Contralateral Structure and Molecular Response to Severe Unilateral Brain Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Right Motor Cortex Resection
2.3. Magnetic Resonance Imaging (MRI)
2.4. RNA Extraction
2.5. mRNA Library Construction and Sequencing
2.6. Data Processing and Analysis
2.7. Differentially Expressed Gene (DEG) Analysis
2.8. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analysis
2.9. Gene Set Enrichment Analysis (GSEA)
2.10. Transcription Factor (TF) Analysis and Hub Genes with Protein–Protein Network Analysis
2.11. Golgi Staining
2.12. Electron Microscopy (EM)
2.13. Immunofluorescence Staining
2.14. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR)
| Cacna1c: | forward 5′-AAGCGGCAGCAGTATGGGAAAC-3′, |
| reverse 5′-TCAGAGTCAGGCAGAGCAGAGC-3′ | |
| Slc2a1: | forward 5′- CATCCACCACACTCACCACACTC-3′, |
| reverse 5′-GCCTGCCAAAGCGATTAACAAAGAG-3′ | |
| Tgfb1: | forward 5′- GACCGCAACAACGCAATCTATGAC-3′, |
| reverse 5′-CTGGCACTGCTTCCCGAATGTC-3′ | |
| β-Actin: | forward 5′- TGTCACCAACTGGGACGATA-3′, |
| reverse 5′- GGGGTGTTGAAGGTCTCAAA-3′ |
2.15. Western Blot (WB) Analysis
3. Results
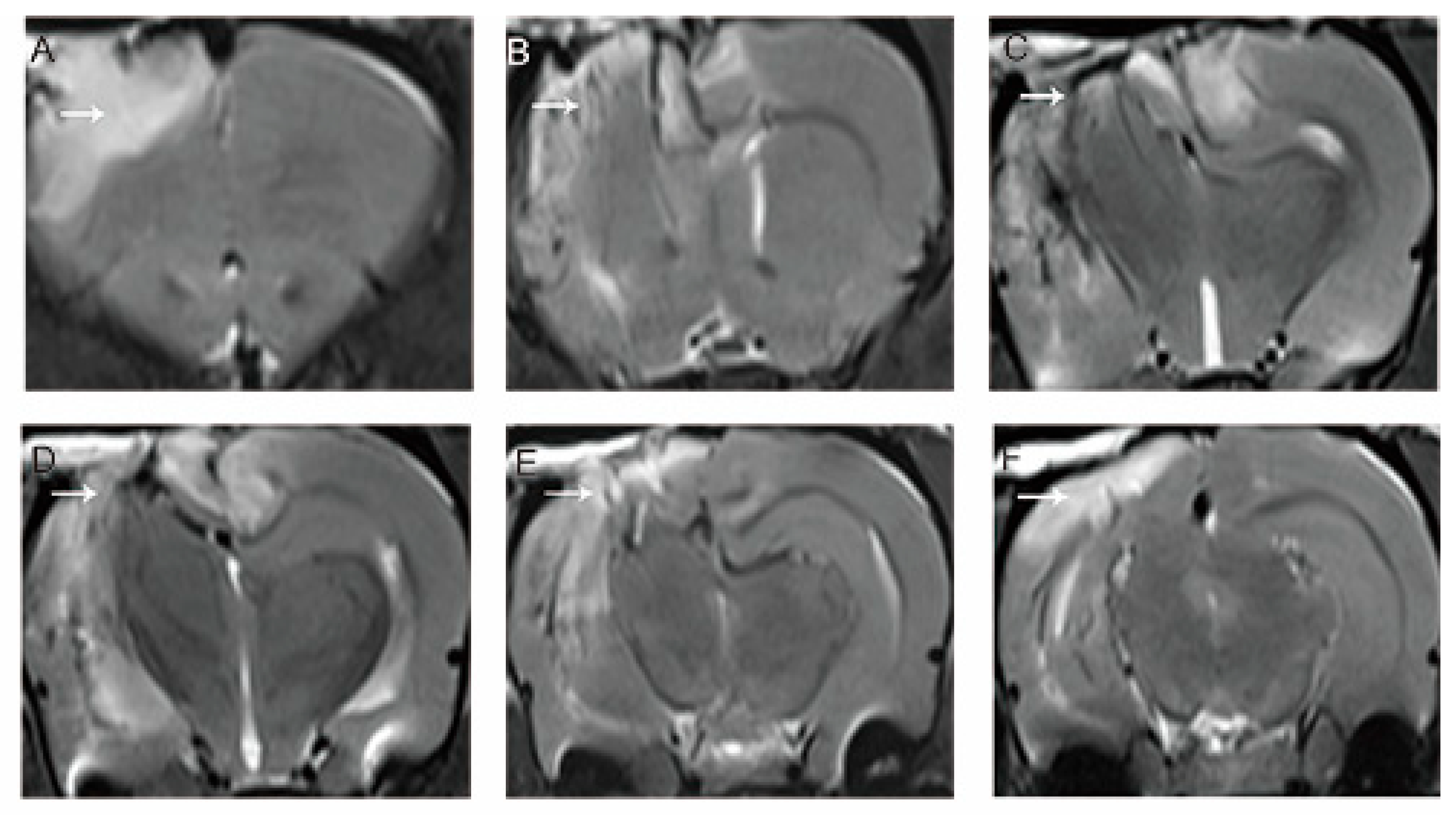
3.1. MRI Evaluation of the Model
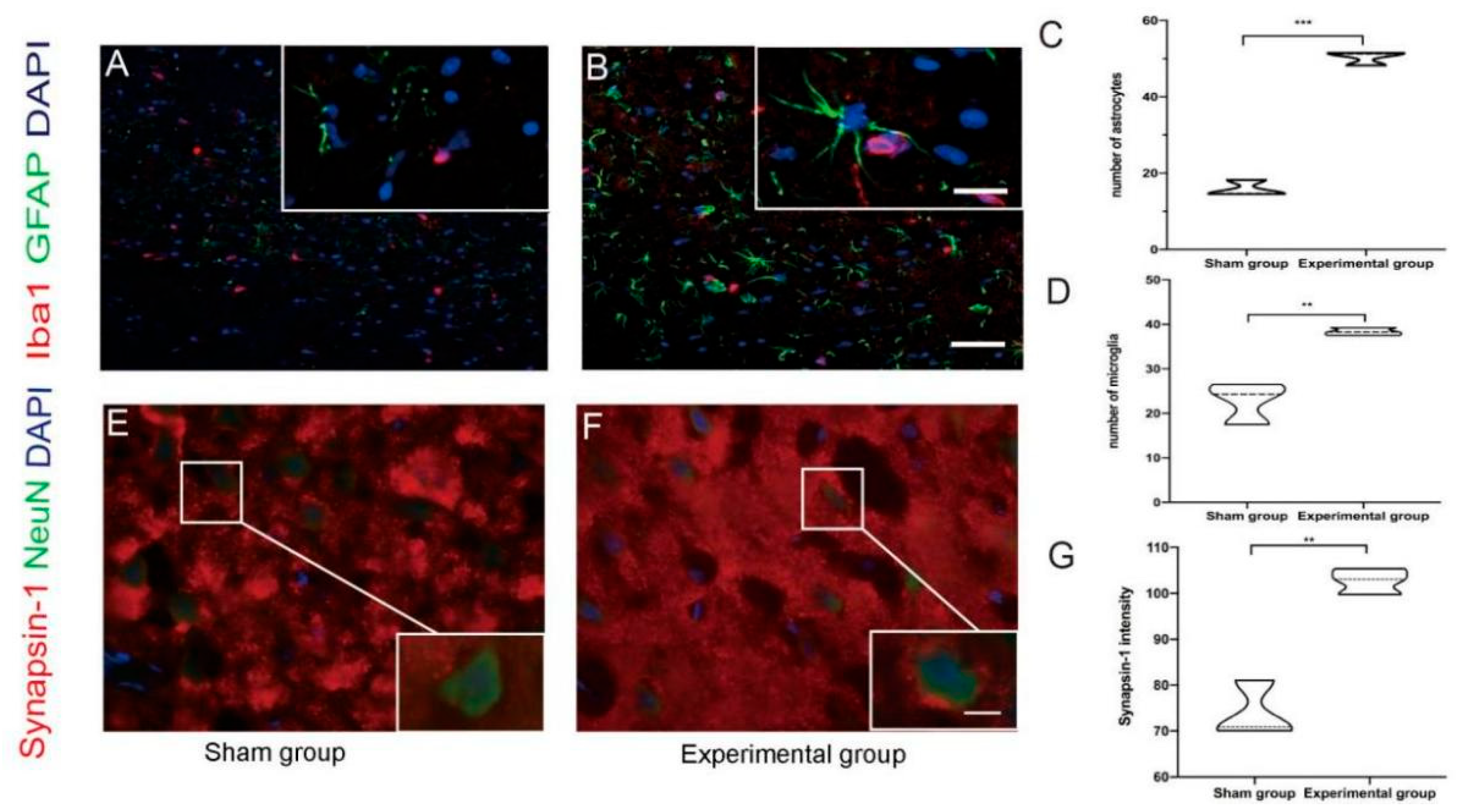
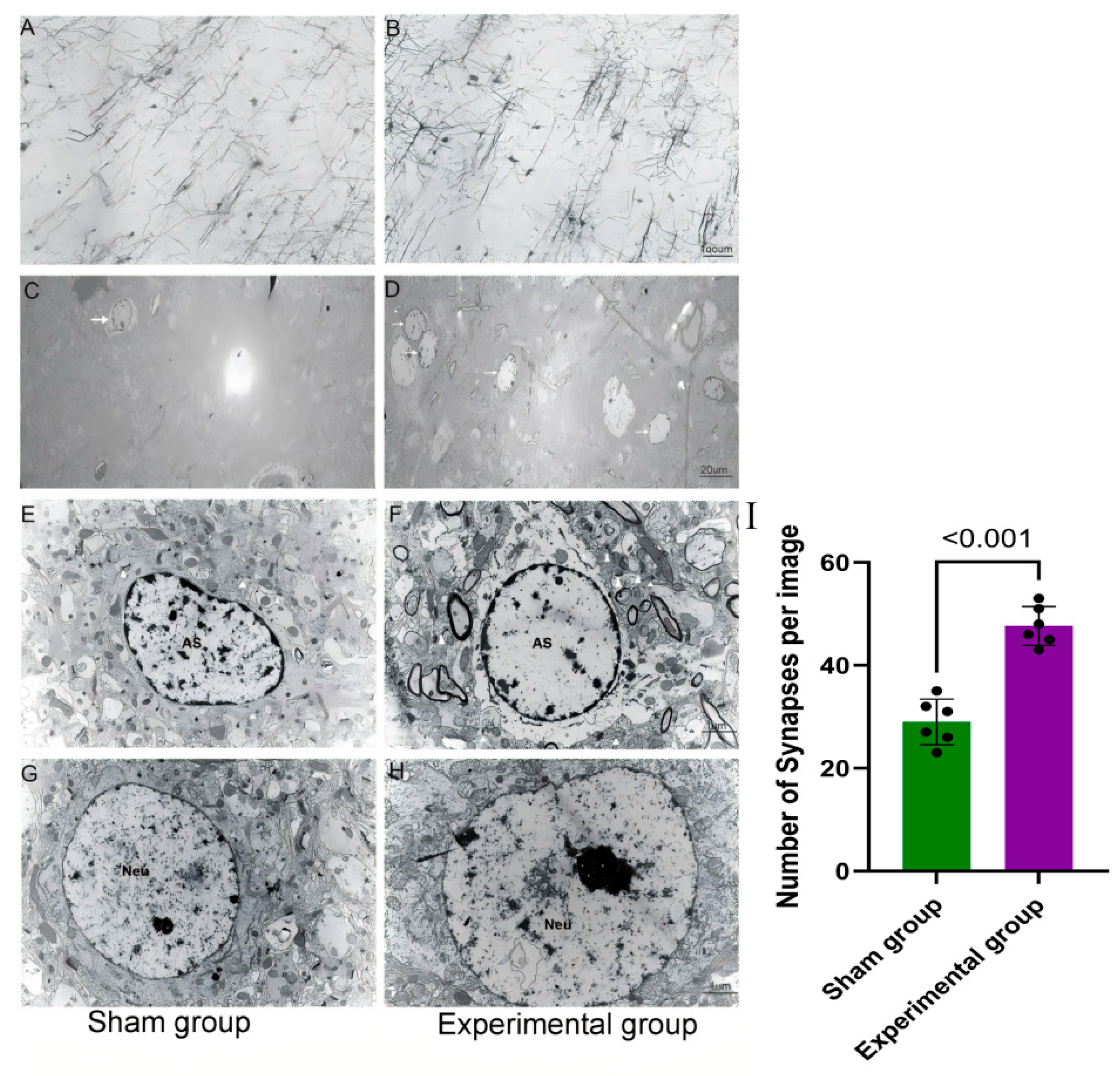
3.2. Right Motor Cortex Resection Resulted in Structural and Molecular Changes in the Contralateral Motor Cortex
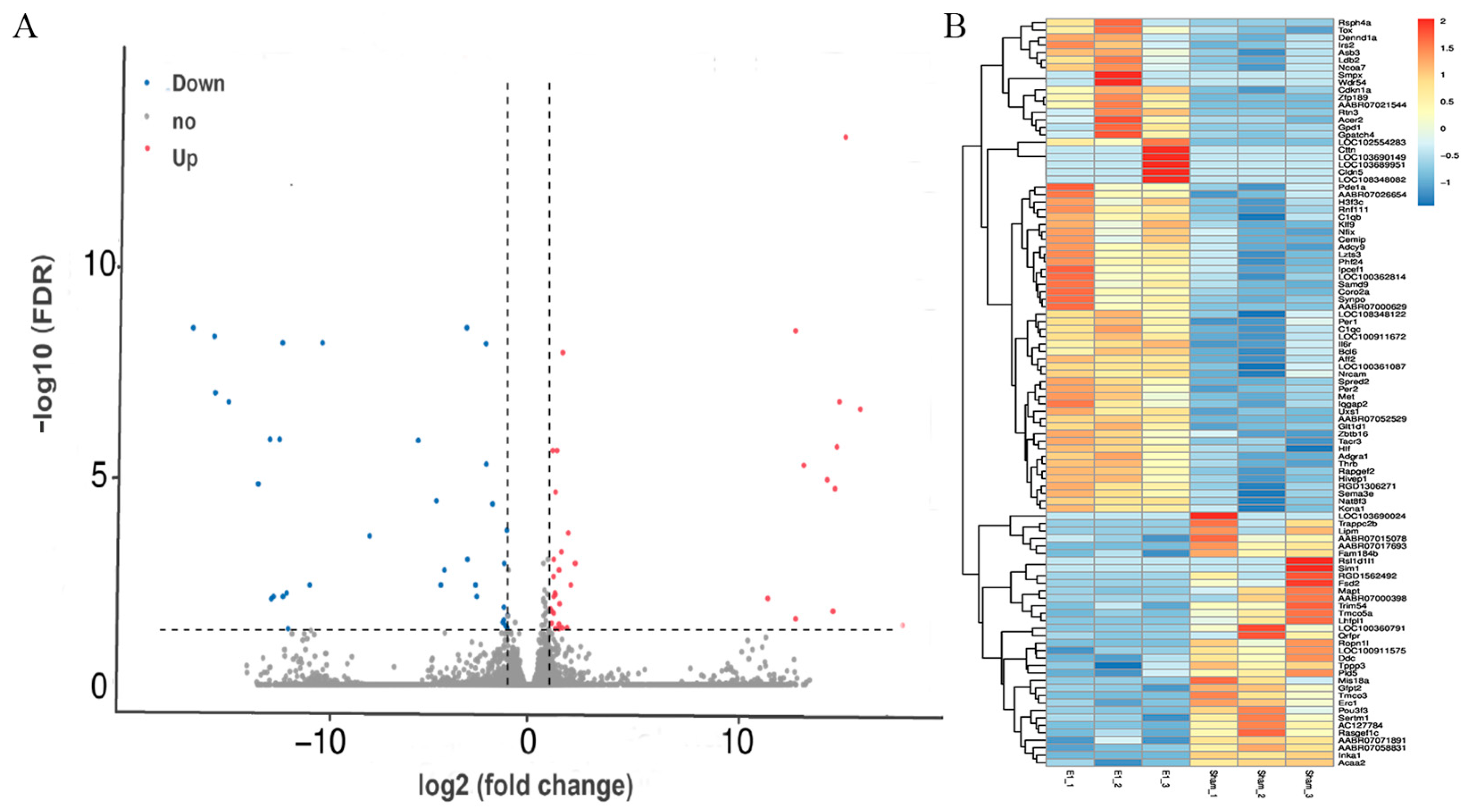
3.3. Gene Expression Changed in the Contralateral Motor Cortex 7 Days After Right Motor Cortex Resection
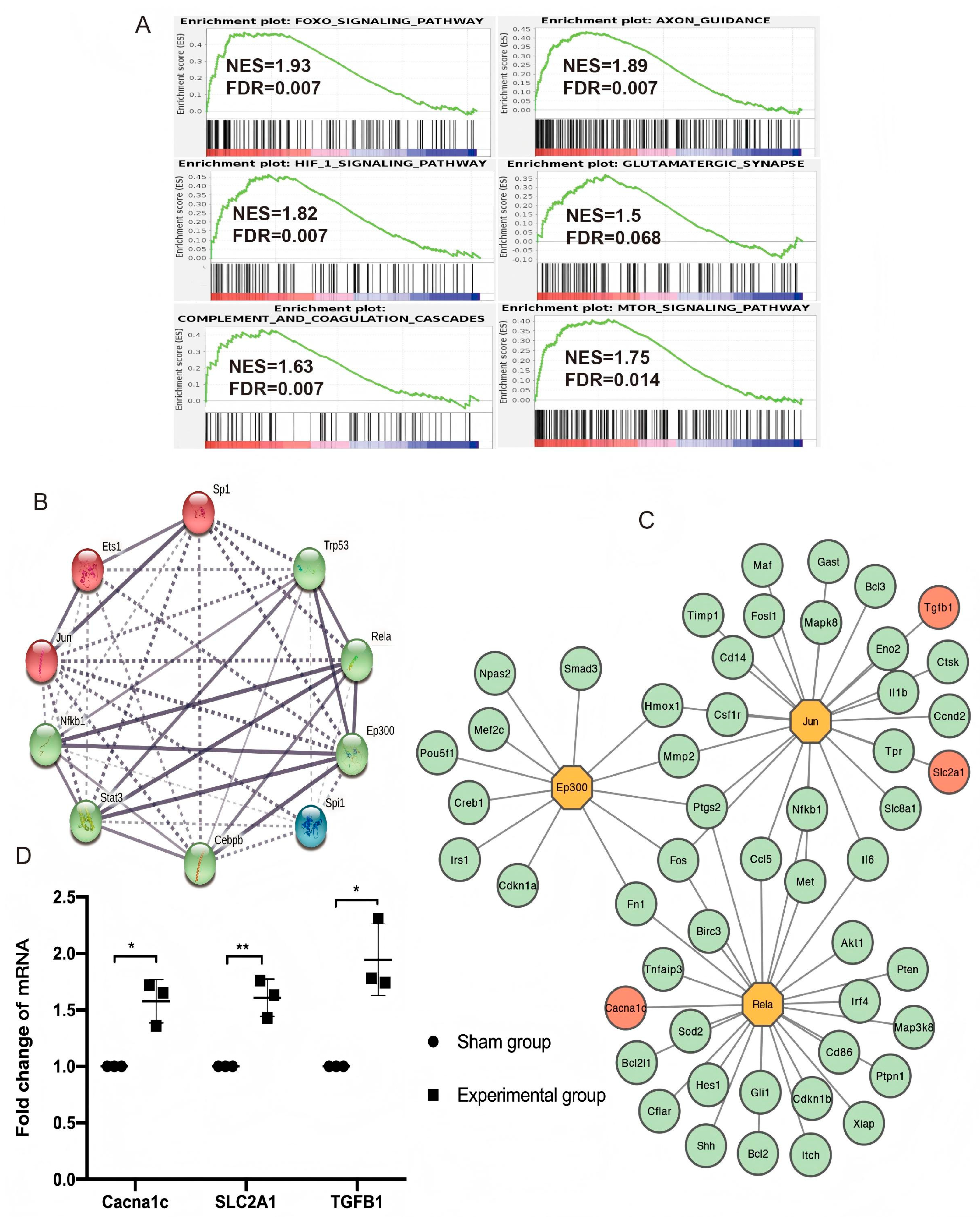
3.4. Enriched Pathways in Gene Set Enrichment Analysis (GSEA)
3.5. TF Regulatory Network Analysis and Hub Gene Identification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. N. Am. 2020, 104, 213–238. [Google Scholar] [CrossRef]
- Marklund, N.; Bellander, B.M.; Godbolt, A.K.; Levin, H.; McCrory, P.; Thelin, E.P. Treatments and rehabilitation in the acute and chronic state of traumatic brain injury. J. Intern. Med. 2019, 285, 608–623. [Google Scholar] [CrossRef]
- Thapa, K.; Khan, H.; Singh, T.G.; Kaur, A. Traumatic Brain Injury: Mechanistic Insight on Pathophysiology and Potential Therapeutic Targets. J. Mol. Neurosci. 2021, 71, 1725–1742. [Google Scholar] [CrossRef]
- Javalgekar, M.; Jupp, B.; Vivash, L.; O’Brien, T.J.; Wright, D.K.; Jones, N.C.; Ali, I. Inflammasomes at the crossroads of traumatic brain injury and post-traumatic epilepsy. J. Neuroinflamm. 2024, 21, 172. [Google Scholar] [CrossRef]
- Godoy, D.A.; Fossi, F.; Robba, C. Neuroworsening in Moderate Traumatic Brain Injury. Neurol. Clin. 2025, 43, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Brett, B.L.; Gardner, R.C.; Godbout, J.; Dams-O’Connor, K.; Keene, C.D. Traumatic Brain Injury and Risk of Neurodegenerative Disorder. Biol. Psychiatry 2022, 91, 498–507. [Google Scholar] [CrossRef]
- Mira, R.G.; Lira, M.; Cerpa, W. Traumatic Brain Injury: Mechanisms of Glial Response. Front. Physiol. 2021, 12, 740939. [Google Scholar] [CrossRef]
- Jamjoom, A.A.B.; Rhodes, J.; Andrews, P.J.D.; Grant, S.G.N. The synapse in traumatic brain injury. Brain 2021, 144, 18–31. [Google Scholar] [CrossRef]
- Rosa, J.M.; Farré-Alins, V.; Ortega, M.C.; Navarrete, M.; Lopez-Rodriguez, A.B.; Palomino-Antolín, A.; Fernández-López, E.; Vila-Del Sol, V.; Decouty, C.; Narros-Fernández, P.; et al. TLR4 pathway impairs synaptic number and cerebrovascular functions through astrocyte activation following traumatic brain injury. Br. J. Pharmacol. 2021, 178, 3395–3413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Malik, A.; Choi, H.B.; Ko, R.W.; Dissing-Olesen, L.; MacVicar, B.A. Microglial CR3 activation triggers long-term synaptic depression in the hippocampus via NADPH oxidase. Neuron 2014, 82, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Krukowski, K.; Chou, A.; Feng, X.; Tiret, B.; Paladini, M.S.; Riparip, L.K.; Chaumeil, M.M.; Lemere, C.; Rosi, S. Traumatic Brain Injury in Aged Mice Induces Chronic Microglia Activation, Synapse Loss, and Complement-Dependent Memory Deficits. Int. J. Mol. Sci. 2018, 19, 3753. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.J.; Tapanes, S.A.; Loris, Z.B.; Balu, D.T.; Sick, T.J.; Coyle, J.T.; Liebl, D.J. Enhanced astrocytic d-serine underlies synaptic damage after traumatic brain injury. J. Clin. Investig. 2017, 127, 3114–3125. [Google Scholar] [CrossRef]
- Nikolakopoulou, A.M.; Koeppen, J.; Garcia, M.; Leish, J.; Obenaus, A.; Ethell, I.M. Astrocytic Ephrin-B1 Regulates Synapse Remodeling Following Traumatic Brain Injury. ASN Neuro 2016, 8, 1759091416630220. [Google Scholar] [CrossRef]
- Shu, H.; Parada, I.; Delgado, A.; Prince, D.A.; Gu, F. Increased excitatory connectivity and epileptiform activity in thrombospondin1/2 knockout mice following cortical trauma. Neurobiol. Dis. 2024, 200, 106634. [Google Scholar] [CrossRef]
- Furman, J.L.; Sompol, P.; Kraner, S.D.; Pleiss, M.M.; Putman, E.J.; Dunkerson, J.; Mohmmad Abdul, H.; Roberts, K.N.; Scheff, S.W.; Norris, C.M. Blockade of Astrocytic Calcineurin/NFAT Signaling Helps to Normalize Hippocampal Synaptic Function and Plasticity in a Rat Model of Traumatic Brain Injury. J. Neurosci. 2016, 36, 1502–1515. [Google Scholar] [CrossRef]
- Axelson, H.W.; Winkler, T.; Flygt, J.; Djupsjö, A.; Hånell, A.; Marklund, N. Plasticity of the contralateral motor cortex following focal traumatic brain injury in the rat. Restor. Neurol. Neurosci. 2013, 31, 73–85. [Google Scholar] [CrossRef]
- Watanabe, H.; Nosova, O.; Sarkisyan, D.; Storm Andersen, M.; Carvalho, L.; Galatenko, V.; Bazov, I.; Lukoyanov, N.; Maia, G.H.; Hallberg, M.; et al. Left-Right Side-Specific Neuropeptide Mechanism Mediates Contralateral Responses to a Unilateral Brain Injury. Eneuro 2021, 8, ENEURO.0548-20.2021. [Google Scholar] [CrossRef]
- Empl, L.; Chovsepian, A.; Chahin, M.; Kan, W.Y.V.; Fourneau, J.; Van Steenbergen, V.; Weidinger, S.; Marcantoni, M.; Ghanem, A.; Bradley, P.; et al. Selective plasticity of callosal neurons in the adult contralesional cortex following murine traumatic brain injury. Nat. Commun. 2022, 13, 2659. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Dong, J.; Ge, Q.; Zhang, B.; Yang, M.; Lu, S.; Li, H.; Niu, F.; Xu, X.; Liu, B. Contralateral synaptic changes following severe unilateral brain injury. Brain Res. Bull. 2022, 188, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Du, F. Golgi-Cox Staining of Neuronal Dendrites and Dendritic Spines with FD Rapid GolgiStain™ Kit. Curr. Protoc. Neurosci. 2019, 88, e69. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Xu, X.; Li, H.; Niu, F.; Yang, M.; Ge, Q.; Lu, S.; Deng, Y.; Wu, H.; Zhang, B.; et al. Megf10-related engulfment of excitatory postsynapses by astrocytes following severe brain injury. CNS Neurosci. Ther. 2023, 29, 2873–2883. [Google Scholar] [CrossRef] [PubMed]
- Francois, M.; Donovan, P.; Fontaine, F. Modulating transcription factor activity: Interfering with protein-protein interaction networks. Semin. Cell Dev. Biol. 2020, 99, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Buzzi, R.M.; Akeret, K.; Schwendinger, N.; Klohs, J.; Vallelian, F.; Hugelshofer, M.; Schaer, D.J. Spatial transcriptome analysis defines heme as a hemopexin-targetable inflammatoxin in the brain. Free Radic. Biol. Med. 2022, 179, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Castranio, E.L.; Wolfe, C.M.; Nam, K.N.; Letronne, F.; Fitz, N.F.; Lefterov, I.; Koldamova, R. ABCA1 haplodeficiency affects the brain transcriptome following traumatic brain injury in mice expressing human APOE isoforms. Acta. Neuropathol. Commun. 2018, 6, 69. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Yu, P.; Shang, X.; Lu, Y.; Li, Y. Transcriptome analysis reveals the mechanism of common carp brain injury after exposure to lead. Sci. Total Environ. 2020, 743, 140796. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liao, X.; Niu, F.; Li, M.; Yan, Y.; He, C.; Wu, X.; Tian, R.; Gao, G. Single-nucleus and spatial signatures of the brainstem in normal brain and mild traumatic brain injury in male mice. Nat. Commun. 2025, 16, 5082. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, X.; Liu, Q.; Xie, Y.; Shi, X.; Chen, J.; Li, Y.; Guo, H.; Sun, R.; Hong, Y.; et al. Microglial TREM-1 receptor mediates neuroinflammatory injury via interaction with SYK in experimental ischemic stroke. Cell Death Dis. 2019, 10, 555. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Wang, R.; Jiang, M.; Ye, Q.; Smith, A.D.; Chen, J.; Shi, Y. Macrophages reprogram after ischemic stroke and promote efferocytosis and inflammation resolution in the mouse brain. CNS Neurosci. Ther. 2019, 25, 1329–1342. [Google Scholar] [CrossRef]
- Todd, B.P.; Chimenti, M.S.; Luo, Z.; Ferguson, P.J.; Bassuk, A.G.; Newell, E.A. Traumatic brain injury results in unique microglial and astrocyte transcriptomes enriched for type I interferon response. J. Neuroinflamm. 2021, 18, 151. [Google Scholar] [CrossRef]
- Toutonji, A.; Mandava, M.; Guglietta, S.; Tomlinson, S. Chronic complement dysregulation drives neuroinflammation after traumatic brain injury: A transcriptomic study. Acta. Neuropathol. Commun. 2021, 9, 126. [Google Scholar] [CrossRef]
- Shi, X.; Luo, L.; Wang, J.; Shen, H.; Li, Y.; Mamtilahun, M.; Liu, C.; Shi, R.; Lee, J.H.; Tian, H.; et al. Stroke subtype-dependent synapse elimination by reactive gliosis in mice. Nat. Commun. 2021, 12, 6943. [Google Scholar] [CrossRef]
- Chung, W.S.; Baldwin, K.T.; Allen, N.J. Astrocyte Regulation of Synapse Formation, Maturation, and Elimination. Cold Spring Harb. Perspect. Biol. 2024, 16, a041352. [Google Scholar] [CrossRef]
- Smith, A.M.; Taylor, E.B.; Brooks, R.J.; Dos Santos, E.S.C.; Grayson, B.E. Cerebral and Peripheral Immune Cell Changes following Rodent Juvenile Traumatic Brain Injury. Brain Sci. 2024, 14, 398. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Han, L.; Zheng, N.; Wang, T.; Xu, H.; Jiang, Y.; Wang, Z.; Liu, Z.; Zheng, Q.; Zhang, X.; et al. Microglial Tmem59 Deficiency Impairs Phagocytosis of Synapse and Leads to Autism-Like Behaviors in Mice. J. Neurosci. 2022, 42, 4958–4979. [Google Scholar] [CrossRef]
- White, T.E.; Ford, G.D.; Surles-Zeigler, M.C.; Gates, A.S.; Laplaca, M.C.; Ford, B.D. Gene expression patterns following unilateral traumatic brain injury reveals a local pro-inflammatory and remote anti-inflammatory response. BMC Genom. 2013, 14, 282. [Google Scholar] [CrossRef]
- Fury, W.; Park, K.W.; Wu, Z.; Kim, E.; Woo, M.S.; Bai, Y.; Macdonald, L.E.; Croll, S.D.; Cho, S. Sustained Increases in Immune Transcripts and Immune Cell Trafficking During the Recovery of Experimental Brain Ischemia. Stroke 2020, 51, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, W.; Zhang, H.M.; Xie, G.Y.; Miao, Y.R.; Xia, M.; Guo, A.Y. hTFtarget: A Comprehensive Database for Regulations of Human Transcription Factors and Their Targets. Genom. Proteom. Bioinform. 2020, 18, 120–128. [Google Scholar] [CrossRef]
- Swanson, L.C.; Trujillo, E.A.; Thiede, G.H.; Katzenberger, R.J.; Shishkova, E.; Coon, J.J.; Ganetzky, B.; Wassarman, D.A. Survival Following Traumatic Brain Injury in Drosophila Is Increased by Heterozygosity for a Mutation of the NF-κB Innate Immune Response Transcription Factor Relish. Genetics 2020, 216, 1117–1136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, D.; Li, H.; Yan, H.; Zhang, Z.; Zhou, C.; Chen, Q.; Ye, Z.; Hang, C. Biphasic activation of nuclear factor-κB and expression of p65 and c-Rel following traumatic neuronal injury. Int. J. Mol. Med. 2018, 41, 3203–3210. [Google Scholar] [CrossRef]
- Kumar, S.; Fritz, Z.; Sulakhiya, K.; Theis, T.; Berthiaume, F. Transcriptional Factors and Protein Biomarkers as Target Therapeutics in Traumatic Spinal Cord and Brain Injury. Curr. Neuropharmacol. 2020, 18, 1092–1105. [Google Scholar] [CrossRef]
- Rehman, S.U.; Ahmad, A.; Yoon, G.H.; Khan, M.; Abid, M.N.; Kim, M.O. Inhibition of c-Jun N-Terminal Kinase Protects Against Brain Damage and Improves Learning and Memory After Traumatic Brain Injury in Adult Mice. Cereb. Cortex 2018, 28, 2854–2872. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Dai, Q.; Han, K.; Hong, W.; Jia, D.; Mo, Y.; Lv, Y.; Tang, H.; Fu, H.; Geng, W. JNK-IN-8, a c-Jun N-terminal kinase inhibitor, improves functional recovery through suppressing neuroinflammation in ischemic stroke. J. Cell Physiol. 2020, 235, 2792–2799. [Google Scholar] [CrossRef]
- Cheng, H.; Men, Y.; An, Y.; Yu, J.; Zhang, G.; Li, J.; Wang, X.; Sun, G.; Wu, Y. Overexpression of endothelial S1pr2 promotes blood-brain barrier disruption via JNK/c-Jun/MMP-9 pathway after traumatic brain injury in both in vivo and in vitro models. Front. Pharmacol. 2024, 15, 1448570. [Google Scholar] [CrossRef]
- Yang, M.; Bai, M.; Zhuang, Y.; Lu, S.; Ge, Q.; Li, H.; Deng, Y.; Wu, H.; Xu, X.; Niu, F.; et al. High-dose dexamethasone regulates microglial polarization via the GR/JAK1/STAT3 signaling pathway after traumatic brain injury. Neural Regen. Res. 2025, 20, 2611–2623. [Google Scholar] [CrossRef]
- Tyzack, G.E.; Sitnikov, S.; Barson, D.; Adams-Carr, K.L.; Lau, N.K.; Kwok, J.C.; Zhao, C.; Franklin, R.J.; Karadottir, R.T.; Fawcett, J.W.; et al. Astrocyte response to motor neuron injury promotes structural synaptic plasticity via STAT3-regulated TSP-1 expression. Nat. Commun. 2014, 5, 4294. [Google Scholar] [CrossRef]
- Wu, Q.; Miao, X.; Zhang, J.; Xiang, L.; Li, X.; Bao, X.; Du, S.; Wang, M.; Miao, S.; Fan, Y.; et al. Astrocytic YAP protects the optic nerve and retina in an experimental autoimmune encephalomyelitis model through TGF-β signaling. Theranostics 2021, 11, 8480–8499. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Weaver, A.M. Astrocyte-derived small extracellular vesicles promote synapse formation via fibulin-2-mediated TGF-β signaling. Cell Rep. 2021, 34, 108829. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, X.; Xu, X.; Li, M.; Tian, R.; Zhuang, Y.; Gao, G. Contralateral Structure and Molecular Response to Severe Unilateral Brain Injury. Brain Sci. 2025, 15, 837. https://doi.org/10.3390/brainsci15080837
Liao X, Xu X, Li M, Tian R, Zhuang Y, Gao G. Contralateral Structure and Molecular Response to Severe Unilateral Brain Injury. Brain Sciences. 2025; 15(8):837. https://doi.org/10.3390/brainsci15080837
Chicago/Turabian StyleLiao, Xixian, Xiaojian Xu, Ming Li, Runfa Tian, Yuan Zhuang, and Guoyi Gao. 2025. "Contralateral Structure and Molecular Response to Severe Unilateral Brain Injury" Brain Sciences 15, no. 8: 837. https://doi.org/10.3390/brainsci15080837
APA StyleLiao, X., Xu, X., Li, M., Tian, R., Zhuang, Y., & Gao, G. (2025). Contralateral Structure and Molecular Response to Severe Unilateral Brain Injury. Brain Sciences, 15(8), 837. https://doi.org/10.3390/brainsci15080837








