Methylphenidate as a Novel Adjunct in Opioid-Taking Patients: Insights into Dopaminergic Neuroadaptation and Hypoactive Delirium
Abstract
1. Introduction
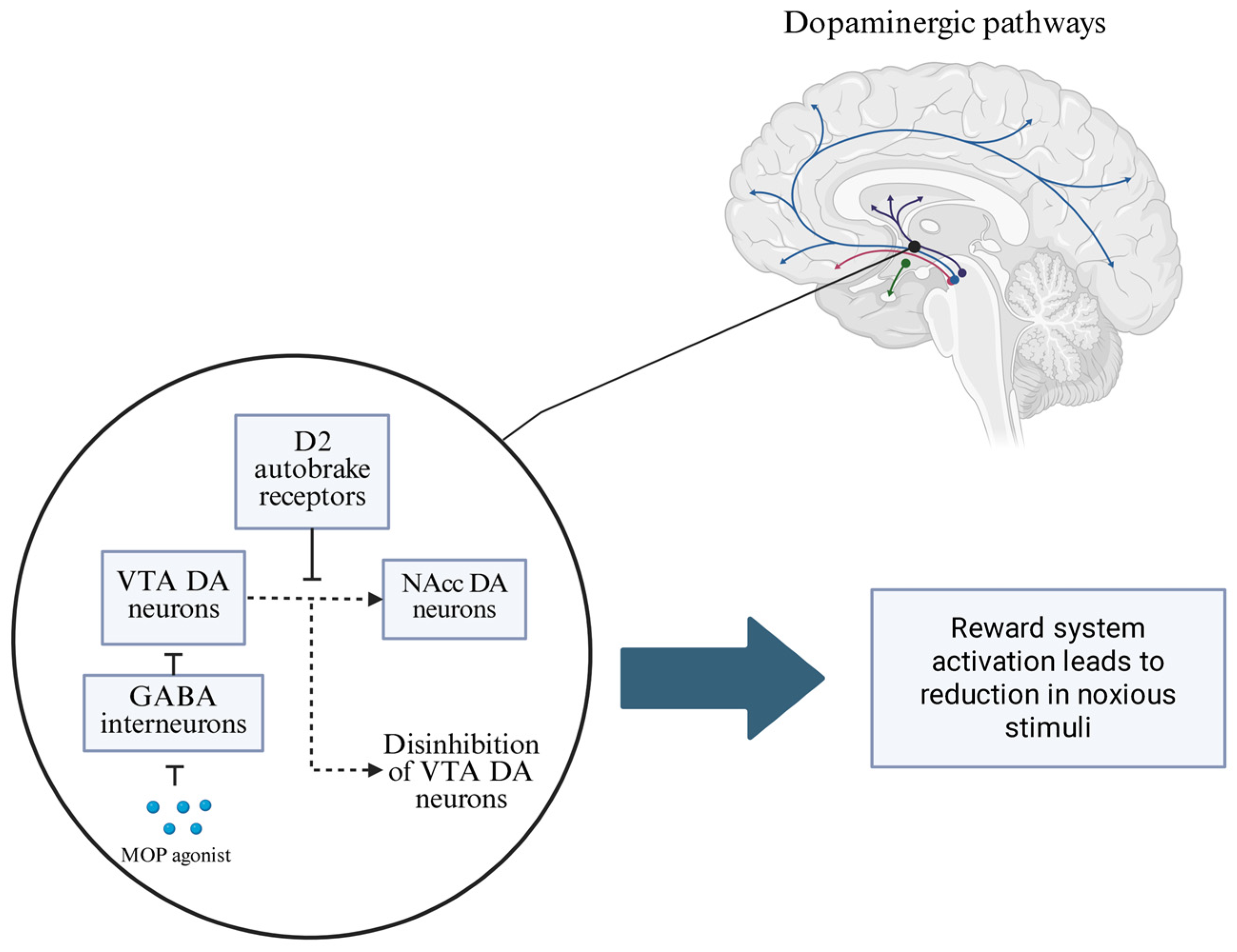
2. The Mechanism of Action of Opioids and the Neurobiological Basis of OUD Development
3. Pharmacokinetics and Pharmacodynamics of Methylphenidate
4. Role of Methylphenidate in Stabilizing Dopaminergic Pathways
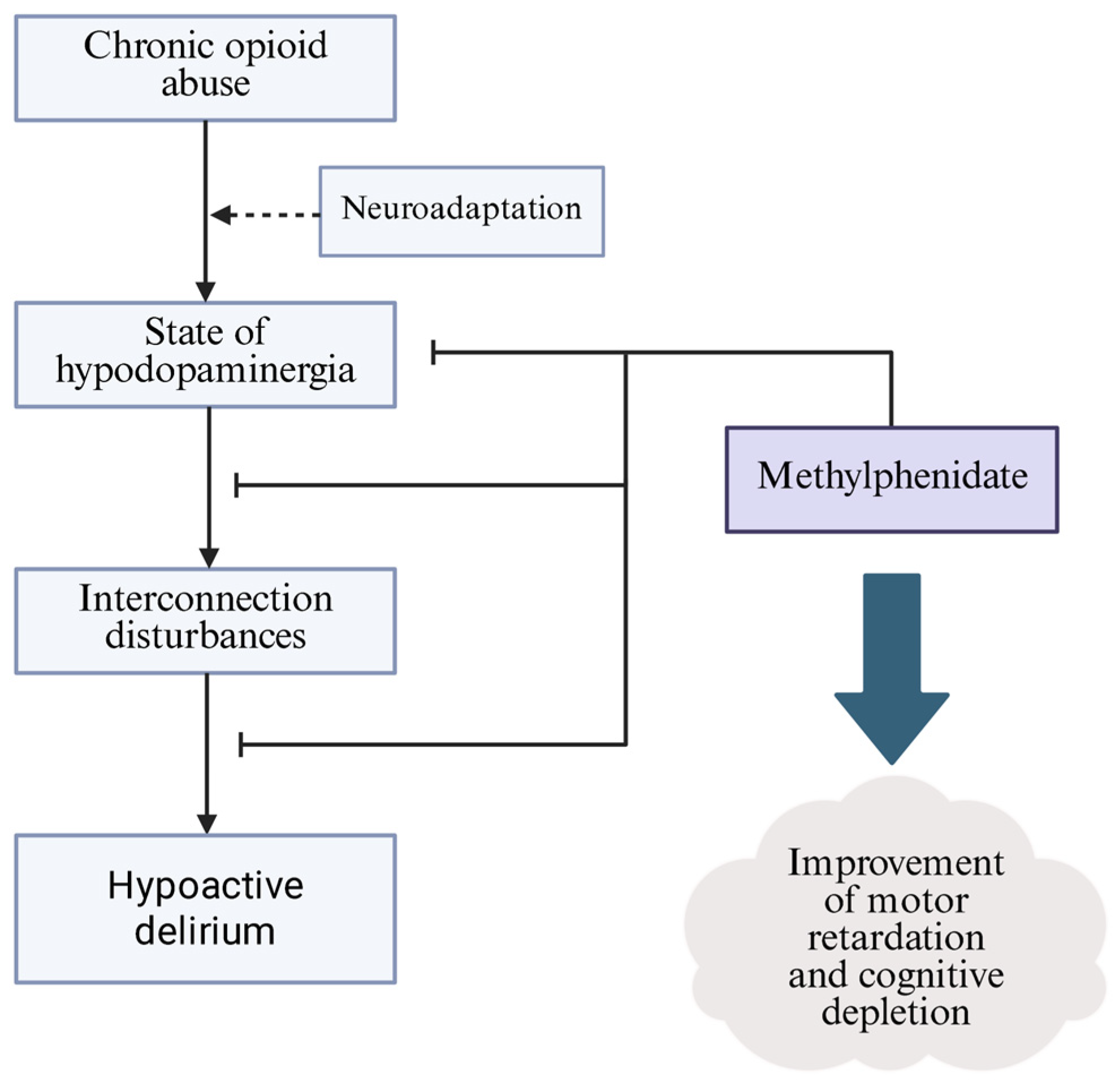
5. Off-Label Prevention and Treatment of Opioids Withdrawal-Induced Hypoactive Delirium
6. Discussion
7. Conclusions
- •
- The development and course of OUD is dependent on the neuroadaptive processes of the dopaminergic pathway of the mesocorticolimbic system.
- •
- These pathways are involved in cognitive and motivational processes and determine the propensity to relapse into addiction.
- •
- MPH, as a dopamine reuptake inhibitor and modulator of VMAT-2, may promote the tonic balance of dopaminergic pathways in the hypodopaminergic environment induced by opioid abuse.
- •
- Despite promising molecular correlations, well-designed clinical trials are needed to determine the preferred recipient group, the safety of adjuvant MPH therapy, and its potential benefits.
- •
- MPH has shown efficacy in reducing hypoactive delirium in patients with terminal cancer, which may share a clinical picture and potential pathogenesis with delirium induced by opioid withdrawal. The current state of knowledge does not allow for the routine use of MPH in this indication, whereas etiological similarities have prompted expanded clinical studies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roberts, A.-O.; Richards, G.C. Is England Facing an Opioid Epidemic? Br. J. Pain 2023, 17, 320–324. [Google Scholar] [CrossRef]
- Højsted, J.; Sjøgren, P. Addiction to Opioids in Chronic Pain Patients: A Literature Review. Eur. J. Pain 2007, 11, 490–518. [Google Scholar] [CrossRef] [PubMed]
- Balyan, R.; Hahn, D.; Huang, H.; Chidambaran, V. Pharmacokinetic and Pharmacodynamic Considerations in Developing a Response to the Opioid Epidemic. Expert Opin. Drug Metab. Toxicol. 2020, 16, 125–141. [Google Scholar] [CrossRef]
- Traynor, J.R.; Moron, J.A. Opioid Research in the Time of the Opioid Crisis. Br. J. Pharmacol. 2023, 180, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Kampman, K.; Jarvis, M. American Society of Addiction Medicine (ASAM) National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. J. Addict. Med. 2015, 9, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Viswanath, O.; Peck, J.; Kaye, A.D.; Gill, J.S.; Simopoulos, T.T. A Brief History of the Opioid Epidemic and Strategies for Pain Medicine. Pain Ther. 2018, 7, 13–21. [Google Scholar] [CrossRef]
- Hoffman, K.A.; Ponce Terashima, J.; McCarty, D. Opioid Use Disorder and Treatment: Challenges and Opportunities. BMC Health Serv. Res. 2019, 19, 884. [Google Scholar] [CrossRef]
- Dowell, D.; Brown, S.; Gyawali, S.; Hoenig, J.; Ko, J.; Mikosz, C.; Ussery, E.; Baldwin, G.; Jones, C.M.; Olsen, Y.; et al. Treatment for Opioid Use Disorder: Population Estimates—United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 567–574. [Google Scholar] [CrossRef]
- Florence, C.; Luo, F.; Rice, K. The Economic Burden of Opioid Use Disorder and Fatal Opioid Overdose in the United States, 2017. Drug Alcohol Depend. 2021, 218, 108350. [Google Scholar] [CrossRef]
- Drug-Induced Deaths—The Current Situation in Europe (European Drug Report 2025). Available online: https://www.euda.europa.eu/publications/european-drug-report/2025/drug-induced-deaths_en (accessed on 3 August 2025).
- Connor, M.; Bagley, E.E.; Chieng, B.C.; Christie, M.J. β-Arrestin-2 Knockout Prevents Development of Cellular Μ-opioid Receptor Tolerance but Does Not Affect Opioid-withdrawal-related Adaptations in Single PAG Neurons. Br. J. Pharmacol. 2015, 172, 492–500. [Google Scholar] [CrossRef]
- Grond, S.; Sablotzki, A. Clinical Pharmacology of Tramadol. Clin. Pharmacokinet. 2004, 43, 879–923. [Google Scholar] [CrossRef]
- Cheng, W.; Lam, R.P.K.; Chan, C.K. Factors Associated with Seizure in Tramadol Overdose: A 12-Year Retrospective Study in Hong Kong. Clin. Toxicol. 2022, 60, 1220–1226. [Google Scholar] [CrossRef]
- Alshehri, F.S. Tapentadol: A Review of Experimental Pharmacology Studies, Clinical Trials, and Recent Findings. Drug Des. Dev. Ther. 2023, 17, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Roulet, L.; Rollason, V.; Desmeules, J.; Piguet, V. Tapentadol Versus Tramadol: A Narrative and Comparative Review of Their Pharmacological, Efficacy and Safety Profiles in Adult Patients. Drugs 2021, 81, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Acharya, M.; Hayes, C.J.; Li, C.; Painter, J.T.; Dayer, L.; Martin, B.C. Development of a Potential Opioid Misuse Measure from Administrative Dispensing Data and Contrasting Opioid Misuse among Individuals on Long-Term Tramadol, Long-Term Short-Acting Hydrocodone or Long-Term Short-Acting Oxycodone Therapy in Arkansas. Curr. Med. Res. Opin. 2022, 38, 1947–1957. [Google Scholar] [CrossRef]
- Miranda, H.F.; Pinardi, G. Antinociception, Tolerance, and Physical Dependence Comparison Between Morphine and Tramadol. Pharmacol. Biochem. Behav. 1998, 61, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Hassamal, S.; Miotto, K.; Dale, W.; Danovitch, I. Tramadol: Understanding the Risk of Serotonin Syndrome and Seizures. Am. J. Med. 2018, 131, 1382.e1–1382.e6. [Google Scholar] [CrossRef]
- Prekupec, M.P.; Mansky, P.A.; Baumann, M.H. Misuse of Novel Synthetic Opioids: A Deadly New Trend. J. Addict. Med. 2017, 11, 256–265. [Google Scholar] [CrossRef]
- Lovrecic, B.; Lovrecic, M.; Gabrovec, B.; Carli, M.; Pacini, M.; Maremmani, A.G.I.; Maremmani, I. Non-Medical Use of Novel Synthetic Opioids: A New Challenge to Public Health. Int. J. Environ. Res. Public Health 2019, 16, 177. [Google Scholar] [CrossRef]
- Navratilova, E.; Xie, J.Y.; Okun, A.; Qu, C.; Eyde, N.; Ci, S.; Ossipov, M.H.; King, T.; Fields, H.L.; Porreca, F. Pain Relief Produces Negative Reinforcement through Activation of Mesolimbic Reward–Valuation Circuitry. Proc. Natl. Acad. Sci. USA 2012, 109, 20709–20713. [Google Scholar] [CrossRef] [PubMed]
- Soderman, A.R.; Unterwald, E.M. Cocaine Reward and Hyperactivity in the Rat: Sites of Mu Opioid Receptor Modulation. Neuroscience 2008, 154, 1506–1516. [Google Scholar] [CrossRef]
- Fields, H. Understanding How Opioids Contribute to Reward and Analgesia. Reg. Anesth. Pain Med. 2007, 32, 242–246. [Google Scholar] [CrossRef]
- Dum, J.; Herz, A. Endorphinergic Modulation of Neural Reward Systems Indicated by Behavioral Changes. Pharmacol. Biochem. Behav. 1984, 21, 259–266. [Google Scholar] [CrossRef]
- Chaudun, F.; Python, L.; Liu, Y.; Hiver, A.; Cand, J.; Kieffer, B.L.; Valjent, E.; Lüscher, C. Distinct Μ-Opioid Ensembles Trigger Positive and Negative Fentanyl Reinforcement. Nature 2024, 630, 141–148. [Google Scholar] [CrossRef]
- Schoffelmeer, A.N.M.; Van Vliet, B.J.; De Vries, T.J.; Heijna, M.H.; Mulder, A.H. Regulation of Brain Neurotransmitter Release and of Adenylate Cyclase Activity by Opioid Receptors. Biochem. Soc. Trans. 1992, 20, 449–453. [Google Scholar] [CrossRef]
- Chan, P.; Lutfy, K. Molecular Changes in Opioid Addiction: The Role of Adenylyl Cyclase and cAMP/PKA System. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2016; Volume 137, pp. 203–227. ISBN 978-0-12-803786-7. [Google Scholar]
- Li, X.-H.; Chen, Q.-Y.; Zhuo, M. Neuronal Adenylyl Cyclase Targeting Central Plasticity for the Treatment of Chronic Pain. Neurotherapeutics 2020, 17, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F. Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia, and Negative Reinforcement. Biol. Psychiatry 2020, 87, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.P. The Role of D2-Autoreceptors in Regulating Dopamine Neuron Activity and Transmission. Neuroscience 2014, 282, 13–22. [Google Scholar] [CrossRef]
- Chen, R.; Ferris, M.J.; Wang, S. Dopamine D2 Autoreceptor Interactome: Targeting the Receptor Complex as a Strategy for Treatment of Substance Use Disorder. Pharmacol. Ther. 2020, 213, 107583. [Google Scholar] [CrossRef]
- Rougé-Pont, F.; Usiello, A.; Benoit-Marand, M.; Gonon, F.; Piazza, P.V.; Borrelli, E. Changes in Extracellular Dopamine Induced by Morphine and Cocaine: Crucial Control by D2 Receptors. J. Neurosci. 2002, 22, 3293–3301. [Google Scholar] [CrossRef] [PubMed]
- Wanat, M.; Willuhn, I.; Clark, J.; Phillips, P. Phasic Dopamine Release in Appetitive Behaviors and Drug Addiction. Curr. Drug Abus. Rev. 2009, 2, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.E.; Berridge, K.C. The Psychology and Neurobiology of Addiction: An Incentive–Sensitization View. Addiction 2000, 95, 91–117. [Google Scholar] [CrossRef]
- Breiter, H.C.; Gollub, R.L.; Weisskoff, R.M.; Kennedy, D.N.; Makris, N.; Berke, J.D.; Goodman, J.M.; Kantor, H.L.; Gastfriend, D.R.; Riorden, J.P.; et al. Acute Effects of Cocaine on Human Brain Activity and Emotion. Neuron 1997, 19, 591–611. [Google Scholar] [CrossRef]
- Sanjari Moghaddam, H.; Shadloo, B.; Shahkhah, H.; Tafakhori, A.; Haghshomar, M.; Meshkat, S.; Aghamollaii, V. Cognitive Impairment in Opium Use Disorder. Behav. Neurol. 2021, 2021, 5548623. [Google Scholar] [CrossRef]
- Hochheimer, M.; Strickland, J.C.; Rabinowitz, J.A.; Ellis, J.D.; Bergeria, C.L.; Hobelmann, J.G.; Huhn, A.S. The Impact of Opioid-Stimulant Co-Use on Tonic and Cue-Induced Craving. J. Psychiatr. Res. 2023, 164, 15–22. [Google Scholar] [CrossRef]
- McHugh, R.K.; Park, S.; Weiss, R.D. Cue-induced Craving in Dependence upon Prescription Opioids and Heroin. Am. J. Addict. 2014, 23, 453–458. [Google Scholar] [CrossRef]
- Childress, A.R.; Mozley, P.D.; McElgin, W.; Fitzgerald, J.; Reivich, M.; O’Brien, C.P. Limbic Activation During Cue-Induced Cocaine Craving. Am. J. Psychiatry 1999, 156, 11–18. [Google Scholar] [CrossRef]
- Perry, B.L.; Odabaş, M.; Yang, K.; Lee, B.; Kaminski, P.; Aronson, B.; Ahn, Y.; Oser, C.B.; Freeman, P.R.; Talbert, J.C. New Means, New Measures: Assessing Prescription Drug-seeking Indicators over 10 Years of the Opioid Epidemic. Addiction 2022, 117, 195–204. [Google Scholar] [CrossRef]
- Zhou, K.; Xu, H.; Lu, S.; Jiang, S.; Hou, G.; Deng, X.; He, M.; Zhu, Y. Reward and Aversion Processing by Input-Defined Parallel Nucleus Accumbens Circuits in Mice. Nat. Commun. 2022, 13, 6244. [Google Scholar] [CrossRef]
- Ding, Y.-S.; Fowler, J.S.; Volkow, N.D.; Dewey, S.L.; Wang, G.-J.; Logan, J.; Gatley, S.J.; Pappas, N. Chiral Drugs: Comparison of the Pharmacokinetics of [ 11 C] d-Threo and l-Threo -Methylphenidate in the Human and Baboon Brain. Psychopharmacology 1997, 131, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Heal, D.J.; Pierce, D.M. Methylphenidate and Its Isomers: Their Role in the Treatment of Attention-Deficit Hyperactivity Disorder Using a Transdermal Delivery System. CNS Drugs 2006, 20, 713–738. [Google Scholar] [CrossRef] [PubMed]
- Shellenberg, T.P.; Stoops, W.W.; Lile, J.A.; Rush, C.R. An Update on the Clinical Pharmacology of Methylphenidate: Therapeutic Efficacy, Abuse Potential and Future Considerations. Expert Rev. Clin. Pharmacol. 2020, 13, 825–833. [Google Scholar] [CrossRef]
- Williard, R.L.; Middaugh, L.D.; Zhu, H.-J.B.; Patrick, K.S. Methylphenidate and Its Ethanol Transesterification Metabolite Ethylphenidate: Brain Disposition, Monoamine Transporters and Motor Activity. Behav. Pharmacol. 2007, 18, 39–51. [Google Scholar] [CrossRef]
- Riddle, E.L.; Hanson, G.R.; Fleckenstein, A.E. Therapeutic Doses of Amphetamine and Methylphenidate Selectively Redistribute the Vesicular Monoamine Transporter-2. Eur. J. Pharmacol. 2007, 571, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Zaczek, R.; Battaglia, G.; Contrera, J.F.; Culp, S.; De Souza, E.B. Methylphenidate and Pemoline Do Not Cause Depletion of Rat Brain Monoamine Markers Similar to That Observed with Methamphetamine. Toxicol. Appl. Pharmacol. 1989, 100, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Morciano, M.; Burré, J.; Corvey, C.; Karas, M.; Zimmermann, H.; Volknandt, W. Immunoisolation of Two Synaptic Vesicle Pools from Synaptosomes: A Proteomics Analysis. J. Neurochem. 2005, 95, 1732–1745. [Google Scholar] [CrossRef]
- Fleckenstein, A.E.; Hanson, G.R. Impact of Psychostimulants on Vesicular Monoamine Transporter Function. Eur. J. Pharmacol. 2003, 479, 283–289. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Fowler, J.S.; Gatley, S.J.; Logan, J.; Ding, Y.-S.; Hitzemann, R.; Pappas, N. Dopamine Transporter Occupancies in the Human Brain Induced by Therapeutic Doses of Oral Methylphenidate. Am. J. Psychiatry 1998, 155, 1325–1331. [Google Scholar] [CrossRef]
- Iversen, L. Neurotransmitter Transporters and Their Impact on the Development of Psychopharmacology. Br. J. Pharmacol. 2006, 147, S82–S88. [Google Scholar] [CrossRef]
- Schweri, M.M.; Skolnick, P.; Rafferty, M.F.; Rice, K.C.; Janowsky, A.J.; Paul, S.M. [3H]Threo-(±)-Methylphenidate Binding to 3,4-Dihydroxyphenylethylamine Uptake Sites in Corpus Striatum: Correlation with the Stimulant Properties of Ritalinic Acid Esters. J. Neurochem. 1985, 45, 1062–1070. [Google Scholar] [CrossRef]
- Izenwasser, S.; Werling, L.L.; Cox, B.M. Comparison of the Effects of Cocaine and Other Inhibitors of Dopamine Uptake in Rat Striatum, Nucleus Accumbens, Olfactory Tubercle, and Medial Prefrontal Cortex. Brain Res. 1990, 520, 303–309. [Google Scholar] [CrossRef]
- Golmirzaei, J.; Mahboobi, H.; Yazdanparast, M.; Mushtaq, G.; Kamal, M.A.; Hamzei, E. Psychopharmacology of Attention-Deficit Hyperactivity Disorder: Effects and Side Effects. Curr. Pharm. Des. 2016, 22, 590–594. [Google Scholar] [CrossRef]
- Bassetti, C.L.A.; Kallweit, U.; Vignatelli, L.; Plazzi, G.; Lecendreux, M.; Baldin, E.; Dolenc-Groselj, L.; Jennum, P.; Khatami, R.; Manconi, M.; et al. European Guideline and Expert Statements on the Management of Narcolepsy in Adults and Children. J. Sleep Res. 2021, 30, e13387. [Google Scholar] [CrossRef]
- Kordon, A.; Stollhoff, K.; Niederkirchner, K.; Mattejat, F.; Rettig, K.; Schäuble, B. Exploring the Impact of Once-Daily OROS® Methylphenidate (MPH) on Symptoms and Quality of Life in Children and Adolescents with ADHD Transitioning from Immediate-Release MPH. Postgrad. Med. 2011, 123, 27–38. [Google Scholar] [CrossRef]
- Repantis, D.; Bovy, L.; Ohla, K.; Kühn, S.; Dresler, M. Cognitive Enhancement Effects of Stimulants: A Randomized Controlled Trial Testing Methylphenidate, Modafinil, and Caffeine. Psychopharmacology 2021, 238, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Lavretsky, H.; Reinlieb, M.; St. Cyr, N.; Siddarth, P.; Ercoli, L.M.; Senturk, D. Citalopram, Methylphenidate, or Their Combination in Geriatric Depression: A Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Psychiatry 2015, 172, 561–569. [Google Scholar] [CrossRef]
- Sullivan, D.R.; Mongoue-Tchokote, S.; Mori, M.; Goy, E.; Ganzini, L. Randomized, Double-blind, Placebo-controlled Study of Methylphenidate for the Treatment of Depression in SSRI-treated Cancer Patients Receiving Palliative Care. Psycho-Oncology 2017, 26, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Lund, L.; Petersen, M.A.; Sjogren, P.; Groenvold, M. Methylphenidate as Needed for Fatigue in Patients With Advanced Cancer. A Prospective, Double-Blind, and Placebo-Controlled Study. J. Pain Symptom Manag. 2020, 60, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Escalante, C.P.; Meyers, C.; Reuben, J.M.; Wang, X.; Qiao, W.; Manzullo, E.; Alvarez, R.H.; Morrow, P.K.; Gonzalez-Angulo, A.M.; Wang, X.S.; et al. A Randomized, Double-Blind, 2-Period, Placebo-Controlled Crossover Trial of a Sustained-Release Methylphenidate in the Treatment of Fatigue in Cancer Patients. Cancer J. 2014, 20, 8–14. [Google Scholar] [CrossRef]
- Tardy, J.; Pariente, J.; Leger, A.; Dechaumont-Palacin, S.; Gerdelat, A.; Guiraud, V.; Conchou, F.; Albucher, J.-F.; Marque, P.; Franceries, X.; et al. Methylphenidate Modulates Cerebral Post-Stroke Reorganization. NeuroImage 2006, 33, 913–922. [Google Scholar] [CrossRef]
- Godfrey, J. Safety of Therapeutic Methylphenidate in Adults: A Systematic Review of the Evidence. J. Psychopharmacol. 2009, 23, 194–205. [Google Scholar] [CrossRef]
- Storebø, O.J.; Storm, M.R.O.; Pereira Ribeiro, J.; Skoog, M.; Groth, C.; Callesen, H.E.; Schaug, J.P.; Darling Rasmussen, P.; Huus, C.-M.L.; Zwi, M.; et al. Methylphenidate for Children and Adolescents with Attention Deficit Hyperactivity Disorder (ADHD). Cochrane Database Syst. Rev. 2023, 2023, CD009885. [Google Scholar] [CrossRef]
- Storebø, O.J.; Krogh, H.B.; Ramstad, E.; Moreira-Maia, C.R.; Holmskov, M.; Skoog, M.; Nilausen, T.D.; Magnusson, F.L.; Zwi, M.; Gillies, D.; et al. Methylphenidate for Attention-Deficit/Hyperactivity Disorder in Children and Adolescents: Cochrane Systematic Review with Meta-Analyses and Trial Sequential Analyses of Randomised Clinical Trials. BMJ 2015, 351, h5203. [Google Scholar] [CrossRef]
- Storebø, O.J.; Pedersen, N.; Ramstad, E.; Kielsholm, M.L.; Nielsen, S.S.; Krogh, H.B.; Moreira-Maia, C.R.; Magnusson, F.L.; Holmskov, M.; Gerner, T.; et al. Methylphenidate for Attention Deficit Hyperactivity Disorder (ADHD) in Children and Adolescents—Assessment of Adverse Events in Non-Randomised Studies. Cochrane Database Syst. Rev. 2018, 5, CD012069. [Google Scholar] [CrossRef]
- Ugwendum, D.; Mbome, Y.; Arrey Agbor, D.B.; Burkhanova, U.; Offor, R.; Okorie, I.J.; Gorantla, A.; Amokaye, F.A.; Atere, M.; Nfonoyim, J. Methylphenidate-Induced Non-Ischemic Heart Failure With Reduced Ejection Fraction and Mild Pulmonary Hypertension. Cureus 2024, 16, e55604. [Google Scholar] [CrossRef]
- Hay, E.; Shklovski, V.; Blaer, Y.; Shlakhover, V.; Katz, A. Intravenous Methylphenidate: An Unusual Way to Provoke ST-Elevation Myocardial Infarction. Am. J. Emerg. Med. 2015, 33, 313.e1–313.e3. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Aguayo, J.C.; Arancibia, M.; Meza-Concha, N.; Bustamante, C.; Pérez-Bracchiglione, J.; Madrid, E. Brief Psychosis Induced by Methylphenidate in a Child with Attention Deficit Disorder: A Case Report and Literature Review. Medwave 2017, 17, e6980. [Google Scholar] [CrossRef] [PubMed]
- Bieś, R.; Fojcik, J.; Warchala, A.; Trędzbor, B.; Krysta, K.; Piekarska-Bugiel, K.; Krzystanek, M. The Risk of Methylphenidate Pharmacotherapy for Adults with ADHD. Pharmaceuticals 2023, 16, 1292. [Google Scholar] [CrossRef]
- Clemow, D.B.; Walker, D.J. The Potential for Misuse and Abuse of Medications in ADHD: A Review. Postgrad. Med. 2014, 126, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Bloom, F.E. Cellular and Molecular Mechanisms of Drug Dependence. Science 1988, 242, 715–723. [Google Scholar] [CrossRef]
- Koob, G.F.; Le Moal, M. Addiction and the Brain Antireward System. Annu. Rev. Psychol. 2008, 59, 29–53. [Google Scholar] [CrossRef]
- Williams, J.T.; Ingram, S.L.; Henderson, G.; Chavkin, C.; Von Zastrow, M.; Schulz, S.; Koch, T.; Evans, C.J.; Christie, M.J. Regulation of Μ-Opioid Receptors: Desensitization, Phosphorylation, Internalization, and Tolerance. Pharmacol. Rev. 2013, 65, 223–254. [Google Scholar] [CrossRef]
- Cahill, C.M.; Walwyn, W.; Taylor, A.M.W.; Pradhan, A.A.A.; Evans, C.J. Allostatic Mechanisms of Opioid Tolerance Beyond Desensitization and Downregulation. Trends Pharmacol. Sci. 2016, 37, 963–976. [Google Scholar] [CrossRef]
- Diana, M. The Dopamine Hypothesis of Drug Addiction and Its Potential Therapeutic Value. Front. Psychiatry 2011, 2, 64. [Google Scholar] [CrossRef]
- Nam, M.-H.; Won, W.; Han, K.-S.; Lee, C.J. Signaling Mechanisms of μ-Opioid Receptor (MOR) in the Hippocampus: Disinhibition versus Astrocytic Glutamate Regulation. Cell. Mol. Life Sci. 2021, 78, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Jain, R.; Chawla, N.; Raghav, R. Serum Corticotrophin Releasing Factor (CRF) and Its Correlation with Stress and Craving in Detoxified Opioid-Dependent Subjects. Asian J. Psychiatry 2022, 68, 102964. [Google Scholar] [CrossRef] [PubMed]
- Pomrenze, M.B.; Cardozo Pinto, D.F.; Neumann, P.A.; Llorach, P.; Tucciarone, J.M.; Morishita, W.; Eshel, N.; Heifets, B.D.; Malenka, R.C. Modulation of 5-HT Release by Dynorphin Mediates Social Deficits during Opioid Withdrawal. Neuron 2022, 110, 4125–4143.e6. [Google Scholar] [CrossRef]
- Curtis, K.; Viswanath, H.; Velasquez, K.M.; Molfese, D.L.; Harding, M.J.; Aramayo, E.; Baldwin, P.R.; Ambrosi, E.; Madan, A.; Patriquin, M.; et al. Increased Habenular Connectivity in Opioid Users Is Associated with an A5 Subunit Nicotinic Receptor Genetic Variant. Am. J. Addict. 2017, 26, 751–759. [Google Scholar] [CrossRef]
- Schwartz, R.P.; Mitchell, M.M.; O’Grady, K.E.; Kelly, S.M.; Gryczynski, J.; Mitchell, S.G.; Gordon, M.S.; Jaffe, J.H. Pharmacotherapy for Opioid Addiction in Community Corrections. Int. Rev. Psychiatry 2018, 30, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Hosker, C.; Ward, D. Hypoactive Delirium. BMJ 2017, 357, j2047. [Google Scholar] [CrossRef]
- Meagher, D. Motor Subtypes of Delirium: Past, Present and Future. Int. Rev. Psychiatry 2009, 21, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Fuller, V. Delirium Recall—An Integrative Review. J. Clin. Nurs. 2016, 25, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Darbyshire, J.L.; Greig, P.R.; Vollam, S.; Young, J.D.; Hinton, L. “I Can Remember Sort of Vivid People…but to Me They Were Plasticine.” Delusions on the Intensive Care Unit: What Do Patients Think Is Going On? PLoS ONE 2016, 11, e0153775. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, S.-W.; Kim, J.-M.; Shin, I.-S.; Bae, K.-Y.; Shim, H.-J.; Bae, W.-K.; Cho, S.-H.; Chung, I.-J.; Yoon, J.-S. Differential Associations Between Delirium and Mortality According to Delirium Subtype and Age: A Prospective Cohort Study. Psychosom. Med. 2015, 77, 903–910. [Google Scholar] [CrossRef]
- Parkar, S.; Seethalakshmi, R.; Adarkar, S.; Kharawala, S. Is This ′complicated′ Opioid Withdrawal? Indian J. Psychiatry 2006, 48, 121. [Google Scholar] [CrossRef]
- Hanna, J.; Swetter, S. A Case of Delirium and Rhabdomyolysis in Severe Iatrogenic Opioid Withdrawal. Psychosomatics 2018, 59, 405–407. [Google Scholar] [CrossRef]
- Aggarwal, A.; Choudhary, S.; Jiloha, R.C. Opioid Withdrawal Delirium. J. Neuropsychiatry Clin. Neurosci. 2011, 23, E37. [Google Scholar] [CrossRef]
- Das, S.; Sah, D.; Nandi, S.; Das, P. Opioid Withdrawal Presenting as Delirium and Role of Buprenorphine: A Case Series. Indian J. Psychol. Med. 2017, 39, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Sanders, R.D. Hypothesis for the Pathophysiology of Delirium: Role of Baseline Brain Network Connectivity and Changes in Inhibitory Tone. Med. Hypothes. 2011, 77, 140–143. [Google Scholar] [CrossRef]
- Choi, S.-H.; Lee, H.; Chung, T.-S.; Park, K.-M.; Jung, Y.-C.; Kim, S.I.; Kim, J.-J. Neural Network Functional Connectivity During and After an Episode of Delirium. Am. J. Psychiatry 2012, 169, 498–507. [Google Scholar] [CrossRef]
- Kinomura, S.; Larsson, J.; Gulyás, B.; Roland, P.E. Activation by Attention of the Human Reticular Formation and Thalamic Intralaminar Nuclei. Science 1996, 271, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Gaudreau, J.-D.; Gagnon, P. Psychotogenic Drugs and Delirium Pathogenesis: The Central Role of the Thalamus. Med. Hypothes. 2005, 64, 471–475. [Google Scholar] [CrossRef]
- Gagnon, B.; Low, G.; Schreier, G. Methylphenidate hydrochloride improves cognitive function in patients with advanced cancer and hypoactive delirium: A prospective clinical study. J. Psychiatry Neurosci. 2005, 30, 100–107. [Google Scholar]
- Morita, T.; Otani, H.; Tsunoda, J.; Inoue, S.; Chihara, S. Successful Palliation of Hypoactive Delirium Due to Multi-Organ Failure by Oral Methylphenidate. Support. Care Cancer 2000, 8, 134–137. [Google Scholar] [CrossRef]
- Berridge, C.W.; Devilbiss, D.M. Psychostimulants as Cognitive Enhancers: The Prefrontal Cortex, Catecholamines, and Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2011, 69, e101–e111. [Google Scholar] [CrossRef]
- Spencer, T.J.; Brown, A.; Seidman, L.J.; Valera, E.M.; Makris, N.; Lomedico, A.; Faraone, S.V.; Biederman, J. Effect of Psychostimulants on Brain Structure and Function in ADHD: A Qualitative Literature Review of Magnetic Resonance Imaging–Based Neuroimaging Studies. J. Clin. Psychiatry 2013, 74, 902–917. [Google Scholar] [CrossRef]
- Ersche, K.D.; Turton, A.J.; Chamberlain, S.R.; Müller, U.; Bullmore, E.T.; Robbins, T.W. Cognitive Dysfunction and Anxious-Impulsive Personality Traits Are Endophenotypes for Drug Dependence. Am. J. Psychiatry 2012, 169, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Verdejo-García, A.; Alcázar-Córcoles, M.A.; Albein-Urios, N. Neuropsychological Interventions for Decision-Making in Addiction: A Systematic Review. Neuropsychol. Rev. 2019, 29, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Bickel, W.K.; Koffarnus, M.N.; Moody, L.; Wilson, A.G. The Behavioral- and Neuro-Economic Process of Temporal Discounting: A Candidate Behavioral Marker of Addiction. Neuropharmacology 2014, 76, 518–527. [Google Scholar] [CrossRef]
- Wilens, T.E.; Martelon, M.; Joshi, G.; Bateman, C.; Fried, R.; Petty, C.; Biederman, J. Does ADHD Predict Substance-Use Disorders? A 10-Year Follow-up Study of Young Adults With ADHD. J. Am. Acad. Child Adolesc. Psychiatry 2011, 50, 543–553. [Google Scholar] [CrossRef]
- Kollins, S.H. Comparing the Abuse Potential of Methylphenidate versus Other Stimulants: A Review of Available Evidence and Relevance to the ADHD Patient. J. Clin. Psychiatry 2003, 64 (Suppl. 11), 14–18. [Google Scholar] [PubMed]
- Konstenius, M.; Jayaram-Lindström, N.; Guterstam, J.; Beck, O.; Philips, B.; Franck, J. Methylphenidate for Attention Deficit Hyperactivity Disorder and Drug Relapse in Criminal Offenders with Substance Dependence: A 24-week Randomized Placebo-controlled Trial. Addiction 2014, 109, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Ware, O.; Geiger, G.; Rivas, V.; Macias Burgos, M.; Nehme-Kotocavage, L.; Bautista, T. Risk of Relapse Following Discharge from Non-Hospital Residential Opioid Use Disorder Treatment: A Systematic Review of Studies Published from 2018 to 2022. Subst. Abus. Rehabil. 2025, 16, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. Is There a Common Molecular Pathway for Addiction? Nat. Neurosci. 2005, 8, 1445–1449. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świderski, N.; Rodek, P.; Kucia, K. Methylphenidate as a Novel Adjunct in Opioid-Taking Patients: Insights into Dopaminergic Neuroadaptation and Hypoactive Delirium. Brain Sci. 2025, 15, 850. https://doi.org/10.3390/brainsci15080850
Świderski N, Rodek P, Kucia K. Methylphenidate as a Novel Adjunct in Opioid-Taking Patients: Insights into Dopaminergic Neuroadaptation and Hypoactive Delirium. Brain Sciences. 2025; 15(8):850. https://doi.org/10.3390/brainsci15080850
Chicago/Turabian StyleŚwiderski, Nikodem, Patryk Rodek, and Krzysztof Kucia. 2025. "Methylphenidate as a Novel Adjunct in Opioid-Taking Patients: Insights into Dopaminergic Neuroadaptation and Hypoactive Delirium" Brain Sciences 15, no. 8: 850. https://doi.org/10.3390/brainsci15080850
APA StyleŚwiderski, N., Rodek, P., & Kucia, K. (2025). Methylphenidate as a Novel Adjunct in Opioid-Taking Patients: Insights into Dopaminergic Neuroadaptation and Hypoactive Delirium. Brain Sciences, 15(8), 850. https://doi.org/10.3390/brainsci15080850







