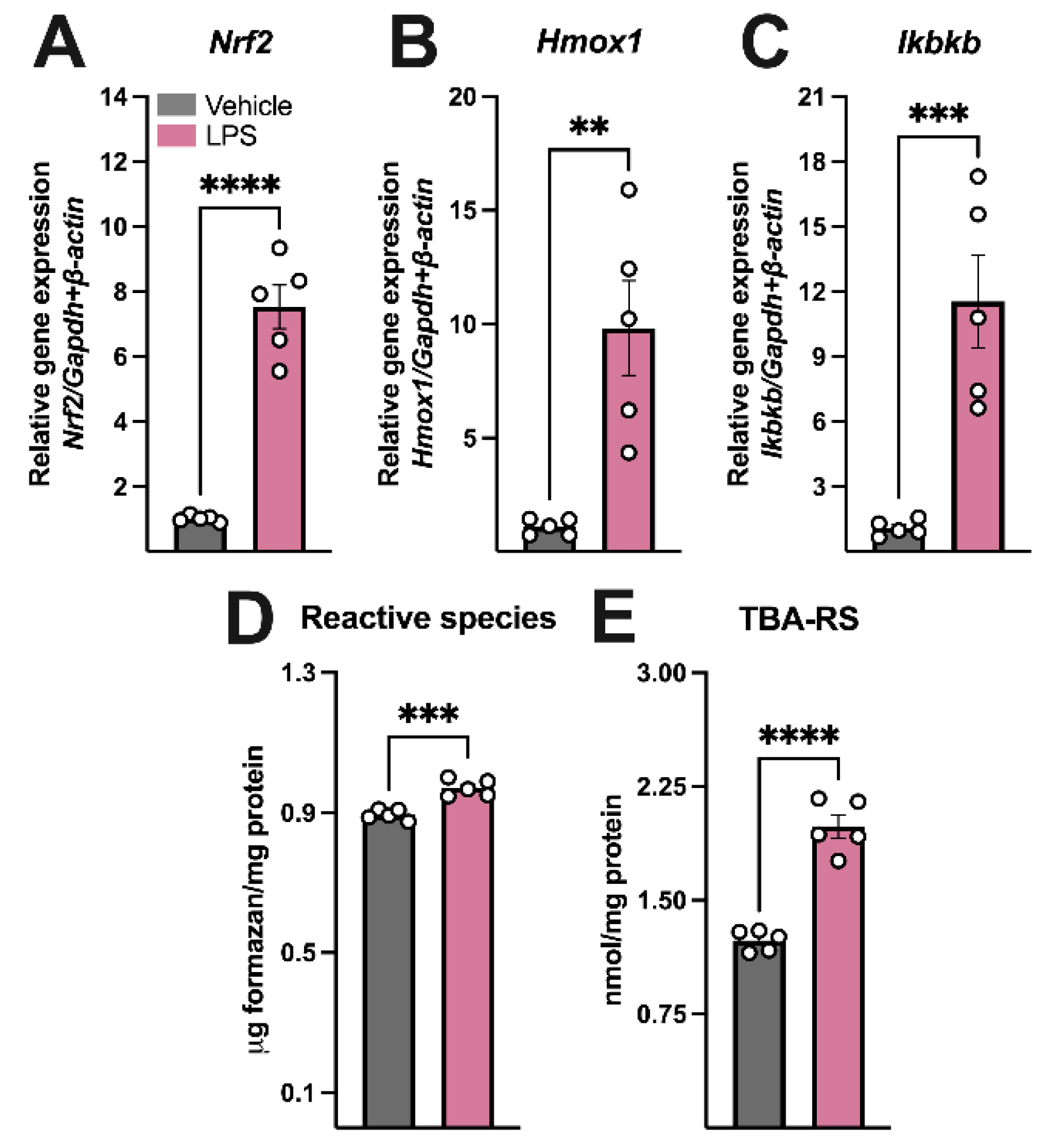
3.1. LPS Administration Induced Sickness Behavior, Hippocampal Inflammation, and Oxidative Stress, Increased BH4 Synthesis, and Disrupted Dopamine Turnover
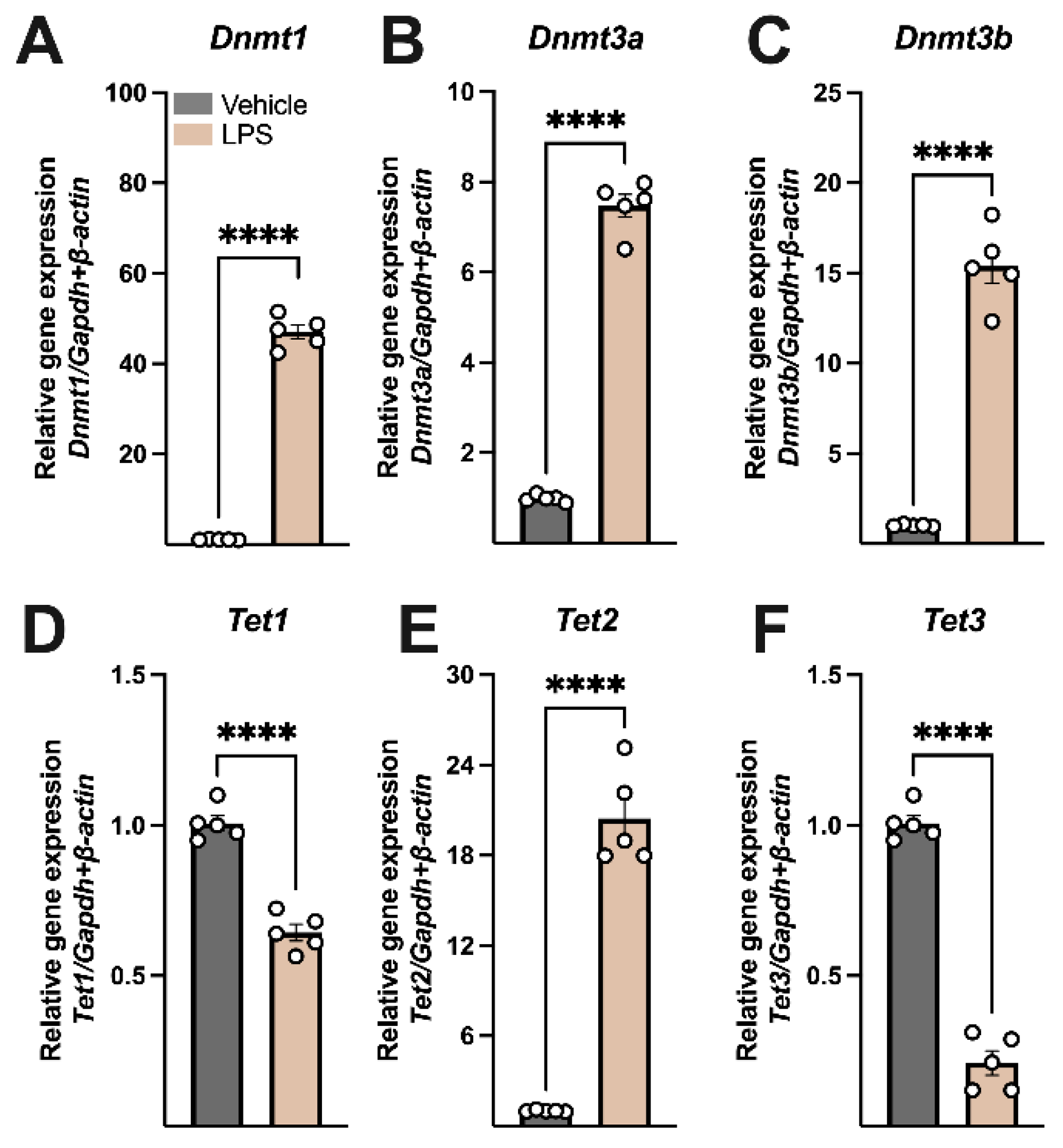
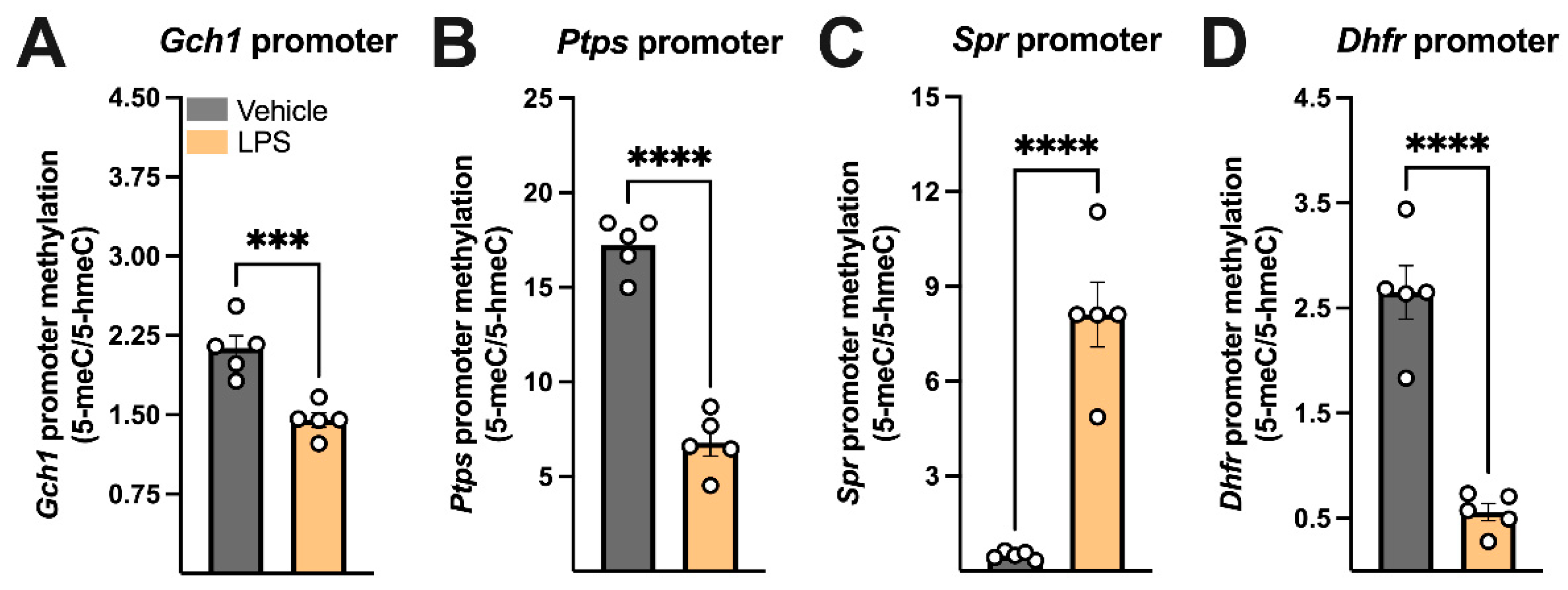
3.2. LPS Modulated the Expression of Key Regulators of DNA Methylation
4. Discussion
5. Conclusions, Limitations, and Further Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eichwald, T.; da Silva, L.d.B.; Staats Pires, A.C.; Niero, L.; Schnorrenberger, E.; Filho, C.C.; Espíndola, G.; Huang, W.L.; Guillemin, G.J.; Abdenur, J.E.; et al. Tetrahydrobiopterin: Beyond Its Traditional Role as a Cofactor. Antioxidants 2023, 12, 1037. [Google Scholar] [CrossRef] [PubMed]
- Werner, E.R.; Blau, N.; Thöny, B. Tetrahydrobiopterin: Biochemistry and Pathophysiology. Biochem. J. 2011, 438, 397–414. [Google Scholar] [CrossRef]
- Werner, E.R.; Werner-Felmayer, G.; Fuchs, D.; Hausen, A.; Reibnegger, G.; Yim, J.J.; Pfleiderer, W.; Wachter, H. Tetrahydrobiopterin biosynthetic activities in human macrophages, fibroblasts, THP-1, and T 24 cells. GTP-cyclohydrolase I is stimulated by interferon-gamma, and 6-pyruvoyl tetrahydropterin synthase and sepiapterin reductase are constitutively present. J. Biol. Chem. 1990, 265, 3189–3192. Available online: http://www.ncbi.nlm.nih.gov/pubmed/2154472 (accessed on 28 June 2025). [CrossRef]
- Fuchs, D.; Spira, T.J.; Hausen, A.; Reibnegger, G.; Werner, E.R.; Felmayer, G.W.; Wachter, H. Neopterin as a Predictive Marker for Disease Progression in Human Immunodeficiency Virus Type 1 Infection. Clin. Chem. 1989, 35, 1746–1749. [Google Scholar] [CrossRef] [PubMed]
- Wirleitner, B.; Reider, D.; Ebner, S.; Böck, G.; Widner, B.; Jaeger, M.; Schennach, H.; Romani, N.; Fuchs, D. Monocyte-Derived Dendritic Cells Release Neopterin. J. Leukoc. Biol. 2002, 72, 1148–1153. [Google Scholar] [CrossRef]
- Bonafé, L.; Thöny, B.; Penzien, J.M.; Czarnecki, B.; Blau, N. Mutations in the Sepiapterin Reductase Gene Cause a Novel Tetrahydrobiopterin-Dependent Monoamine-Neurotransmitter Deficiency without Hyperphenylalaninemia. Am. J. Human. Genet. 2001, 69, 269–277. [Google Scholar] [CrossRef]
- Tayeh, M.A.; Marletta, M.A. Macrophage Oxidation of L-Arginine to Nitric Oxide, Nitrite, and Nitrate. Tetrahydrobiopterin Is Required as a Cofactor. J. Biol. Chem. 1989, 264, 19654–19658. [Google Scholar] [CrossRef]
- Kaufman, S.; Berlow, S.; Summer, G.K.; Milstien, S.; Schulman, J.D.; Orloff, S.; Spielberg, S.; Pueschel, S. Hyperphenylalaninemia Due to a Deficiency of Biopterin. A Variant Form of Phenylketonuria. N. Engl. J. Med. 1978, 299, 673–679. [Google Scholar] [CrossRef]
- Storm, C.B.; Kaufman, S. The Effect of Variation of Cofactor and Substrate Structure on the Action of Phenylalanine Hydroxylase. Biochem. Biophys. Res. Commun. 1968, 32, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, T.; Weiner, N. Isolation and Characterization of a Tyrosine Hydroxylase Cofactor from Bovine Adrenal Medulla. Mol. Pharmacol. 1971, 7, 569–580. [Google Scholar] [CrossRef]
- Cronin, S.J.F.F.; Seehus, C.; Weidinger, A.; Talbot, S.; Reissig, S.; Seifert, M.; Pierson, Y.; McNeill, E.; Longhi, M.S.; Turnes, B.L.; et al. The Metabolite BH4 Controls T Cell Proliferation in Autoimmunity and Cancer. Nature 2018, 563, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Tost, J. DNA Methylation: An Introduction to the Biology and the Disease-Associated Changes of a Promising Biomarker. Mol. Biotechnol. 2010, 44, 71–81. [Google Scholar] [CrossRef]
- Sawan, C.; Vaissiere, T.; Murr, R.; Herceg, Z. Epigenetic Drivers and Genetic Passengers on the Road to Cancer. Mutat. Res. 2008, 642, 1–13. [Google Scholar] [CrossRef]
- Loenarz, C.; Schofield, C.J. Oxygenase Catalyzed 5-Methylcytosine Hydroxylation. Chem. Biol. 2009, 16, 580–583. [Google Scholar] [CrossRef]
- Chestnut, B.A.; Chang, Q.; Price, A.; Lesuisse, C.; Wong, M.; Martin, L.J. Epigenetic Regulation of Motor Neuron Cell Death through DNA Methylation. J. Neurosci. 2011, 31, 16619–16636. [Google Scholar] [CrossRef]
- Su, X.; Chu, Y.; Kordower, J.H.; Li, B.; Cao, H.; Huang, L.; Nishida, M.; Song, L.; Wang, D.; Federoff, H.J. PGC−1α Promoter Methylation in Parkinson’s Disease. PLoS ONE 2015, 10, e0134087. [Google Scholar] [CrossRef]
- Delgado-Morales, R.; Agís-Balboa, R.C.; Esteller, M.; Berdasco, M. Epigenetic Mechanisms during Ageing and Neurogenesis as Novel Therapeutic Avenues in Human Brain Disorders. Clin Epigenet. 2017, 9, 67. [Google Scholar] [CrossRef]
- Pieper, H.C.; Evert, B.O.; Kaut, O.; Riederer, P.F.; Waha, A.; Wüllner, U. Different Methylation of the TNF-Alpha Promoter in Cortex and Substantia Nigra: Implications for Selective Neuronal Vulnerability. Neurobiol. Dis. 2008, 32, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Kaut, O.; Ramirez, A.; Pieper, H.; Schmitt, I.; Jessen, F.; Wüllner, U. DNA Methylation of the TNF-α Promoter Region in Peripheral Blood Monocytes and the Cortex of Human Alzheimer’s Disease Patients. Dement. Geriatr. Cogn. Disord. 2014, 38, 10–15. [Google Scholar] [CrossRef]
- Nicolia, V.; Cavallaro, R.A.; Lopez-Gonzalez, I.; Maccarrone, M.; Scarpa, S.; Ferrer, I.; Fuso, A. DNA Methylation Profiles of Selected Pro-Inflammatory Cytokines in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2017, 76, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Ghisoni, K.; de Paula Martins, R.; Barbeito, L.; Latini, A. Neopterin as a Potential Cytoprotective Brain Molecule. J. Psychiatr. Res. 2015, 71, 134–139. [Google Scholar] [CrossRef]
- de Paula Martins, R.; Ghisoni, K.; Lim, C.K.; Aguiar, A.S.; Guillemin, G.J.; Latini, A. Neopterin Preconditioning Prevents Inflammasome Activation in Mammalian Astrocytes. Free Radic. Biol. Med. 2018, 115, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.S.; Moreira, E.L.G.; Hoeller, A.A.; Oliveira, P.A.; Córdova, F.M.; Glaser, V.; Walz, R.; Cunha, R.A.; Leal, R.B.; Latini, A.; et al. Exercise Attenuates Levodopa-Induced Dyskinesia in 6-Hydroxydopamine-Lesioned Mice. Neuroscience 2013, 243, 46–53. [Google Scholar] [CrossRef]
- Scheffer, D.d.L.; Ghisoni, K.; Aguiar, A.S., Jr.; Latini, A. Moderate Running Exercise Prevents Excessive Immune System Activation. Physiol. Behav. 2019, 204, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, D.d.L.; Freitas, F.C.; Aguiar, A.S., Jr.; Ward, C.; Guglielmo, L.G.A.; Prediger, R.D.; Cronin, S.J.F.; Walz, R.; Andrews, N.A.; Latini, A. Impaired Dopamine Metabolism Is Linked to Fatigability in Mice and Fatigue in Parkinson’s Disease Patients. Brain Commun. 2021, 3, fcab116. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Kim, J.W.; Cha, Y.-N.; Kim, C. A Quantitative Nitroblue Tetrazolium Assay for Determining Intracellular Superoxide Anion Production in Phagocytic Cells. J. Immunoass. Immunochem. 2006, 27, 31–44. [Google Scholar] [CrossRef]
- Glaser, V.; Moritz, B.; Schmitz, A.; Dafré, A.L.; Nazari, E.M.; Rauh Müller, Y.M.; Feksa, L.; Straliottoa, M.R.; De Bem, A.F.; Farina, M.; et al. Protective Effects of Diphenyl Diselenide in a Mouse Model of Brain Toxicity. Chem. Biol. Interact. 2013, 206, 18–26. [Google Scholar] [CrossRef]
- Griess, J.P.; Bemerkungen, Z.A.H.H. Ueber Einige Azoverbindungen. Ber. Deutch Chem. Ges. 1879, 12, 426–428. [Google Scholar] [CrossRef]
- Remor, A.P.; da Silva, R.A.; de Matos, F.J.; Glaser, V.; de Paula Martins, R.; Ghisoni, K.; da Luz Scheffer, D.; Andia, D.C.; Portinho, D.; de Souza, A.P.; et al. Chronic Metabolic Derangement-Induced Cognitive Deficits and Neurotoxicity Are Associated with REST Inactivation. Mol. Neurobiol. 2019, 56, 1539–1557. [Google Scholar] [CrossRef]
- Eichwald, T.; Solano, A.F.; Souza, J.; de Miranda, T.B.; Carvalho, L.B.; dos Santos Sanna, P.L.; da Silva, R.A.F.; Latini, A. Anti-Inflammatory Effect of Caffeine on Muscle under Lipopolysaccharide-Induced Inflammation. Antioxidants 2023, 12, 554. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bertogliat, M.J.; Morris-Blanco, K.C.; Vemuganti, R. Epigenetic Mechanisms of Neurodegenerative Diseases and Acute Brain Injury. Neurochem. Int. 2020, 133, 104642. [Google Scholar] [CrossRef] [PubMed]
- Pogribny, I.P.; Beland, F.A. DNA Hypomethylation in the Origin and Pathogenesis of Human Diseases. Cell. Mol. Life Sci. 2009, 66, 2249–2261. [Google Scholar] [CrossRef]
- Tulloch, J.; Leong, L.; Thomson, Z.; Chen, S.; Lee, E.G.; Keene, C.D.; Millard, S.P.; Yu, C.E. Glia-Specific APOE Epigenetic Changes in the Alzheimer’s Disease Brain. Brain Res. 2018, 1698, 179–186. [Google Scholar] [CrossRef]
- Velmeshev, D.; Magistri, M.; Mazza, E.M.C.; Lally, P.; Khoury, N.; D’Elia, E.R.; Bicciato, S.; Faghihi, M.A. Cell-Type-Specific Analysis of Molecular Pathology in Autism Identifies Common Genes and Pathways Affected Across Neocortical Regions. Mol. Neurobiol. 2020, 57, 2279–2289. [Google Scholar] [CrossRef]
- Hernandez, D.G.; Nalls, M.A.; Gibbs, J.R.; Arepalli, S.; van der brug, M.; Chong, S.; Moore, M.; Longo, D.L.; Cookson, M.R.; Traynor, B.J.; et al. Distinct DNA Methylation Changes Highly Correlated with Chronological Age in the Human Brain. Hum. Mol. Genet. 2011, 20, 1164–1172. [Google Scholar] [CrossRef]
- Costa, M.R.; dos Santos, A.Y.I.; de Miranda, T.B.; Aires, R.; de Camargo Coque, A.; Hurtado, E.C.P.; Bernardi, M.M.; Pecorari, V.G.A.; Andia, D.C.; Birbrair, A.; et al. Impact of Neuroinflammation on Epigenetic Transcriptional Control of Sonic Hedgehog Members in the Central Nervous System. Brain Res. 2023, 1799, 148180. [Google Scholar] [CrossRef] [PubMed]
- Zamiri, K.; Kesari, S.; Paul, K.; Hwang, S.H.; Hammock, B.; Kaczor-Urbanowicz, K.E.; Urbanowicz, A.; Gao, L.; Whitelegge, J.; Fiala, M. Therapy of Autoimmune Inflammation in Sporadic Amyotrophic Lateral Sclerosis: Dimethyl Fumarate and H-151 Downregulate Inflammatory Cytokines in the CGAS-STING Pathway. FASEB J. 2023, 37, e23068. [Google Scholar] [CrossRef] [PubMed]
- Adamu, A.; Li, S.; Gao, F.; Xue, G. The Role of Neuroinflammation in Neurodegenerative Diseases: Current Understanding and Future Therapeutic Targets. Front. Aging Neurosci. 2024, 16, 1347987. [Google Scholar] [CrossRef]
- Wu, A.; Zhang, J. Neuroinflammation, Memory, and Depression: New Approaches to Hippocampal Neurogenesis. J. Neuroinflamm. 2023, 20, 283. [Google Scholar] [CrossRef]
- D’Sa, C.; Hirayama, K.; West, A.; Hahn, M.; Min, Z.; Kapatos, G. Tetrahydrobiopterin Biosynthesis in C6 Glioma Cells: Induction of GTP Cyclohydrolase T Gene Expression by Lipopolysaccharide and Cytokine Treatment. Mol. Brain Res. 1996, 41, 105–110. [Google Scholar] [CrossRef]
- Werner, E.R.; Werner-Felmayer, G.; Mayer, B. Tetrahydrobiopterin, Cytokines, and Nitric Oxide Synthesis. Proc. Soc. Exp. Biol. Med. 1998, 219, 171–182. [Google Scholar] [CrossRef]
- Niedbala, W.; Wei, X.Q.; Piedrafita, D.; Xu, D.; Liew, F.Y. Effects of Nitric Oxide on the Induction and Differentiation of Th1 Cells. Eur. J. Immunol. 1999, 29, 2498–2505. [Google Scholar] [CrossRef]
- Latremoliere, A.; Latini, A.; Andrews, N.; Cronin, S.J.; Fujita, M.; Gorska, K.; Hovius, R.; Romero, C.; Chuaiphichai, S.; Painter, M.; et al. Reduction of Neuropathic and Inflammatory Pain through Inhibition of the Tetrahydrobiopterin Pathway. Neuron 2015, 86, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Scheffer, D.d.L.; Turnes, B.L.; Cronin, S.J.F.; Latrémolière, A.; Costigan, M.; Woolf, C.J.; Latini, A.; Andrews, N.A. Sepiapterin Reductase Inhibition Leading to Selective Reduction of Inflammatory Joint Pain in Mice and Increased Urinary Sepiapterin Levels in Humans and Mice. Arthritis Rheumatol. 2020, 72, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Ghisoni, K.; Aguiar, A.S.; de Oliveira, P.A.; Matheus, F.C.; Gabach, L.; Perez, M.; Carlini, V.P.; Barbeito, L.; Mongeau, R.; Lanfumey, L.; et al. Neopterin Acts as an Endogenous Cognitive Enhancer. Brain Behav. Immun. 2016, 56, 156–164. [Google Scholar] [CrossRef]
- Biesmans, S.; Meert, T.F.; Bouwknecht, J.A.; Acton, P.D.; Davoodi, N.; De Haes, P.; Kuijlaars, J.; Langlois, X.; Matthews, L.J.R.; Ver Donck, L.; et al. Systemic Immune Activation Leads to Neuroinflammation and Sickness Behavior in Mice. Mediat. Inflamm. 2013, 2013, 271359. [Google Scholar] [CrossRef]
- Hsiung, S.; Moro, A.; Ban, Y.; Chen, X.; Savio, A.S.; Hernandez, R.; Malek, T.R. Acute Lipopolysaccharide-Induced Inflammation Lowers IL-2R Signaling and the Proliferative Potential of Regulatory T Cells. Immunohorizons 2020, 4, 809–824. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, B.; Zhu, B.; Xiong, B.; Lu, P.; Ai, L.; Yang, N.; Zhao, Y.; Xu, H. Nitric Oxide in the Spinal Cord Is Involved in the Hyperalgesia Induced by Tetrahydrobiopterin in Chronic Restraint Stress Rats. Front. Neurosci. 2021, 15, 593654. [Google Scholar] [CrossRef] [PubMed]
- Thony, B.; Auerbach, G.; Blau, N. Tetrahydrobiopterin Biosynthesis, Regeneration and Functions. Biochem. J. 2000, 347, 1–16. [Google Scholar] [CrossRef]
- Ichinose, H.; Inoue, K.; Arakawa, S.; Watanabe, Y.; Kurosaki, H.; Koshiba, S.; Hustad, E.; Takada, M.; Aasly, J.O. Alterations in the Reduced Pteridine Contents in the Cerebrospinal Fluids of LRRK2 Mutation Carriers and Patients with Parkinson’s Disease. J. Neural Transm. 2018, 125, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Jang, Y.J.; Kim, H.J.; Hwang, O. Tetrahydrobiopterin Is Released from and Causes Preferential Death of Catecholaminergic Cells by Oxidative Stress. Mol. Pharmacol. 2000, 58, 633–640. [Google Scholar] [CrossRef]
- Lee, S.Y.; Moon, Y.; Hee Choi, D.; Jin Choi, H.; Hwang, O. Particular Vulnerability of Rat Mesencephalic Dopaminergic Neurons to Tetrahydrobiopterin: Relevance to Parkinson’s Disease. Neurobiol. Dis. 2007, 25, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.-W.; Bang, Y.J.; Kim, K.-M.; Lee, K.Y.; Kang, B.Y.; Kim, E.M.; Inoue, H.; Hwang, O.; Choi, H.J. Role of Cyclooxygenase-2 in Tetrahydrobiopterin-Induced Dopamine Oxidation. Biochem. Biophys. Res. Commun. 2007, 359, 735–741. [Google Scholar] [CrossRef]
- Ni, A.; Ernst, C. Evidence That Substantia Nigra Pars Compacta Dopaminergic Neurons Are Selectively Vulnerable to Oxidative Stress Because They Are Highly Metabolically Active. Front. Cell Neurosci. 2022, 16, 826193. [Google Scholar] [CrossRef]
- Pacelli, C.; Giguère, N.; Bourque, M.-J.; Lévesque, M.; Slack, R.S.; Trudeau, L.-É. Elevated Mitochondrial Bioenergetics and Axonal Arborization Size Are Key Contributors to the Vulnerability of Dopamine Neurons. Curr. Biol. 2015, 25, 2349–2360. [Google Scholar] [CrossRef]
- Hüls, A.; Robins, C.; Conneely, K.N.; Edgar, R.; De Jager, P.L.; Bennett, D.A.; Wingo, A.P.; Epstein, M.P.; Wingo, T.S. Brain DNA Methylation Patterns in CLDN5 Associated with Cognitive Decline. Biol. Psychiatry 2022, 91, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, X.; Zhang, Y.; Qu, C.; Zhou, X.; Zhang, S. Histamine Induces Microglia Activation and the Release of Proinflammatory Mediators in Rat Brain Via H1R or H4R. J. Neuroimmune Pharmacol. 2020, 15, 280–291. [Google Scholar] [CrossRef]


| Gene (ID) | Primer | 5′-3′ Sequence | Reaction Conditions | Product Size (bp) |
|---|---|---|---|---|
| Nrf2 (18024) | Forward | ATG GAG CAA GTT TGG CAG GA | 95 °C-15 s; 61 °C-30 s; 72 °C-30 s | 456 |
| Reverse | GCT GGG AAC AGC GGT AGT AT | |||
| Nlrp3 (216799) | Forward | ATT ACC CGC CCG AGA AAG G | 95 °C-15 s; 58 °C-30 s; 72 °C-30 s | 141 |
| Reverse | TCG CAG CAA AGA TCC ACA CAG | |||
| Asc1 (104385) | Forward | AGA CAT GGG CTT ACA GGA | 95 °C-15 s; 60 °C-30 s; 72 °C-30 s | 256 |
| Reverse | CTC CCT CAT CTT GTC TTG G | |||
| Caspase 1 (12362) | Forward | TGA AAG AGG TGA AAG AAT T | 95 °C-15 s; 59 °C-30 s; 72 °C-30 s | 386 |
| Reverse | TCT CCA AGA CAC ATT ATC T | |||
| Il1b (16176) | Forward | GAC CTT GGA TGA GGA CA | 95 °C-15 s; 60 °C-30 s; 72 °C-30 s | 183 |
| Reverse | AGC TCA TAT GGG TCC GAC AG | |||
| Il10 (16153) | Forward | CCA AGC CCT TAT CGG AAA TGA | 95 °C-15 s; 60 °C-30 s; 72 °C-30 s | 163 |
| Reverse | TTT TCA CAG GGG AGA AAT CG | |||
| Il18 (16173) | Forward | ACT TTG GCC GAC TTC ACT GT | 95 °C-15 s; 60 °C-30 s; 72 °C-30 s | 125 |
| Reverse | GGG TTC ACT GGC ACT TTG AT | |||
| Hmox1 (15368) | Forward | CACGCATATACCCGCTACCT | 95 °C-15 s; 61 °C-30 s; 72 °C-30 s | 175 |
| Reverse | CCAGAGTGTTCATTCGAGCA | |||
| Ikbkb (16150) | Forward | TCACTTGTCTCCTGGTGCTG | 95 °C-15 s; 61 °C-30 s; 72 °C-30 s | 234 |
| Reverse | TGTGCAGCAGATTTCCTTTG | |||
| Dnmt1 (13433) | Forward | CCT TTG TGG GAA CCT GGA A | 95 °C-15 s; 63 °C-30 s; 72 °C-30 s | 240 |
| Reverse | CTG TCG TCT GCG GTG ATT | |||
| Dnmt3A (13435) | Forward | GAG GGA ACT GAG ACC CCA C | 95 °C-15 s; 63 °C-30 s; 72 °C-30 s | 216 |
| Reverse | CTG GAA GGT GAG TCT TGG CA | |||
| Dnmt3B (13436) | Forward | AGC GGG TAT GAG GAG TGC AT | 95 °C-15 s; 63 °C-30 s; 72 °C-30 s | 91 |
| Reverse | GGG AGC ATC CTT CGT GTC TG | |||
| Tet1 (52463) | Forward | GAG CCT GTT CCT CGA TGT GG | 95 °C-15 s; 65 °C-30 s; 72 °C-30 s | 367 |
| Reverse | CAA ACC CAC CTG AGG CTG TT | |||
| Tet2 (214133) | Forward | AAC CTG GCT ACT GTC ATT GCT CCA | 95 °C-15 s; 65 °C-30 s; 72 °C-30 s | 211 |
| Reverse | ATG TTC TGC TGG TCT CTG TGG GAA | |||
| Tet3 (194388) | Forward | GTC TCC CCA AGT CCT ACC TCC G | 95 °C-15 s; 63 °C-30 s; 72 °C-30 s | 137 |
| Reverse | GTC AGT GCC CCA CGC TTC A | |||
| Gch1 (14528) | Forward | TGT TGG TGT GTG CCT TGT CT | 95 °C-15 s; 60 °C-30 s; 72 °C-30 s | 191 |
| Reverse | TGT CAG CCA GAG GGA GAA CT | |||
| Ptps (19286) | Forward | CTG CAT GTG GTT TTG GTG AG | 95 °C-15 s; 60 °C-30 s; 72 °C-30 s | 178 |
| Reverse | TTA ACG GCT GAG CCA TCT CT | |||
| Spr (20751) | Forward | TAG AGG TGG GAA GAG CAG GA | 95 °C-15 s; 59 °C-30 s; 72 °C-30 s | 190 |
| Reverse | CCA GTG AAC ACC ACA GGA TG | |||
| Dhfr (13361) | Forward | GAG GTC CAG GAG GAA AAA GG | 95 °C-15 s; 60 °C-30 s; 72 °C-30 s | 209 |
| Reverse | ATC CCC AGG ATC ACA AAA CA | |||
| b-Actin (11461) | Forward | TCT TGG GTA TGG AAT CCT GTG | 95 °C-15 s; 58 °C-30 s; 72 °C-30 s | 82 |
| Reverse | AGG TCT TTA CGG ATG TCA ACG | |||
| Gapdh (14433) | Forward | AGG CCG GTG CTG AGT ATG TC | 95 °C-15 s; 58 °C-30 s; 72 °C-30 s | 530 |
| Reverse | TGC CTG CTT CAC CAC CTT CT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, J.; da Luz Scheffer, D.; Solano, A.F.; Veloso, S.; Cruz, L.; Foganholi-Silva, R.; Latini, A. Resolution of Lipopolysaccharide-Induced Inflammation Followed by DNA Hypomethylation and Increased Tetrahydrobiopterin Biosynthesis in Mouse Hippocampus. Brain Sci. 2025, 15, 880. https://doi.org/10.3390/brainsci15080880
Souza J, da Luz Scheffer D, Solano AF, Veloso S, Cruz L, Foganholi-Silva R, Latini A. Resolution of Lipopolysaccharide-Induced Inflammation Followed by DNA Hypomethylation and Increased Tetrahydrobiopterin Biosynthesis in Mouse Hippocampus. Brain Sciences. 2025; 15(8):880. https://doi.org/10.3390/brainsci15080880
Chicago/Turabian StyleSouza, Jennyffer, Debora da Luz Scheffer, Alexandre Francisco Solano, Samantha Veloso, Luisa Cruz, Rodrigo Foganholi-Silva, and Alexandra Latini. 2025. "Resolution of Lipopolysaccharide-Induced Inflammation Followed by DNA Hypomethylation and Increased Tetrahydrobiopterin Biosynthesis in Mouse Hippocampus" Brain Sciences 15, no. 8: 880. https://doi.org/10.3390/brainsci15080880
APA StyleSouza, J., da Luz Scheffer, D., Solano, A. F., Veloso, S., Cruz, L., Foganholi-Silva, R., & Latini, A. (2025). Resolution of Lipopolysaccharide-Induced Inflammation Followed by DNA Hypomethylation and Increased Tetrahydrobiopterin Biosynthesis in Mouse Hippocampus. Brain Sciences, 15(8), 880. https://doi.org/10.3390/brainsci15080880






