Clomipramine Induced Oxidative Stress and Morphological Alterations in the Prefrontal Cortex and Limbic System of Neonatal Rats
Abstract
1. Introduction
2. Materials and Methods
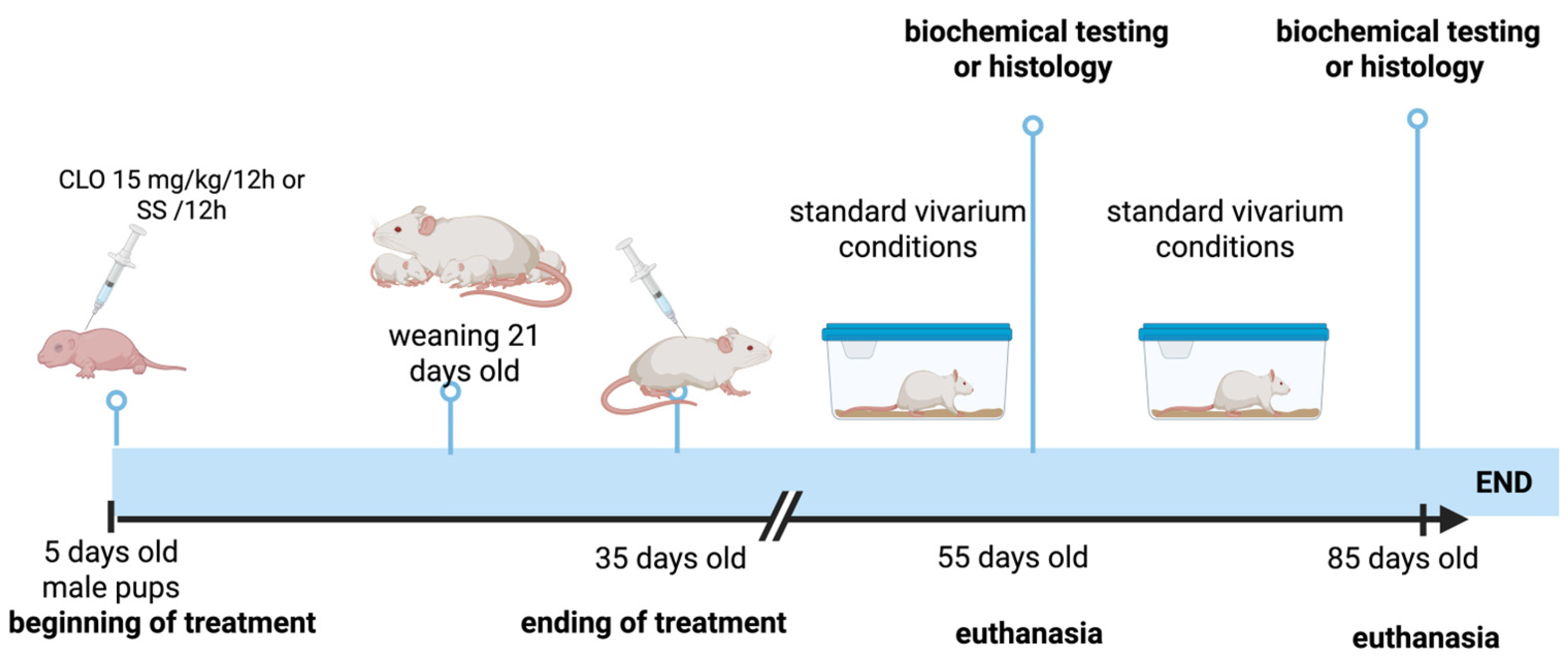
2.1. Animals and Treatment
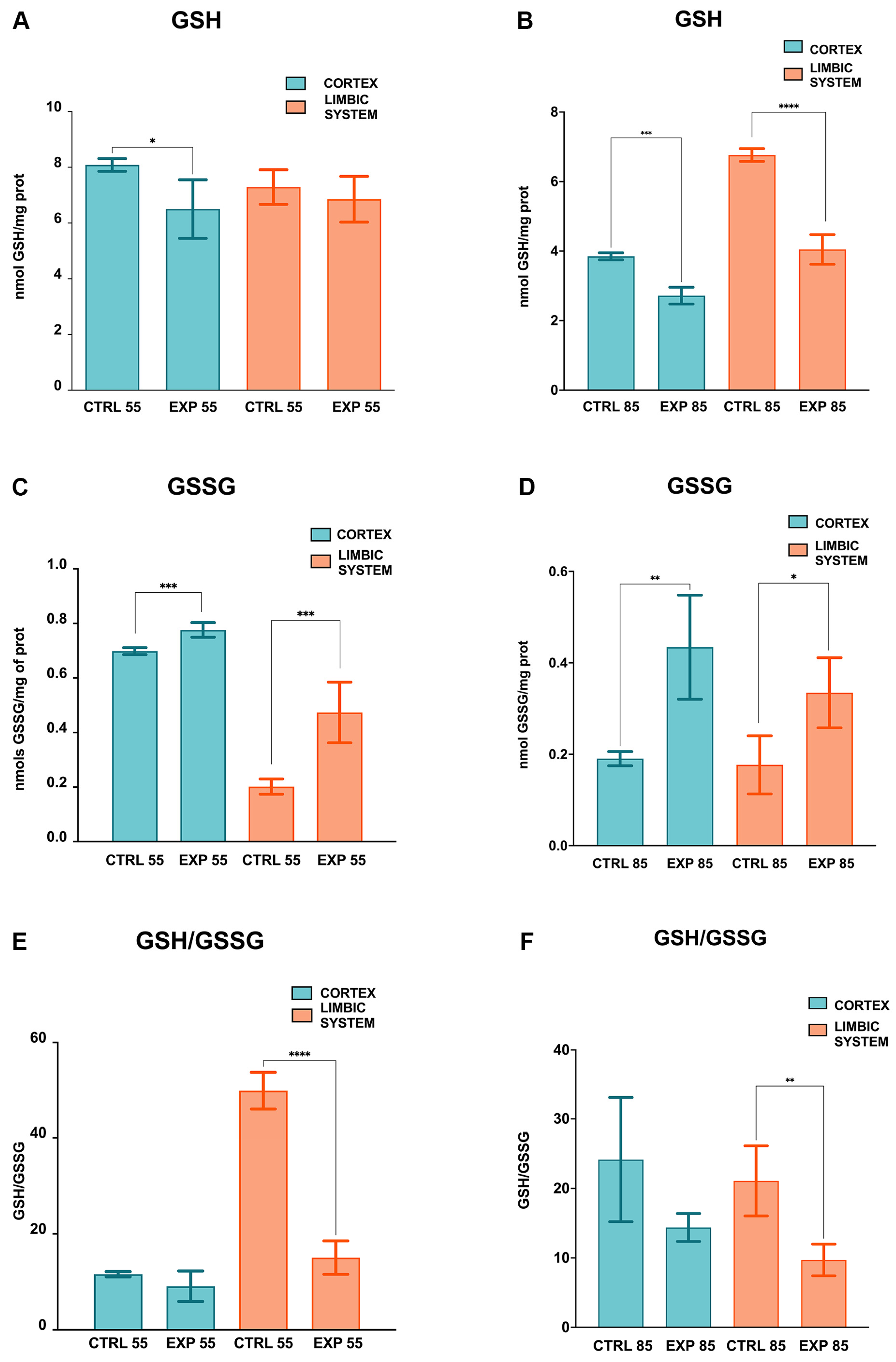
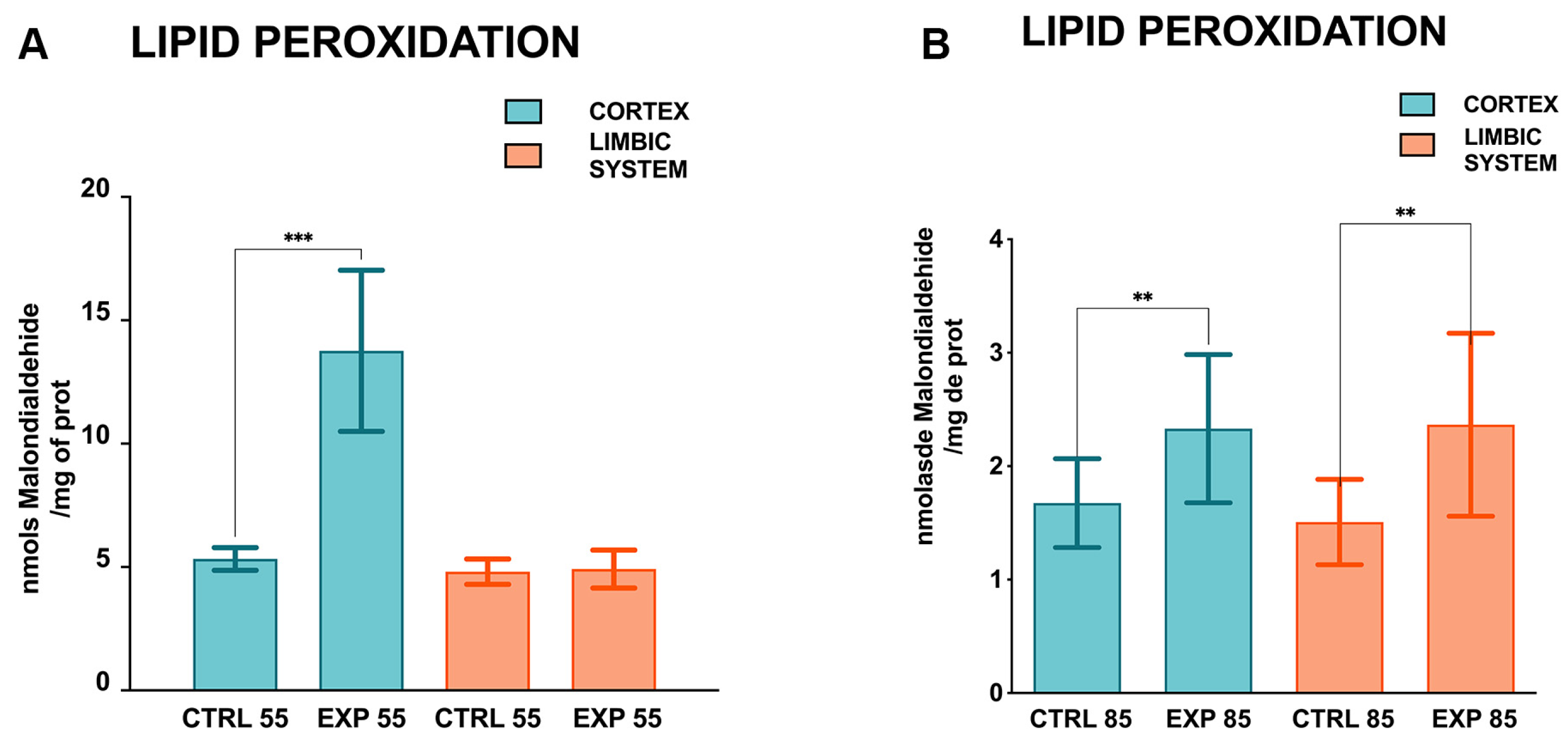
2.2. Biochemical Determinations
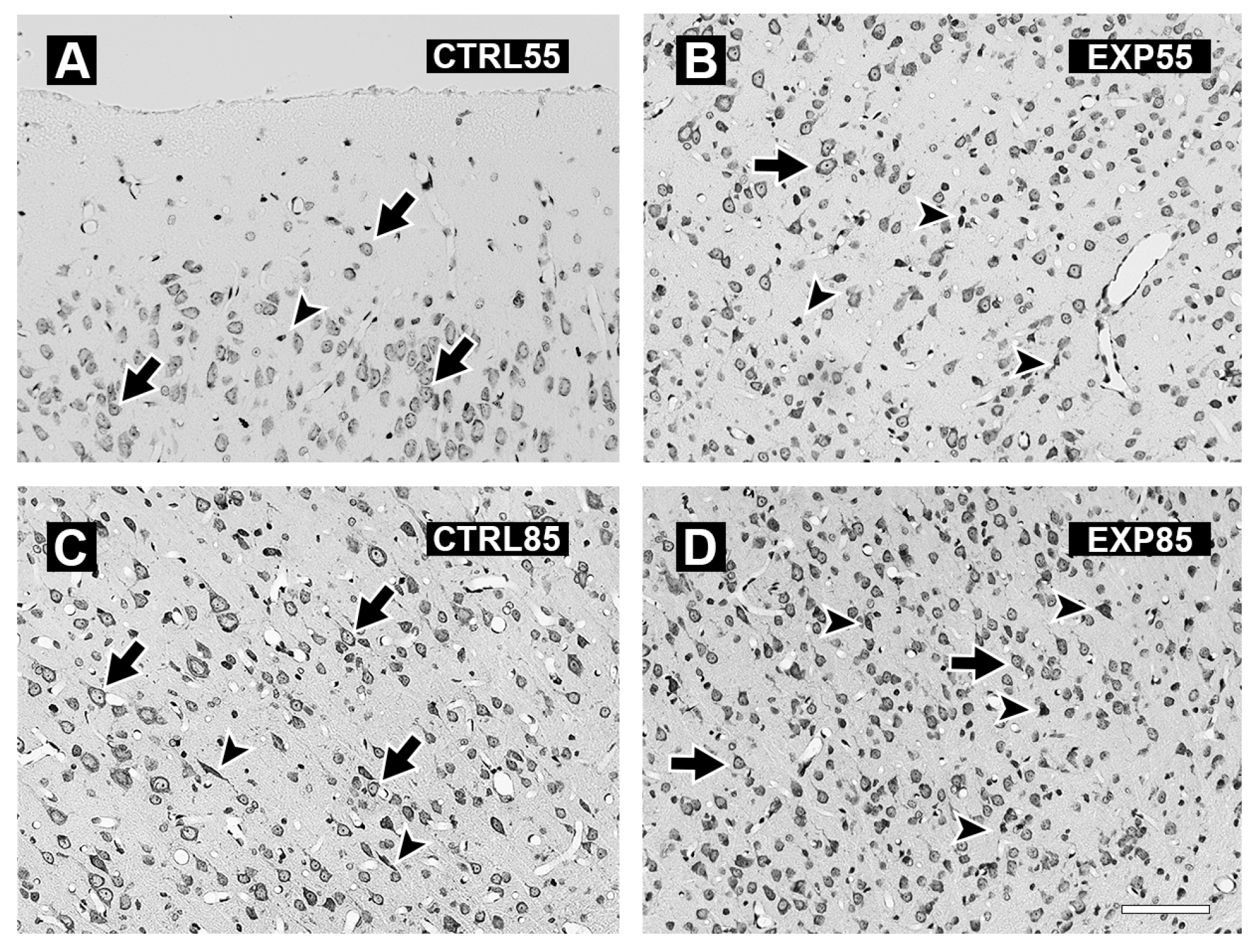
2.3. Histology
2.4. Statistical Analysis
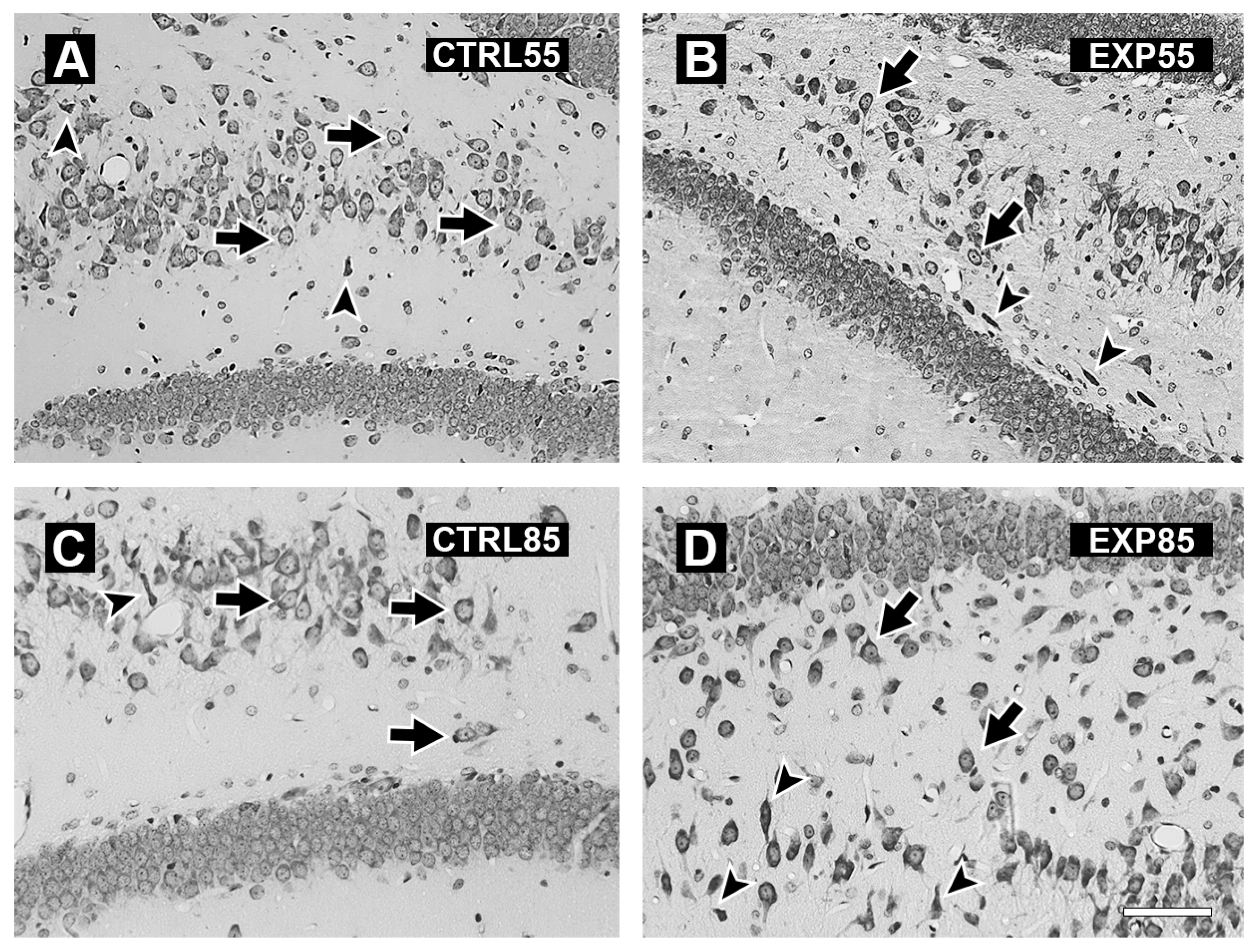
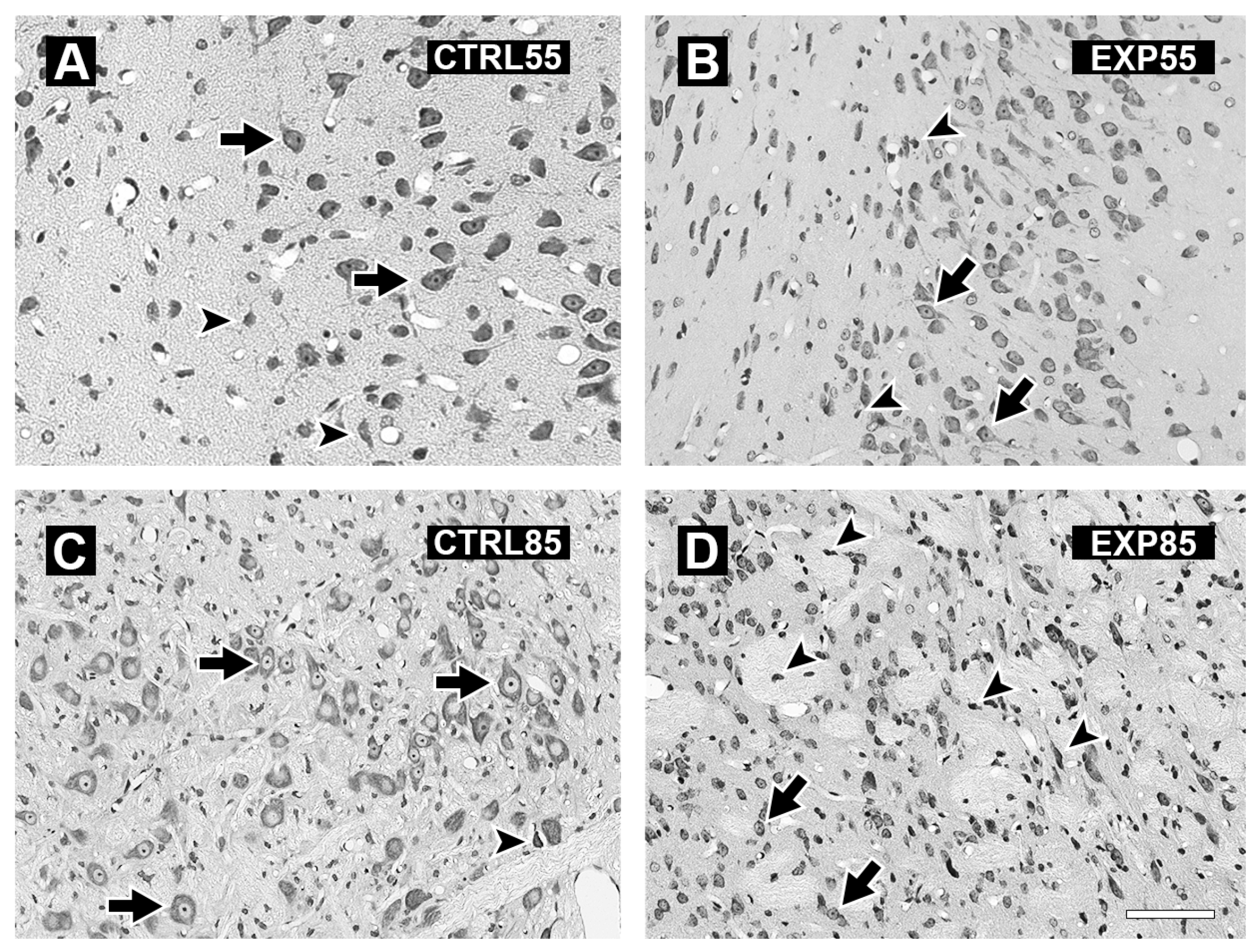
3. Results
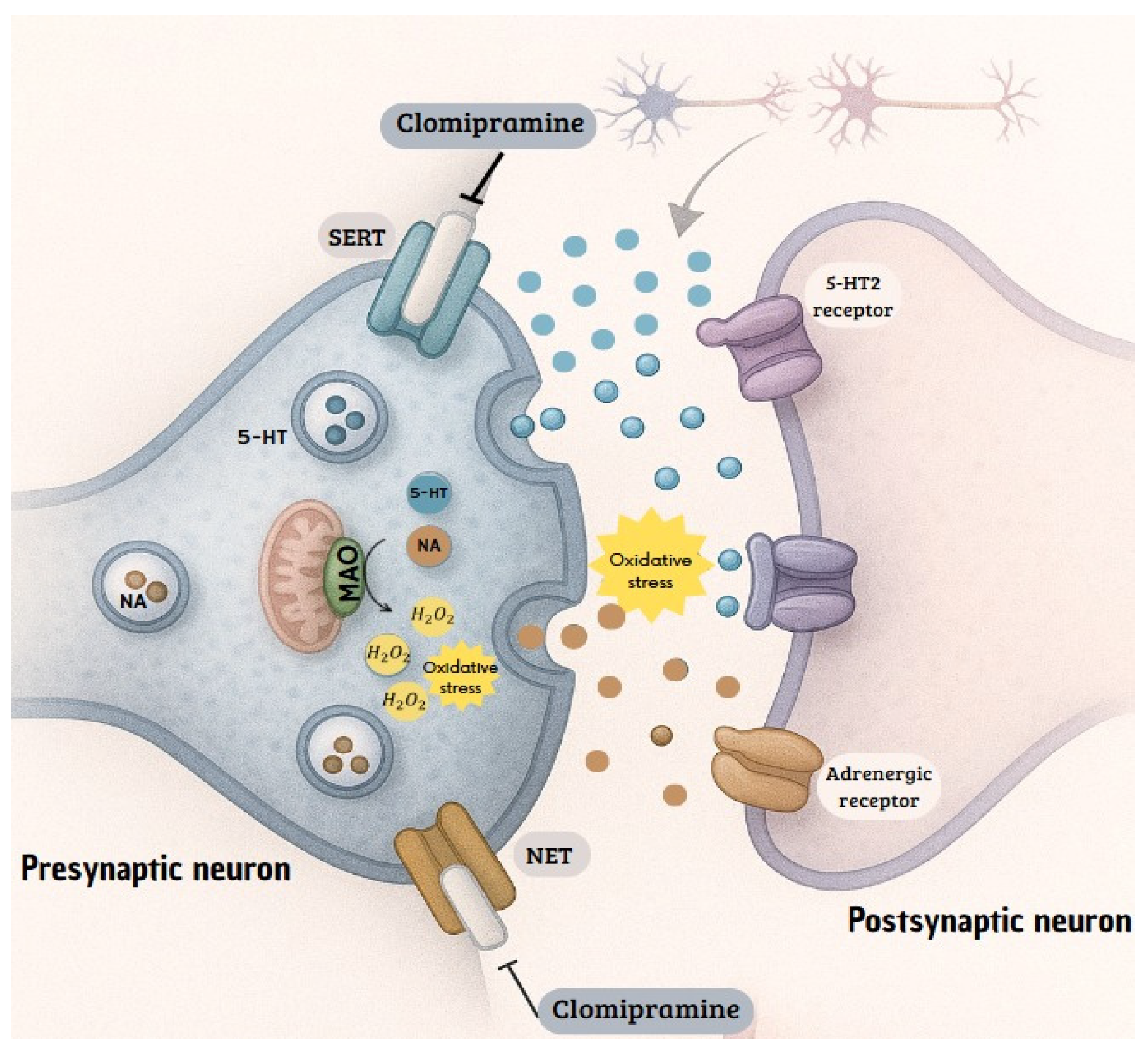
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAT | Catalase |
| CLO | Clomipramine |
| CTRL | Control |
| EXP | Experimental |
| GPx | Glutathione peroxidase |
| GSH | Reduced glutathione |
| GSSG | Oxidized glutathione |
| H2O2 | Hydrogen peroxide |
| HO• | Hydroxyl radical |
| INP | Instituto Nacional de Pediatría |
| LDE | Lipid-derived electrophiles |
| LIMB | Limbic system |
| NET | Noradrenaline transporter |
| MAO | Monoamine oxidase |
| MDA | Malondialdehyde |
| OCD | Obsessive–compulsive disorder |
| OPA | O-phthalaldehyde |
| OS | Oxidative stress |
| PCX | Prefrontal cortex |
| ROS | Reactive oxygen species |
| SD | Standard deviation |
| SERT | Serotonin transporter |
| SOD | Superoxide dismutase |
| TBARS | Thiobarbituric acid |
References
- Drugbank. Available online: https://go.drugbank.com/drugs/DB01242 (accessed on 8 August 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 2801, Clomipramine. 12 March 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Clomipramine (accessed on 8 August 2025).
- Wilson, M.; Tripp, J. Clomipramine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541006 (accessed on 8 August 2025).
- Hollander, E.; Kaplan, A.; Allen, A.; Cartwright, C. Pharmacotherapy for obsessive-compulsive disorder. Psychiatr. Clin. N. Am. 2000, 23, 643–656. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; Clomipramine; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548878/ (accessed on 8 August 2025).
- Hansen, H.H.; Mikkelsen, J.D. Long-term effects on serotonin transporter mRNA expression of chronic neonatal exposure to a serotonin reuptake inhibitor. Eur. J. Pharmacol. 1998, 352, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Cassano, P.; Argibay, P. La enfermedad depresiva y sus modelos animales. Rev. Hosp. Ital. B. Aires 2009, 29, 117–120. [Google Scholar]
- Neil, D.; Vogel, G.; Hagler, M.; Kors, D.; Hennessey, A. Diminished sexual activity in a new animal model of endogenous depression. Neurosci. Biobehav. Rev. 1990, 14, 73–76. [Google Scholar] [CrossRef]
- Vogel, G.; Hartely, P.; Neill, D.; Hagler, M.; Kors, D. Animal depression model by neonatal clomipramine: Reduction of shock induced aggression. Pharmacol. Biochem. Behav. 1988, 31, 103–106. [Google Scholar] [CrossRef]
- Vogel, G.; Neill, D.; Hagler, M.; Kors, D. A new animal model of endogenous depression: A summary of present findings. Neurosci. Biobehav. Rev. 1990, 14, 85–91. [Google Scholar] [CrossRef]
- Velazquez-Moctezuma, J.; Ruiz, O.D. Neonatal treatment with clomipramine increased immobility in forced swim test: An attribute of animal models of depression. Pharmacol. Biochem. Behav. 1992, 42, 737–739. [Google Scholar] [CrossRef]
- Vogel, G.; Neill, D.; Kors, D.; Hagler, M. REM sleep abnormalities in a new animal model of endogenous depression. Neurosci. Biobehav. Rev. 1990, 14, 77–83. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Meti, B.L. Alterations in the levels of monoamines in discrete brain regions of clomipramine-induced animal model of endogenous depression. Neurochem. Res. 1999, 24, 345–349. [Google Scholar] [CrossRef]
- Thanacoody, H.K.; Thomas, S.H. Tricyclic antidepressant poisoning: Cardiovascular toxicity. Toxicol Rev. 2005, 24, 205–214. [Google Scholar] [CrossRef]
- El-Demerdash, E.; Mohamadin, A.M. Does oxidative stress contribute in tricyclic antidepressants-induced cardiotoxicity? Toxicol. Lett. 2004, 152, 159–166. [Google Scholar] [CrossRef]
- Bizerra, P.F.V.; Itou da Silva, F.S.; Gilglioni, E.H.; Nanami, L.F.; Klosowski, E.M.; de Souza, B.T.L.; Raimundo, A.F.G.; Dos Santos, K.B.P.; Mewes, J.M.; Constantin, R.P.; et al. The harmful acute effects of clomipramine in the rat liver: Impairments in mitochondrial bioenergetics. Toxicol. Lett. 2023, 383, 1–16. [Google Scholar] [CrossRef]
- Xia, Z.; Lundgren, B.; Bergstrand, A.; DePierre, J.W.; Nässberger, L. Changes in the generation of reactive oxygen species and in mitochondrial membrane potential during apoptosis induced by the antidepressants imipramine, clomipramine, and citalopram and the effects on these changes by Bcl-2 and Bcl-X(L). Biochem. Pharmacol. 1999, 57, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Daley, E.; Wilkie, D.; Loesch, A.; Hargreaves, I.P.; Kendall, D.A.; Pilkington, G.J.; Bates, T.E. Chlorimipramine: A novel anticancer agent with a mitochondrial target. Biochem. Biophys. Res. Commun. 2005, 328, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Higgins, S.C.; Pilkington, G.J. The in vitro effects of tricyclic drugs and dexamethasone on cellular respiration of malignant glioma. Anticancer Res. 2010, 30, 391–397. [Google Scholar] [PubMed]
- Balk, R.D.S.; Bridi, J.C.; de Lima Portella, R.; Carvalho, N.R.; Dobrachinski, F.; da Silva, M.H.; Amaral, G.P.; Dias, G.R.; de Vargas Barbosa, N.; Soares, F.A. Clomipramine treatment and repeated restraint stress alter parameters of oxidative stress in brain regions of male rats. Neurochem. Res. 2010, 35, 1761–1770. [Google Scholar] [CrossRef]
- Jimenez, A.G.; Winward, J.D.; Smith, D.M.; Ragan, C.M. Effects of short-term clomipramine on anxiety-like behavior, cellular metabolism, and oxidative stress in primary fibroblast cells of male and female rats. Physiol. Rep. 2018, 6, e13615. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R.A. fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Gutteridge, J.M.; Halliwell, B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem. Sci. 1990, 15, 129–135. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.A.; Eleiwa, N.Z.; Khairy, G.M.; El-Aziz, R.M.A.; Metwally, M.M.M.; Galal, A.A.A. Comparative effect of administration and discontinuation of sildenafil and/or clomipramine on the hepatic, cardiac and testicular tissues of male rats. Andrologia 2021, 53, e13983. [Google Scholar] [CrossRef]
- Othman, H.; Ammari, M.; Lassoued, A.; Sakly, M.; Abdelmelek, H. Zinc improves clomipramine effects on 21depressive and locomotor behavior and reverses its oxidative stress in rats. Behav. Brain Res. 2019, 374, 112122. [Google Scholar] [CrossRef] [PubMed]
- Balant-Gorgia, A.E.; Gex-Fabry, M.; Balant, L.P. Clinical pharmacokinetics of clomipramine. Clin. Pharmacokinet. 1991, 20, 447–462. [Google Scholar] [CrossRef] [PubMed]
- de Vos, A.; van der Weide, J.; Loovers, H.M. Association between CYP2C19*17 and metabolism of amitriptyline, citalopram and clomipramine in Dutch hospitalized patients. Pharmacogenomics J. 2011, 11, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.K.; Flinois, J.P.; Beaune, P.; Brøsen, K. The biotransformation of clomipramine in vitro, identification of the cytochrome P450s responsible for the separate metabolic pathways. J. Pharmacol. Exp. Ther. 1996, 277, 1659–1664. [Google Scholar] [CrossRef]
- Wu, Z.L.; Huang, S.L.; Ou-Yang, D.S.; Xu, Z.H.; Xie, H.G.; Zhou, H.H. Clomipramine N-demethylation metabolism in human liver microsomes. Zhongguo Yao Li Xue Bao 1998, 19, 433–436. [Google Scholar]
- Kobuchi, S.; Fukushima, K.; Shibata, M.; Ito, Y.; Sugioka, N.; Takada, K. Phar-macokinetics of clomipramine, an antidepressant, in poloxamer 407-induced hyperlipidaemic model rats. J. Pharm. Pharmacol. 2011, 63, 515–523. [Google Scholar] [CrossRef]
- Kobuchi, S.; Fukushima, K.; Aoyama, H.; Matsuda, T.; Ito, Y.; Sugioka, N.; Takada, K. Effect of oxidative stress on the pharmacokinetics of clomipramine in rats treated with ferric-nitrilotriacetate. Drug Metab. Lett. 2011, 5, 243–252. [Google Scholar] [CrossRef]
- Kurata, K.; Kishitani, K.; Kido, H.; Kurachi, M.; Yamaguchi, N. A pharmacokinetic study of clomipramine in regions of the brain. Jpn J. Psychiatry Neurol. 1986, 40, 631–638. [Google Scholar] [CrossRef]
- Aitchison, K.; Datla, K.; Rooprai, H.; Fernando, J.; Dexter, D. Regional distribution of clomipramine and desmethylclomipramine in rat brain and peripheral organs on chronic clomipramine administration. J. Psychopharmacol. 2010, 24, 1261–1268. [Google Scholar] [CrossRef]
- Detcheverry, F.; Senthil, S.; Narayanan, S.; Badhwar, A. Changes in levels of the antioxidant glutathione in brain and blood across the age span of healthy adults: A systematic review. Neuroimage Clin. 2023, 40, 103503. [Google Scholar] [CrossRef]
- Nanda, D.; Tolputt, J.; Collard, K.J. Changes in brain glutathione levels during postnatal development in the rat. Brain Res. Dev. Brain Res. 1996, 94, 238–241. [Google Scholar] [CrossRef]
- Anand, P.; Nehru, B. Alterations in glutathione system in adult and pup rat brains following chronic aluminum exposure. Indian J. Occup. Environ. Med. 2006, 10, 128–132. [Google Scholar] [CrossRef]
- El-Fiky, S.A.; Abou-Zaid, F.A.; Farag, I.M.; Fahmy, M.A.; El-Fiky, N.M. Genotoxic effect of the tricyclic antidepressant drug clomipra-mine hydrochloride in somatic and germ cells of male mice. Asian Pac. J. Trop. Dis. 2016, 6, 321–327. [Google Scholar] [CrossRef]
- Kolachana, P.; Subrahmanyam, V.V.; Meyer, K.B.; Zhang, L.; Smith, M.T. Benzene and its phenolic metabolites produce oxidative DNA damage in HL60 cells in vitro and in the bone marrow in vivo. Cancer Res. 1993, 53, 1023–1026. [Google Scholar] [PubMed]
- Várkonyi, A.; Kelsey, K.; Semey, K.; Bodell, W.J.; Lévay, G.; Mark, E.; Wain, J.C.; Christiani, D.C.; Wiencke, J.K. Polyphenol associated-DNA adducts in lung and blood mononuclear cells from lung cancer patients. Cancer Lett. 2006, 236, 24–31. [Google Scholar] [CrossRef]
- Poganik, J.R.; Aye, Y. Electrophile Signaling and Emerging Immuno- and Neuro-modulatory Electrophilic Pharmaceuticals. Front. Aging Neurosci. 2020, 12, 1. [Google Scholar] [CrossRef]
- Poganik, J.R.; Long, M.J.C.; Disare, M.T.; Liu, X.; Chang, S.H.; Hla, T.; Aye, Y. Post-transcriptional regulation of Nrf2-mRNA by the mRNA-binding proteins HuR and AUF1. FASEB J. 2019, 33, 14636–14652. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.J.; Won, Y.S.; Kim, E.K.; Park, S.I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Korobkova, E.A.; Ng, W.; Venkatratnam, A.; Williams, A.K.; Nizamova, M.; Azar, N. In vitro studies of DNA damage caused by tricyclic antidepressants: A role of peroxidase in the side effects of the drugs. Chem. Res. Toxicol. 2010, 23, 1497–1503. [Google Scholar] [CrossRef]
- Korobkova, E.A.; Nemeth, J.; Cadougan, M.; Venkatratnam, A.; Bassit, M.; Azar, N. Reactive metabolites of desipramine and clomipramine: The kinetics of formation and reactivity with DNA. Bioorg. Med. Chem. 2012, 20, 340–345. [Google Scholar] [CrossRef]
- Bao, F.; Liu, D. Hydroxyl radicals generated in the rat spinal cord at the level produced by impact injury induce cell death by necrosis and apoptosis: Protection by a metalloporphyrin. NeuroSci 2004, 126, 285–295. [Google Scholar] [CrossRef]
- Elmorsy, E.; Al-Ghafari, A.; Almutairi, F.M.; Aggour, A.M.; Carter, W.G. Anti-depressants are cytotoxic to rat primary blood brain barrier endothelial cells at high therapeutic concentrations. Toxicol Vitro 2017, 44, 154–163. [Google Scholar] [CrossRef]
- Eto, K.; Fukuda, T.; Araki, Y.; Inoue, B.; Ogata, M. Effect of tricyclic drugs on mitochondrial membrane. Acta Med. Okayama 1985, 39, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razaq, W.; Kendall, D.A.; Bates, T.E. The effects of antidepressants on mitochondrial function in a model cell system and isolated mitochondria. Neurochem. Res. 2011, 36, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Shih, F.C.; Lin, C.F.; Wu, Y.C.; Hsu, C.C.; Chen, B.C.; Chang, Y.C.; Lin, Y.S.; Satria, R.D.; Lin, P.Y.; Chen, C.L. Desmethylclomipramine triggers mitochondrial damage and death in TGF-β-induced mesenchymal type of A549 cells. Life Sci. 2024, 351, 122817. [Google Scholar] [CrossRef] [PubMed]
- Keatley, K.; Stromei-Cleroux, S.; Wiltshire, T.; Rajala, N.; Burton, G.; Holt, W.V.; Littlewood, D.T.J.; Briscoe, A.G.; Jung, J.; Ashkan, K.; et al. Integrated Approach Reveals Role of Mitochondrial Germ-Line Mutation F18L in Respiratory Chain, Oxidative Alterations, Drug Sensitivity, and Patient Prognosis in Glioblastoma. Int. J. Mol. Sci. 2019, 20, 3364. [Google Scholar] [CrossRef]
- Gellén, B.; Völgyi, K.; Győrffy, B.; Darula, Z.; Hunyadi-Gulyás, É.; Baracskay, P.; Czurkó, A.; Hernádi, I.; Juhász, G.; Dobolyi, Á.; et al. Proteomic investigation of the prefrontal cortex in the rat clomipramine model of depression. J. Proteomics 2017, 153, 53–64. [Google Scholar] [CrossRef][Green Version]
- Bardel, G.; Gross, A.T.; Neacsiu, C.B. The neural circuitry of emotional regulation: Evidence from multimodal imaging. BrainBridge Neurosci. Biomed. Eng. 2024, 1, 27–41. [Google Scholar] [CrossRef]
- Kafetzopoulos, V.; Kokras, N.; Katsaitis, F.; Sousa, N.; Leite-Almeida, H.; Sotiropoulos, I.; Dalla, C. Prefrontal cortex-nucleus reuniens-hippocampus network exhibits sex-differentiated responses to stress and antidepressant treatment in rats. Psychopharmacology 2025, 242, 1627–1639. [Google Scholar] [CrossRef]
- Kokras, N.; Antoniou, K.; Dalla, C.; Bekris, S.; Xagoraris, M.; Ovestreet, D.; Papadopoulou-Daifoti, Z. Sex-related differential response to clomipramine treatment in a rat model of depression. J. Psychopharmacol. 2009, 23, 945–956. [Google Scholar] [CrossRef]
- Limón-Morales, O.; Arteaga-Silva, M.; Rojas-Castañeda, J.; Molina-Jiménez, T.; Guadarrama-Cruz, G.; Cerbón, M.; Bonilla-Jaime, H. Neonatal treatment with clomipramine modifies the expression of estrogen receptors in brain areas of male adult rats. Brain Res. 2019, 1724, 146443. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Wang, Y.; Deng, F.; Deng, Z. Oxidative Stress: Signaling Pathways, Biological Functions, and Disease. MedComm (2020) 2025, 6, e70268. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labra-Ruíz, N.A.; Mendoza-Torreblanca, J.G.; Osnaya-Brizuela, N.; Valenzuela-Peraza, A.; Ortiz-Herrera, M.; Barragán-Mejía, G.; Cárdenas-Rodríguez, N.; Santamaría-Del Ángel, D. Clomipramine Induced Oxidative Stress and Morphological Alterations in the Prefrontal Cortex and Limbic System of Neonatal Rats. Brain Sci. 2025, 15, 1068. https://doi.org/10.3390/brainsci15101068
Labra-Ruíz NA, Mendoza-Torreblanca JG, Osnaya-Brizuela N, Valenzuela-Peraza A, Ortiz-Herrera M, Barragán-Mejía G, Cárdenas-Rodríguez N, Santamaría-Del Ángel D. Clomipramine Induced Oxidative Stress and Morphological Alterations in the Prefrontal Cortex and Limbic System of Neonatal Rats. Brain Sciences. 2025; 15(10):1068. https://doi.org/10.3390/brainsci15101068
Chicago/Turabian StyleLabra-Ruíz, Norma Angélica, Julieta Griselda Mendoza-Torreblanca, Norma Osnaya-Brizuela, Armando Valenzuela-Peraza, Maribel Ortiz-Herrera, Gerardo Barragán-Mejía, Noemí Cárdenas-Rodríguez, and Daniel Santamaría-Del Ángel. 2025. "Clomipramine Induced Oxidative Stress and Morphological Alterations in the Prefrontal Cortex and Limbic System of Neonatal Rats" Brain Sciences 15, no. 10: 1068. https://doi.org/10.3390/brainsci15101068
APA StyleLabra-Ruíz, N. A., Mendoza-Torreblanca, J. G., Osnaya-Brizuela, N., Valenzuela-Peraza, A., Ortiz-Herrera, M., Barragán-Mejía, G., Cárdenas-Rodríguez, N., & Santamaría-Del Ángel, D. (2025). Clomipramine Induced Oxidative Stress and Morphological Alterations in the Prefrontal Cortex and Limbic System of Neonatal Rats. Brain Sciences, 15(10), 1068. https://doi.org/10.3390/brainsci15101068







