Cerebral Microbleeds with a Venous Connection on 3 Tesla Susceptibility-Weighted Imaging in Persons with Alzheimer’s Disease and Healthy Aging Controls
Abstract
1. Introduction
2. Materials and Methods
2.1. Database
2.2. Participants
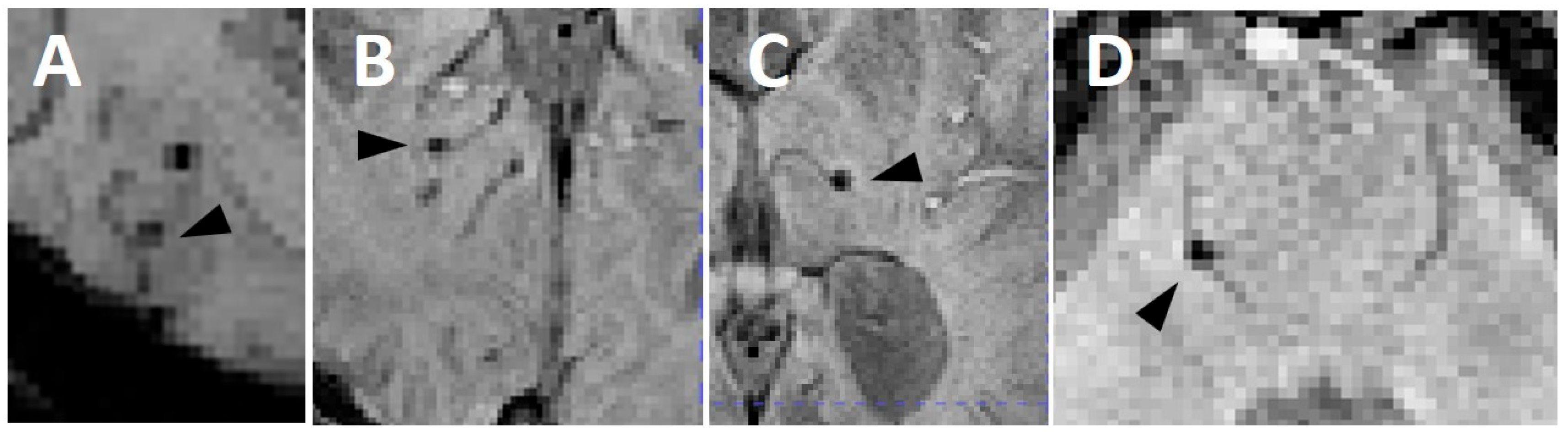
2.3. Image Acquisition and Interpretation
2.4. Clinical Parameters
2.5. Statistics
3. Results
3.1. Entire Cohort
3.2. Cerebral Microbleeds
3.3. AD vs. Healthy Controls
3.4. Cerebral Microbleeds with a Venous Connection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| APOE | Apoliptoprotein E |
| CAA | Cerebral amyloid angiopathy |
| CMB | Cerebral microbleed |
| CMBven | Cerebral microbleeds with a venous connection |
| CVSD | Cerebrovascular small vessel disease |
| MoCA | Montreal cognitive assessment |
| SWI | Susceptibility-weighted imaging |
References
- Zedde, M.; Grisendi, I.; Assenza, F.; Vandelli, G.; Napoli, M.; Moratti, C.; Lochner, P.; Seiffge, D.J.; Piazza, F.; Valzania, F.; et al. The Venular Side of Cerebral Amyloid Angiopathy: Proof of Concept of a Neglected Issue. Biomedicines 2023, 11, 2663. [Google Scholar] [CrossRef] [PubMed]
- Keith, J.; Gao, F.; Noor, R.; Kiss, A.; Balasubramaniam, G.; Au, K.; Rogaeva, E.; Masellis, M.; Black, S.E. Collagenosis of the Deep Medullary Veins: An Underrecognized Pathologic Correlate of White Matter Hyperintensities and Periventricular Infarction? J. Neuropathol. Exp. Neurol. 2017, 76, 299–312. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G.; Gurol, M.E.; Ayata, C.; Bacskai, B.J.; Frosch, M.P.; Viswanathan, A.; Greenberg, S.M. Emerging Concepts in Sporadic Cerebral Amyloid Angiopathy. Brain 2017, 140, 1829–1850. [Google Scholar] [CrossRef]
- Mendel, T.; Wierzba-Bobrowicz, T.; Stępień, T.; Szpak, G.M. Original Article β-Amyloid Deposits in Veins in Patients with Cerebral Amyloid Angiopathy and Intracerebral Haemorrhage. Folia Neuropathol. 2013, 2, 120–126. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G.; Frosch, M.P.; Baron, J.-C.; Pasi, M.; Albucher, J.F.; Banerjee, G.; Barbato, C.; Bonneville, F.; Brandner, S.; et al. The Boston Criteria Version 2.0 for Cerebral Amyloid Angiopathy: A Multicentre, Retrospective, MRI–Neuropathology Diagnostic Accuracy Study. Lancet Neurol. 2022, 21, 714–725. [Google Scholar] [CrossRef]
- Rotta, J.; Perosa, V.; Yakupov, R.; Kuijf, H.J.; Schreiber, F.; Dobisch, L.; Oltmer, J.; Assmann, A.; Speck, O.; Heinze, H.-J.; et al. Detection of Cerebral Microbleeds With Venous Connection at 7-Tesla MRI. Neurology 2021, 96, E2048–E2057. [Google Scholar] [CrossRef] [PubMed]
- LaMontagne, P.J.; Benzinger, T.L.S.; Morris, J.C.; Keefe, S.; Hornbeck, R.; Xiong, C.; Grant, E.; Hassenstab, J.; Moulder, K.; Vlassenko, A.G.; et al. OASIS-3: Longitudinal Neuroimaging, Clinical, and Cognitive Dataset for Normal Aging and Alzheimer Disease. 2019. Available online: https://www.medrxiv.org/content/10.1101/2019.12.13.19014902v1 (accessed on 6 August 2025).
- Gregoire, S.M.; Chaudhary, U.J.; Brown, M.M.; Yousry, T.A.; Kallis, C.; Jäger, H.R.; Werring, D.J. The Microbleed Anatomical Rating Scale (MARS). Neurology 2009, 73, 1759–1766. [Google Scholar] [CrossRef]
- Ayaz, M.; Boikov, A.; McAuley, G.; Schrag, M.; Kido, D.K.; Haacke, E.M.; Kirsch, W. Imaging Cerebral Microbleeds with SWI. In Susceptibility Weighted Imaging in MRI. Basic Concepts and Clinical Applications; Haacke, E.M., Reichenbach, J.R., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 191–214. [Google Scholar]
- Wang, Y.; Fan, X.; Ni, J.; Feng, F. Dilated Juxtacortical Perivascular Spaces and Venous Cerebral Microbleeds in Cerebral Amyloid Angiopathy-Related Inflammation. Ann. Neurol. 2023, 94, 605–607. [Google Scholar] [CrossRef]
- Kepp, K.P.; Robakis, N.K.; Høilund-Carlsen, P.F.; Sensi, S.L.; Vissel, B. The Amyloid Cascade Hypothesis: An Updated Critical Review. Brain 2023, 146, 3969–3990. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Bacskai, B.J.; Hernandez-Guillamon, M.; Pruzin, J.; Sperling, R.; van Veluw, S.J. Cerebral Amyloid Angiopathy and Alzheimer Disease—One Peptide, Two Pathways. Nat. Rev. Neurol. 2020, 16, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Griffin, W.S.T.; de Vos, R.A.I.; Ghebremedhin, E. Cerebral Amyloid Angiopathy and Its Relationship to Alzheimer’s Disease. Acta Neuropathol. 2008, 115, 599–609. [Google Scholar] [CrossRef]
- Cordonnier, C.; van der Flier, W.M. Brain Microbleeds and Alzheimer’s Disease: Innocent Observation or Key Player? Brain 2011, 134, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Moody, D.M.; Brown, W.R.; Challa, V.R.; Anderson, R.L. Periventricular Venous Collagenosis: Association with Leukoaraiosis. Radiology 1995, 194, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.O.; Massey, A.; Newman, T.A.; Hutchings, M.; Kuo, Y.-M.; Roher, A.E. Cerebral Amyloid Angiopathy. Am. J. Pathol. 1998, 153, 725–733. [Google Scholar] [CrossRef]
- Black, S.; Gao, F.; Bilbao, J. Understanding White Matter Disease. Stroke 2009, 40, S48–S52. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N. Linking Cerebrovascular Defense Mechanisms in Brain Ageing and Alzheimer’s Disease. Neurobiol. Aging 2009, 30, 1512–1514. [Google Scholar] [CrossRef]
- Morrone, C.D.; Bishay, J.; McLaurin, J. Potential Role of Venular Amyloid in Alzheimer’s Disease Pathogenesis. Int. J. Mol. Sci. 2020, 21, 1985. [Google Scholar] [CrossRef]
- Shih, A.Y.; Blinder, P.; Tsai, P.S.; Friedman, B.; Stanley, G.; Lyden, P.D.; Kleinfeld, D. The Smallest Stroke: Occlusion of One Penetrating Vessel Leads to Infarction and a Cognitive Deficit. Nat. Neurosci. 2013, 16, 55–63. [Google Scholar] [CrossRef]
- Bouvy, W.H.; Kuijf, H.J.; Zwanenburg, J.J.M.; Koek, H.L.; Kappelle, L.J.; Luijten, P.R.; Ikram, M.K.; Biessels, G.J. Abnormalities of Cerebral Deep Medullary Veins on 7 Tesla MRI in Amnestic Mild Cognitive Impairment and Early Alzheimer’s Disease: A Pilot Study. J. Alzheimer’s Dis. 2017, 57, 705–710. [Google Scholar] [CrossRef]
- Jochems, A.C.C.; Blair, G.W.; Stringer, M.S.; Thrippleton, M.J.; Clancy, U.; Chappell, F.M.; Brown, R.; Jaime Garcia, D.; Hamilton, O.K.L.; Morgan, A.G.; et al. Relationship Between Venules and Perivascular Spaces in Sporadic Small Vessel Diseases. Stroke 2020, 51, 1503–1506. [Google Scholar] [CrossRef]
- Okudera, T.; Huang, Y.P.; Fukusumi, A.; Nakamura, Y.; Hatazawa, J.; Uemura, K. Micro-Angiographical Studies of the Medullary Venous System of the Cerebral Hemisphere. Neuropathology 1999, 19, 93–111. [Google Scholar] [CrossRef] [PubMed]
- van Veluw, S.J.; Charidimou, A.; van der Kouwe, A.J.; Lauer, A.; Reijmer, Y.D.; Costantino, I.; Gurol, M.E.; Biessels, G.J.; Frosch, M.P.; Viswanathan, A.; et al. Microbleed and Microinfarct Detection in Amyloid Angiopathy: A High-Resolution MRI-Histopathology Study. Brain 2016, 139, 3151–3162. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T. Quantitative Susceptibility Mapping (QSM): Decoding MRI Data for a Tissue Magnetic Biomarker. Magn. Reson. Med. 2015, 73, 82–101. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wardlaw, J.M. Update on Cerebral Small Vessel Disease: A Dynamic Whole-Brain Disease. Stroke Vasc. Neurol. 2016, 1, 83–92. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 571) | AD (n = 140) | No AD (n = 431) | p-Value | |
|---|---|---|---|---|
| Age, mean ± SD | 69.3 ± 9.2 | 77 ± 6.7 years | 67 ± 8.7 years | <0.001 # |
| Female sex, n (%) | 339 (59.4) | 69 (49.3) | 270 (62.6) | 0.004 § |
| Race | ||||
| Asian, n (%) | 3 (0.5) | 0 (0) | 3 (0.7) | 1 * |
| Black, n (%) | 80 (14) | 14 (10) | 66 (15.3) | 0.11 § |
| White, n (%) | 487 (85.3) | 126 (90) | 361 (83.8) | 0.094 § |
| APOE | ||||
| ε2/2, n (%) | 3 (0.5) | 1 (0.7) | 2 (0.46) | 0.732 § |
| ε2/3, n (%) | 66 (11.5) | 13 (9.3) | 53 12.3) | 0.324 § |
| ε2/4, n (%) | 12 (2.1) | 1 (0.7) | 11 (2.6) | 0.185 § |
| ε3/3, n (%) | 296 (51.8) | 60 (42.9) | 236 (54.8) | 0.012 § |
| ε3/4, n (%) | 166 (29) | 56 (40) | 110 (25.5) | 0.001 § |
| ε4/4, n (%) | 25 (4.4) | 8 (5.7) | 17 (3.9) | 0.380 § |
| History of stroke/TIA, n (%) | 25 (4.4) | 10 (7.1) | 15 (3.5) | 0.065 § |
| Arterial hypertension, n (%) | 260 (45.5) | 73 (52.1) | 187 (43.4) | 0.07 § |
| Diabetes, n (%) | 55 (9.6) | 15 (10.7) | 40 (9.3) | 0.61 § |
| Hypercholesterinemia, n (%) | 263 (46.1) | 66 (47.1) | 197 (45.7) | 0.767 § |
| Antithrombotic treatment, n (%) | 291 (50.9) | 90 (64) | 201 (47) | <0.001 § |
| MoCA, median (IQR), range | 26 (23, 28), 7–30 | 21 (17.5, 24), 7–30 | 27 (25, 28), 15–30 | <0.001 $ |
| Total (n = 571) | AD (n = 140) | No AD (n = 431) | p-Value | |
|---|---|---|---|---|
| Persons with any CMB, n (%) | 116 (20.3) | 36 (25.7) | 80 (18.6) | 0.067 § |
| Total CMB, n | 367 | 151 | 216 | NA |
| CMB per person, median (IQR), range | 0 (0, 0), 0–28 | 0 (0, 1), 0–22 | 0 (0, 0), 0–28 | 0.034 $ |
| Deep CMB total, n (%) | 48 (13.1) | 33 (21.9) | 15 (6.9) | <0.001 § |
| Deep CMB per person, median (IQR), range | 0 (0, 0), 0–6 | 0 (0, 0), 0–6 | 0 (0, 0), 0–3 | <0.001 $ |
| Lobar CMB total, n (%) | 277 (75.5) | 103 (68.2) | 174 (80.5) | 0.006 § |
| Lobar CMB per person, median (IQR), range | 0 (0, 0), 0–28 | 0 (0, 0), 0–16 | 0 (0, 0), 0–28 | 0.124 $ |
| Persons with mixed CMB, n (%) | 15 (2.6) | 9 (6.4) | 6 (1.4) | 0.001 § |
| Cerebellar CMB total, n (%) | 40 (10.9) | 15 (9.9) | 25 (11.6) | 0.084 § |
| Cerebellar CMB per person, median (IQR), range | 0 (0, 0), 0–13 | 0 (0, 0), 0–3 | 0 (0, 0), 0–13 | 0.029 $ |
| Persons with any CMBven, n (%) | 26 (4.5) | 10 (6.6) | 16 (7.4) | 0.773 § |
| Total CMBven, n (%) | 40 (10.9) | 12 (7.9) | 28 (12.9) | 0.403 § |
| Deep CMBven, n (%) | 2 (0.5) | 2 (1.3) | 0 (0) | 0.059 * |
| Deep CMBven per person, median (IQR), range | 0 (0, 0), 0–1 | 0 (0, 0), 0–1 | 0 (0, 0), 0 | 0.013 $ |
| Lobar CMBven, n (%) | 37 (10.1) | 10 (6.6) | 27 (12.5) | 0.713 § |
| Lobar CMBven per person, median (IQR), range | 0 (0, 0), 0–4 | 0 (0, 0), 0–2 | 0 (0, 0), 0–4 | 0.181 $ |
| Cerebellar CMBven, n (%) | 1 (0.3) | 0 (0) | 1 (0.5) | 1 * |
| Cerebellar CMBven per person, median (IQR), range | 0 (0, 0), 0–1 | 0 (0, 0), 0 | 0 (0, 0), 0–1 | 0.571 $ |
| CMBven Present (n = 26) | CMBven Absent (n = 545) | p-Value | |
|---|---|---|---|
| Age, mean ± SD | 72.3 ± 8.8 years | 68.9 ± 9.2 | 0.069 # |
| Female sex, n (%) | 9 (35) | 324 (59.4) | <0.001 § |
| Race | |||
| Asian, n (%) | 0 (0) | 3 (0.5) | 1 * |
| Black, n (%) | 2 (7.7) | 76 (13.9) | 0.364 § |
| White, n (%) | 24 (92.3) | 465 (85.3) | 0.320 § |
| APOE | |||
| ε2/2, n (%) | 1 (3.8) | 2 (0.55) | 0.016 § |
| ε2/3, n (%) | 2 (7.7) | 63 (11.6) | 0.544 § |
| ε2/4, n (%) | 0 (0) | 12 (2.2) | 1 * |
| ε3/3, n (%) | 16 (62) | 288 (52.8) | 0.385 § |
| ε3/4, n (%) | 5 (19.2) | 153 (28) | 0.324 § |
| ε4/4, n (%) | 2 (7.7) | 24 (4.4) | 0.431 § |
| History of stroke/TIA, n (%) | 2 (7.7) | 23 (4.2) | 0.397 § |
| Arterial hypertension, n (%) | 10 (38.4) | 248 (45.5) | 0.480 § |
| Diabetes, n (%) | 3 (11.5) | 52 (9.5) | 0.735 § |
| Hypercholesterinemia, n (%) | 9 (34.6) | 248 (45.5) | 0.275 § |
| Antithrombotic treatment, n (%) | 19 (73) | 274 (50.2) | 0.005 § |
| MoCA, median (IQR), range | 24 (20, 25.75), 8–28 | 26 (24, 28), 7–30 | 0.014 $ |
| CMB per person, median (IQR), range | 3.5 (2, 5), 1–28 | 0 (0, 0), 0–21 | <0.001 $ |
| Variable | OR (95% CI) | p-Value |
|---|---|---|
| Total CMB number | 1.351 (1.161–1.688) | <0.0014 * |
| Gender | 1.427 (0.392–5.419) | 0.585 |
| APOE ε genotype | 1.558 (0.002–92.812) | 0.874 |
| MoCA score | 0.862 (0.762–0.982) | 0.018 * |
| Age | 0.968 (0.899–1.045) | 0.397 |
| Antithrombotic treatment | 0.916 (0.246–3.470) | 0.894 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jensen-Kondering, U.; Kuhn, V.; Schacht, H.; Neumann, A.; Royl, G.; Schramm, P. Cerebral Microbleeds with a Venous Connection on 3 Tesla Susceptibility-Weighted Imaging in Persons with Alzheimer’s Disease and Healthy Aging Controls. Brain Sci. 2025, 15, 851. https://doi.org/10.3390/brainsci15080851
Jensen-Kondering U, Kuhn V, Schacht H, Neumann A, Royl G, Schramm P. Cerebral Microbleeds with a Venous Connection on 3 Tesla Susceptibility-Weighted Imaging in Persons with Alzheimer’s Disease and Healthy Aging Controls. Brain Sciences. 2025; 15(8):851. https://doi.org/10.3390/brainsci15080851
Chicago/Turabian StyleJensen-Kondering, Ulf, Veronique Kuhn, Hannes Schacht, Alexander Neumann, Georg Royl, and Peter Schramm. 2025. "Cerebral Microbleeds with a Venous Connection on 3 Tesla Susceptibility-Weighted Imaging in Persons with Alzheimer’s Disease and Healthy Aging Controls" Brain Sciences 15, no. 8: 851. https://doi.org/10.3390/brainsci15080851
APA StyleJensen-Kondering, U., Kuhn, V., Schacht, H., Neumann, A., Royl, G., & Schramm, P. (2025). Cerebral Microbleeds with a Venous Connection on 3 Tesla Susceptibility-Weighted Imaging in Persons with Alzheimer’s Disease and Healthy Aging Controls. Brain Sciences, 15(8), 851. https://doi.org/10.3390/brainsci15080851






