Musical Distractions: Music-Based Rhythmic Auditory Stimulation Fails to Improve Gait in Huntington’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Protocol
- Pre Uncued (PRE): The first uncued walking trials were used as the baseline value for all participants. The cadence of steady-state walking, as determined by APDM Mobility Lab software, for each trial was averaged together and multiplied by 1.10 to represent each participant’s individualized cueing tempo for MUSIC and SING tasks.
- Instructions: “…walk at your comfortable pace”.
- Cognitive Dual Task (DT): Direct comparison between baseline walking and DT can be found in [36]. For this study, the verbal fluency cognitive dual task was included as a comparison to the cueing paradigms.
- Instructions: “Name as many words as you can that start with a specific letter while you walk”. No specific instruction for the prioritization of either task was provided.
- 110% Musical Cue (MUSIC): Participants listened to one round of the song at their individualized tempo. After an auditory signal to begin walking, the same song continued to loop continuously for 30 s.
- Instructions: “After one verse, the music will keep playing and you can begin walking. Keep walking on the beat until the music stops”.
- 110% Singing Cue (SING): Similar to MUSIC, participants listened to one round of the song at their individualized tempo. After the auditory signal to begin walking, the music stopped, and participants were asked to begin singing aloud and walking to the beat of their own singing, trying to match the same tempo they had just heard. No specific instructions for prioritization of either singing or walking were provided.
- Instructions: “When the music stops, start singing the song and walking to the beat. Keep walking and singing until the tone sounds”.
- Post Uncued (POST): One final set of three trials was conducted to investigate the effects of the cueing on comfortable pace walking, as results may assist in understanding the role that fatigue may play in this population.
- Instructions: “…you will again walk at your comfortable walking pace”.
2.3. Statistical Analysis
3. Results
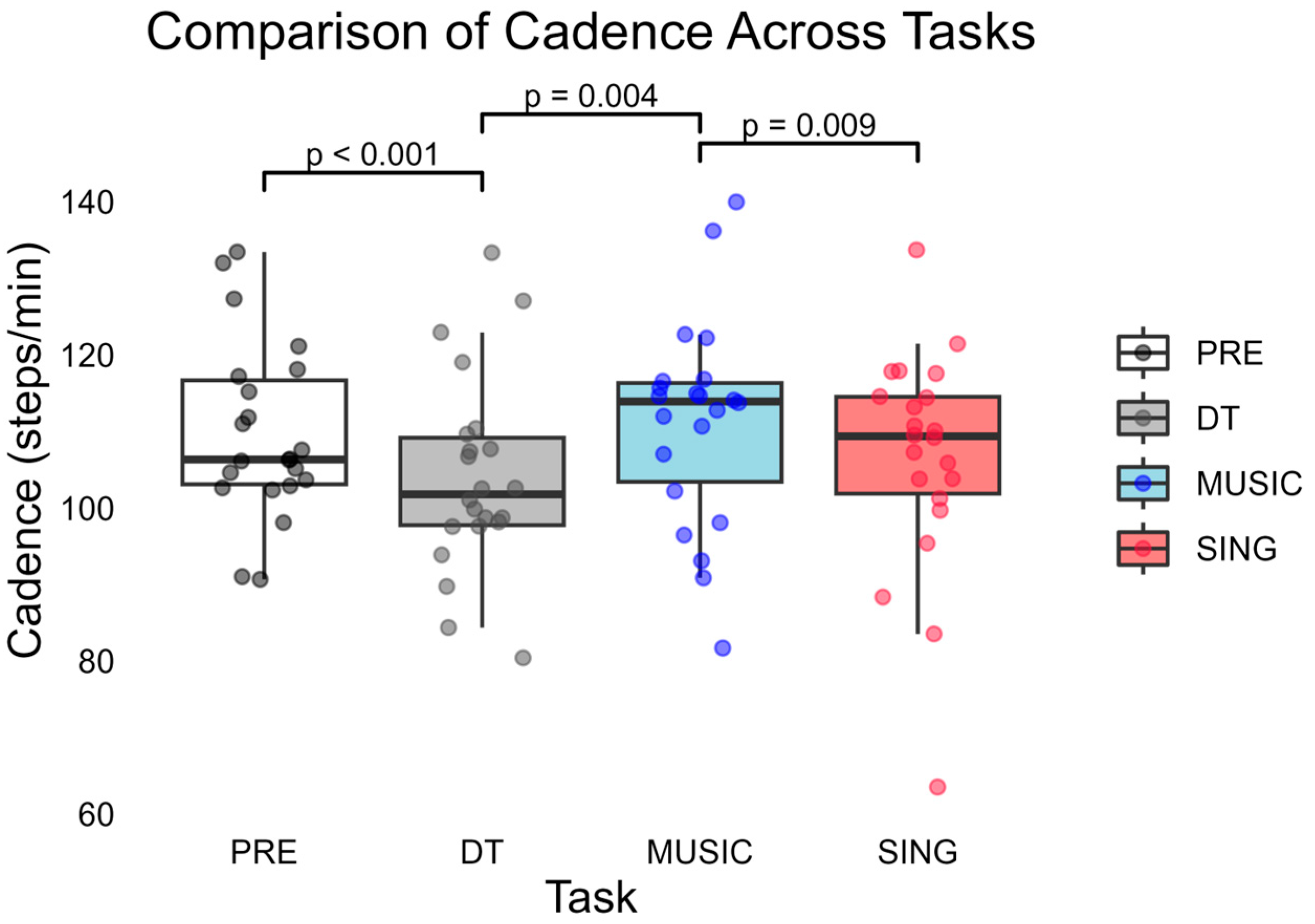
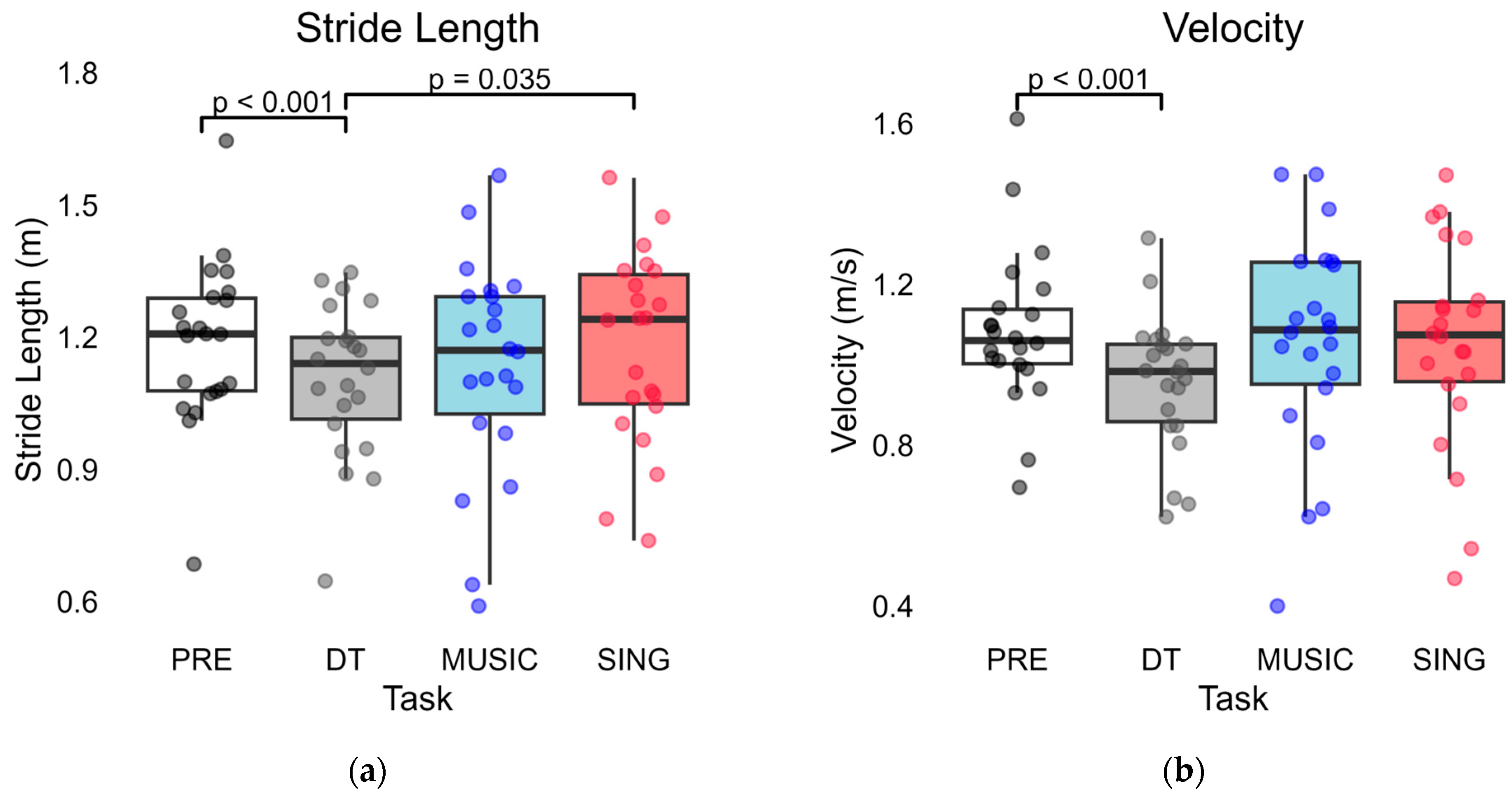
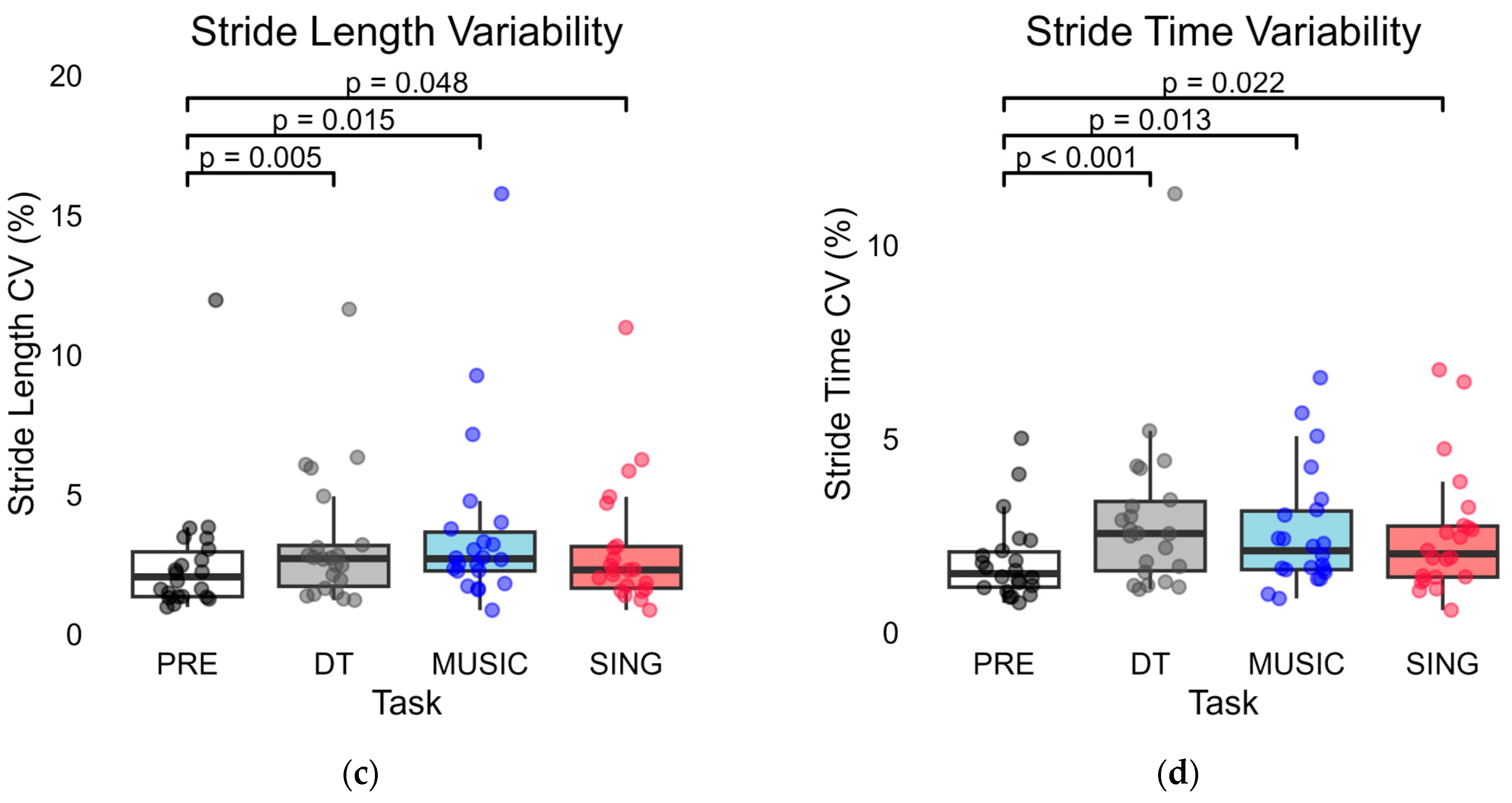
3.1. PRE Compared to DT Trials
3.2. PRE Compared to MUSIC and SING Trials
3.3. MUSIC Compared to SING Trials
3.4. Cue Trials Compared to DT
3.5. PRE Compared to POST
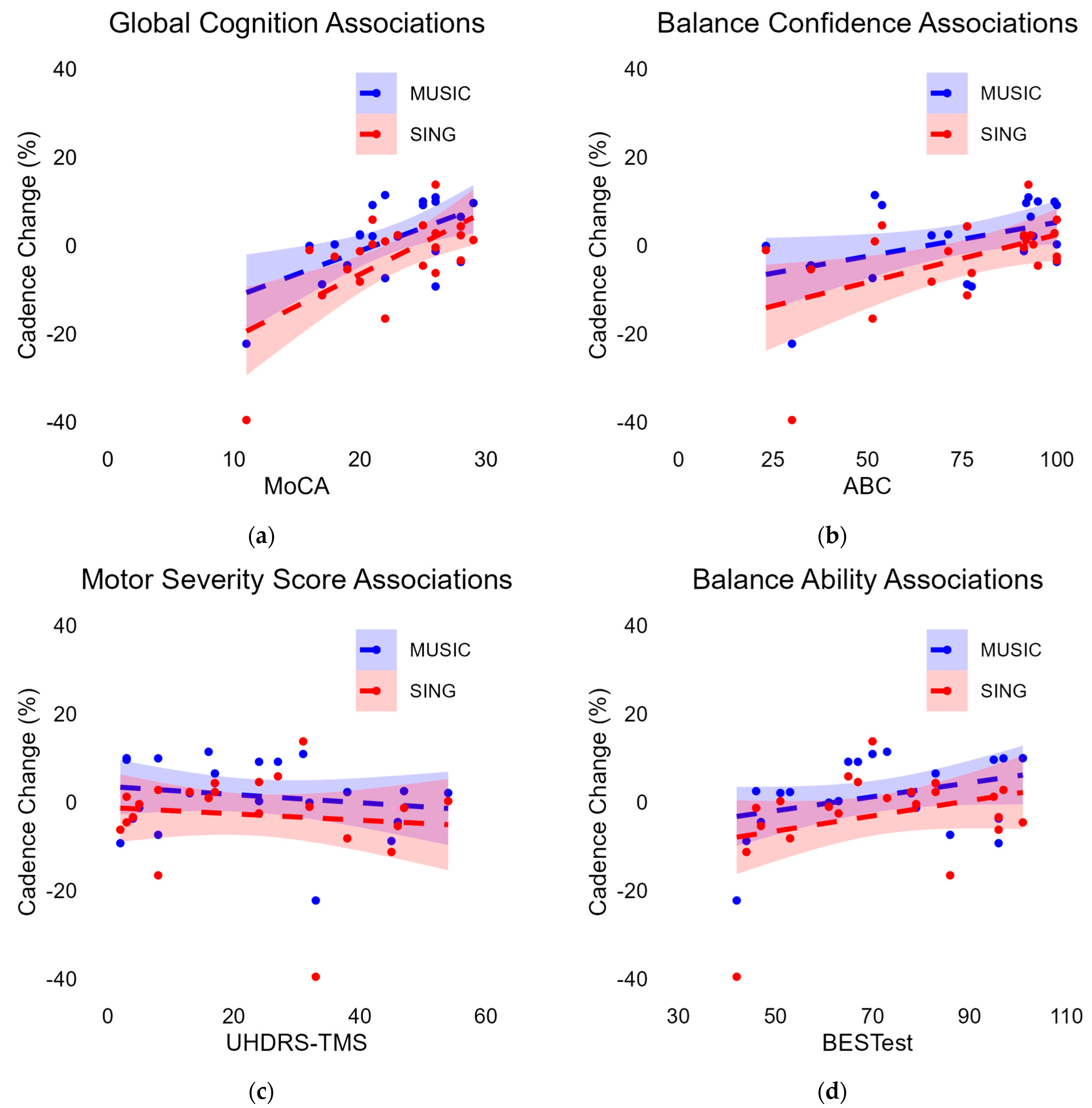
3.6. Associations with Cadence Change
3.6.1. Associations with Cadence Change During MUSIC
3.6.2. Associations with Cadence Change During SING
4. Discussion
4.1. Limitations
4.2. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HD | Huntington’s disease |
| PD | Parkinson’s disease |
| RAS | Rhythmic auditory stimulation |
| MoCA | Montreal Cognitive Assessment |
| UHDRS-TMS | Unified Huntington’s Disease Rating Scale Total Motor Score |
| BESTest | Balance Evaluation Systems Test |
| MUSIC | externally based cue of music being played aloud |
| SING | internally generated cueing of participants singing aloud |
| PRE | baseline, uncued walking trials |
| DT | cognitive dual-task trials |
| POST | final, uncued walking trials |
| CV | coefficient of variation |
| ABC | Activities-Specific Balance Confidence Scale |
| T-MoCA | Telephone MoCA |
Appendix A
| ID | Age | Age of Onset | Gender | Falls in the Last Month | MoCA | UHDRS-TMS | BESTest | ABC | VMATInhibitor | Benzo-Diazepine | Anti-Psychotic | Tetracyclic Antidepressant | SNRI | SSRI | Anticonvulsant |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | 41 | male | 3 | 11 | 33 | 42 | 30 | N | Y | Y | Y | Y | N | N |
| 2 | 63 | 55 | female | 0 | 21 | 27 | 65 | 100 | N | Y | N | Y | N | Y | N |
| 3 | 31 | 28 | female | 2 | 25 | 3 | 101 | 95 | N | N | N | N | N | Y | N |
| 4 | 65 | 61 | male | 0 | 18 | 24 | 63 | 100 | N | N | N | N | N | N | N |
| 5 | 48 | 41 | male | 0 | 28 | 4 | 96 | 100 | N | N | N | N | N | N | N |
| 6 | 74 | 59 | male | 0 | 20 | 38 | 53 | 66.9 | N | N | N | N | N | Y | Y |
| 7 | 43 | 33 | female | 0 | 23 | 13 | 78 | 91.3 | N | N | Y | Y | N | Y | Y |
| 8 | 70 | 48 | male | 0 | 25 | 24 | 67 | 53.8 | N | N | Y | N | N | Y | N |
| 9 | 61 | 50 | female | 0 | 20 | 47 | 46 | 71.3 | N | N | Y | N | N | Y | N |
| 10 | 53 | 46 | male | 6 | 19 | 46 | 47 | 35 | N | Y | Y | Y | N | Y | Y |
| 11 | 56 | 43 | male | 0 | 26 | 31 | 70 | 92.5 | N | N | N | N | N | N | N |
| 12 | 65 | 54 | male | 0 | 21 | 54 | 51 | 93.8 | Y | N | N | N | N | Y | N |
| 13 | 54 | 35 | female | 0 | 28 | 17 | 83 | 93.1 | N | N | N | N | N | N | N |
| 14 | 68 | 59 | female | 0 | 17 | 45 | 44 | 76.3 | N | N | Y | Y | N | Y | N |
| 15 | 62 | 52 | male | 0 | 16 | 32 | 61 | 23.1 | Y | N | N | N | N | Y | Y |
| 16 | 37 | 36 | female | 0 | 29 | 3 | 95 | 91.9 | N | N | N | N | N | N | N |
| 17 | 34 | 29 | female | 0 | 22 | 16 | 73 | 51.9 | N | Y | N | Y | Y | N | N |
| 18 | 41 | 32 | female | 2 | 26 | 2 | 96 | 77.5 | N | N | N | N | N | N | N |
| 19 | 57 | 33 | female | 10 | 22 | 8 | 86 | 51.3 | N | N | N | N | N | Y | N |
| 20 | 45 | 42 | female | 1 | 28 | 17 | 83 | 76.3 | N | Y | N | N | N | Y | N |
| 21 | 50 | 46 | female | 0 | 26 | 5 | 79 | 91.3 | N | N | N | N | Y | N | N |
| 22 | 46 | 34 | male | 1 | 26 | 8 | 97 | 99.4 | N | N | N | N | N | N | N |
Appendix B
| Gait Variable | PRE | DT | MUSIC | SING |
|---|---|---|---|---|
| Log (stride length CV) | −1.67 ± 0.25 | −1.56 ± 0.26 | −1.53 ± 0.27 | −1.60 ± 0.26 |
| Log (stride time CV) | −1.79 ± 0.21 | −1.60 ± 0.25 | −1.65 ± 0.23 | −1.67 ± 0.26 |
Appendix C
| Gait Variable | PRE | POST | t(df) = t, p |
|---|---|---|---|
| Cadence (steps/min) | 109.75 ± 11.52 | 109.04 ± 12.51 | t(21) = 0.630, p = 0.535 |
| Stridelength (m) | 1.19 ± 0.19 | 1.19 ± 0.19 | t(21) = 0.019, p = 0.985 |
| Velocity (m/s) | 1.08 ± 0.20 | 1.08 ± 0.21 | t(21) = 0.229, p = 0.821 |
| Stride length CV (%) * | 2.58 ± 2.28 | 2.53 ± 2.00 | t(21) = 0.352, p = 0.729 |
| Stride time CV (%) * | 1.85 ± 1.07 | 1.98 ± 1.15 | t(21) = −0.866, p = 0.396 |
| Log (stride length CV) * | −1.67 ± 0.25 | −1.68 ± 0.26 | t(21) = 0.321, p = 0.752 |
| Log (stride time CV) * | −1.79 ± 0.21 | −1.77 ± 0.25 | t(21) = −0.530, p = 0.601 |
References
- Medina, A.; Mahjoub, Y.; Shaver, L.; Pringsheim, T. Prevalence and Incidence of Huntington’s Disease: An Updated Systematic Review and Meta-Analysis. Mov. Disord. 2022, 37, 2327–2335. [Google Scholar] [CrossRef]
- Overview of Huntington’s Disease. Available online: https://hdsa.org/what-is-hd/overview-of-huntingtons-disease (accessed on 29 April 2025).
- Shannon, K.M. Huntington’s Disease—Clinical Signs, Symptoms, Presymptomatic Diagnosis, and Diagnosis. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 100, pp. 3–13. ISBN 978-0-444-52014-2. [Google Scholar]
- Girotti, F.; Marano, R.; Soliveri, P.; Geminiani, G.; Scigliano, G. Relationship between Motor and Cognitive Disorders in Huntington’s Disease. J. Neurol. 1988, 235, 454–457. [Google Scholar] [CrossRef]
- Bradshaw, J.L.; Phillips, J.G.; Dennis, C.; Mattingley, J.B.; Andrewes, D.; Chiu, E.; Pierson, J.M.; Bradshaw, J.A. Initiation and Execution of Movement Sequences in Those Suffering from and At-Risk of Developing Huntington’s Disease. J. Clin. Exp. Neuropsychol. 1992, 14, 179–192. [Google Scholar] [CrossRef]
- Rao, A.K.; Muratori, L.; Louis, E.D.; Moskowitz, C.B.; Marder, K.S. Spectrum of Gait Impairments in Presymptomatic and Symptomatic Huntington’s Disease. Mov. Disord. 2008, 23, 1100–1107. [Google Scholar] [CrossRef]
- Kirkwood, S.C.; Su, J.L.; Conneally, P.M.; Foroud, T. Progression of Symptoms in the Early and Middle Stages of Huntington Disease. Arch. Neurol. 2001, 58, 273. [Google Scholar] [CrossRef] [PubMed]
- Grimbergen, Y.A.; Munneke, M.; Bloem, B.R. Falls in Parkinson’s Disease. Curr. Opin. Neurol. 2004, 17, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Delval, A.; Krystkowiak, P.; Blatt, J.-L.; Labyt, E.; Dujardin, K.; Destée, A.; Derambure, P.; Defebvre, L. Role of Hypokinesia and Bradykinesia in Gait Disturbances in Huntington’s Disease: A Biomechanical Study. J. Neurol. 2006, 253, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Vuong, K.; Canning, C.G.; Menant, J.C.; Loy, C.T. Gait, Balance, and Falls in Huntington Disease. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 159, pp. 251–260. ISBN 978-0-444-63916-5. [Google Scholar]
- Dalton, A.; Khalil, H.; Busse, M.; Rosser, A.; Van Deursen, R.; ÓLaighin, G. Analysis of Gait and Balance through a Single Triaxial Accelerometer in Presymptomatic and Symptomatic Huntington’s Disease. Gait Posture 2013, 37, 49–54. [Google Scholar] [CrossRef]
- Gaßner, H.; Jensen, D.; Marxreiter, F.; Kletsch, A.; Bohlen, S.; Schubert, R.; Muratori, L.M.; Eskofier, B.; Klucken, J.; Winkler, J.; et al. Gait Variability as Digital Biomarker of Disease Severity in Huntington’s Disease. J. Neurol. 2020, 267, 1594–1601. [Google Scholar] [CrossRef]
- Grimbergen, Y.A.M.; Knol, M.J.; Bloem, B.R.; Kremer, B.P.H.; Roos, R.A.C.; Munneke, M. Falls and Gait Disturbances in Huntington’s Disease. Mov. Disord. 2008, 23, 970–976. [Google Scholar] [CrossRef]
- Quinn, L.; Rao, A. Physical Therapy for People with Huntington Disease: Current Perspectives and Case Report. J. Neurol. Phys. Ther. 2002, 26, 145–153. [Google Scholar] [CrossRef]
- Moon, Y.; Sung, J.; An, R.; Hernandez, M.E.; Sosnoff, J.J. Gait Variability in People with Neurological Disorders: A Systematic Review and Meta-Analysis. Hum. Mov. Sci. 2016, 47, 197–208. [Google Scholar] [CrossRef]
- Tyagi, S.; Shekhar, N.; Thakur, A.K. Alternative Approaches for the Management of Huntington’s Disease: A Narrative Review. Altern. Ther. Health Med. 2024, 30, 68–75. [Google Scholar]
- Travessa, A.M.; Rodrigues, F.B.; Mestre, T.A.; Ferreira, J.J. Fifteen Years of Clinical Trials in Huntington’s Disease: A Very Low Clinical Drug Development Success Rate. J. Huntingt. Dis. 2017, 6, 157–163. [Google Scholar] [CrossRef]
- Ferreira, J.J.; Rodrigues, F.B.; Duarte, G.S.; Mestre, T.A.; Bachoud-Levi, A.; Bentivoglio, A.R.; Burgunder, J.; Cardoso, F.; Claassen, D.O.; Landwehrmeyer, G.B.; et al. An MDS Evidence-Based Review on Treatments for Huntington’s Disease. Mov. Disord. 2022, 37, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Saft, C.; Burgunder, J.-M.; Dose, M.; Jung, H.H.; Katzenschlager, R.; Priller, J.; Nguyen, H.P.; Reetz, K.; Reilmann, R.; Seppi, K.; et al. Symptomatic Treatment Options for Huntington’s Disease (Guidelines of the German Neurological Society). Neurol. Res. Pr. 2023, 5, 61. [Google Scholar] [CrossRef]
- Quinn, L.; Kegelmeyer, D.; Kloos, A.; Rao, A.K.; Busse, M.; Fritz, N.E. Clinical Recommendations to Guide Physical Therapy Practice for Huntington Disease. Neurology 2020, 94, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Thaut, M.H.; Abiru, M. Rhythmic Auditory Stimulation in Rehabilitation of Movement Disorders: A Review of Current Research. Music. Percept. 2010, 27, 263–269. [Google Scholar] [CrossRef]
- Nieuwboer, A.; Kwakkel, G.; Rochester, L.; Jones, D.; van Wegen, E.; Willems, A.M.; Chavret, F.; Hetherington, V.; Baker, K.; Lim, I. Cueing Training in the Home Improves Gait-Related Mobility in Parkinson’s Disease: The RESCUE Trial. J. Neurol. Neurosurg. Psychiatry 2007, 78, 134–140. [Google Scholar] [CrossRef]
- Muthukrishnan, N.; Abbas, J.J.; Shill, H.A.; Krishnamurthi, N. Cueing Paradigms to Improve Gait and Posture in Parkinson’s Disease: A Narrative Review. Sensors 2019, 19, 5468. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, S.J.; Barber, B.; Colby, M.; Cormack, B.; Mick, T.; Jenkins, M.E. Cueing and Gait Improvement Among People with Parkinson’s Disease: A Meta-Analysis. Arch. Phys. Med. Rehabil. 2013, 94, 562–570. [Google Scholar] [CrossRef]
- Harrison, E.C.; Earhart, G.M. The Effect of Auditory Cues on Gait Variability in People with Parkinson’s Disease and Older Adults: A Systematic Review. Neurodegener. Dis. Manag. 2023, 13, 113–128. [Google Scholar] [CrossRef]
- Thaut, M.H.; Miltner, R.; Lange, H.W.; Hurt, C.P.; Hoemberg, V. Velocity Modulation and Rhythmic Synchronization of Gait in Huntington’s Disease. Mov. Disord. 1999, 14, 808–819. [Google Scholar] [CrossRef]
- Delval, A.; Krystkowiak, P.; Delliaux, M.; Blatt, J.; Derambure, P.; Destée, A.; Defebvre, L. Effect of External Cueing on Gait in Huntington’s Disease. Mov. Disord. 2008, 23, 1446–1452. [Google Scholar] [CrossRef]
- Bilney, B.; Morris, M.E.; Churchyard, A.; Chiu, E.; Georgiou-Karistianis, N. Evidence for a Disorder of Locomotor Timing in Huntington’s Disease. Mov. Disord. 2005, 20, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Churchyard, A.; Morris, M.; Georgiou-Karistianis, N.; Chiu, E.; Cooper, R.; Iansek, R. Gait Dysfunction in Huntington’s Disease: Parkinsonism and a Disorder of Timing. Implic. Mov. Rehabil. Adv. Neurol. 2001, 87, 375–385. [Google Scholar]
- Kim, K.-H.; Song, M.-K. Update of Rehabilitation in Huntington’s Disease: Narrative Review. Brain Neurorehabil. 2023, 16, e28. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.E.; Van Walsem, M.R.; Brean, A.; Frich, J.C. Therapeutic Use of Music, Dance, and Rhythmic Auditory Cueing for Patients with Huntington’s Disease: A Systematic Review. J. Huntingt. Dis. 2019, 8, 393–420. [Google Scholar] [CrossRef]
- De Dreu, M.J.; Van Der Wilk, A.S.D.; Poppe, E.; Kwakkel, G.; Van Wegen, E.E.H. Rehabilitation, Exercise Therapy and Music in Patients with Parkinson’s Disease: A Meta-Analysis of the Effects of Music-Based Movement Therapy on Walking Ability, Balance and Quality of Life. Park. Relat. Disord. 2012, 18, S114–S119. [Google Scholar] [CrossRef] [PubMed]
- Rodger, M.W.M.; Craig, C.M. Beyond the Metronome: Auditory Events and Music May Afford More than Just Interval Durations as Gait Cues in Parkinson’s Disease. Front. Neurosci. 2016, 10, 272. [Google Scholar] [CrossRef]
- Rose, D.; Delevoye-Turrell, Y.; Ott, L.; Annett, L.E.; Lovatt, P.J. Music and Metronomes Differentially Impact Motor Timing in People with and without Parkinson’s Disease: Effects of Slow, Medium, and Fast Tempi on Entrainment and Synchronization Performances in Finger Tapping, Toe Tapping, and Stepping on the Spot Tasks. Park. Dis. 2019, 2019, 6530838. [Google Scholar] [CrossRef]
- Harrison, E.C.; Horin, A.P.; Earhart, G.M. Internal Cueing Improves Gait More than External Cueing in Healthy Adults and People with Parkinson Disease. Sci. Rep. 2018, 8, 15525. [Google Scholar] [CrossRef] [PubMed]
- Tueth, L.E.; Haussler, A.M.; Baudendistel, S.T.; Earhart, G.M. Exploring Relationships among Gait, Balance, and Physical Activity in Individuals with Huntington’s Disease. J. Huntington’s Dis. 2024, 13, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Bezdicek, O.; Majerova, V.; Novak, M.; Nikolai, T.; Ruzicka, E.; Roth, J. Validity of the Montreal Cognitive Assessment in the Detection of Cognitive Dysfunction in Huntington’s Disease. Appl. Neuropsychol. Adult 2013, 20, 33–40. [Google Scholar] [CrossRef]
- Mestre, T.A.; Forjaz, M.J.; Mahlknecht, P.; Cardoso, F.; Ferreira, J.J.; Reilmann, R.; Sampaio, C.; Goetz, C.G.; Cubo, E.; Martinez-Martin, P.; et al. Rating Scales for Motor Symptoms and Signs in Huntington’s Disease: Critique and Recommendations. Mov. Disord. Clin. Pr. 2018, 5, 111–117. [Google Scholar] [CrossRef]
- Horak, F.B.; Wrisley, D.M.; Frank, J. The Balance Evaluation Systems Test (BESTest) to Differentiate Balance Deficits. Phys. Ther. 2009, 89, 484–498. [Google Scholar] [CrossRef]
- Mancini, M.; King, L.; Salarian, A.; Holmstrom, L.; McNames, J.; Horak, F.B. Mobility Lab to Assess Balance and Gait with Synchronized Body-Worn Sensors. J. Bioeng. Biomed. Sci. 2011, S1, 007. [Google Scholar] [CrossRef]
- Harrison, E.C.; McNeely, M.E.; Earhart, G.M. The Feasibility of Singing to Improve Gait in Parkinson Disease. Gait Posture 2017, 53, 224–229. [Google Scholar] [CrossRef]
- Park, K.S.; Hass, C.J.; Janelle, C.M. Familiarity with Music Influences Stride Amplitude and Variability during Rhythmically-Cued Walking in Individuals with Parkinson’s Disease. Gait Posture 2021, 87, 101–109. [Google Scholar] [CrossRef]
- Harrison, E.C.; Tueth, L.E.; Haussler, A.M.; Rawson, K.S.; Earhart, G.M. Personalized Auditory Rhythmic Cues to Optimize Gait in Older Adults and People with Parkinson Disease. J. Neurol. Phys. Ther. 2025, 49, 162–170. [Google Scholar] [CrossRef]
- Browning, S.; Holland, S.; Wellwood, I.; Bilney, B. Spatiotemporal Gait Parameters in Adults with Premanifest and Manifest Huntington’s Disease: A Systematic Review. J. Mov. Disord. 2023, 16, 307–320. [Google Scholar] [CrossRef]
- Powell, L.E.; Myers, A.M. The Activities-Specific Balance Confidence (ABC) Scale. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1995, 50A, M28–M34. [Google Scholar] [CrossRef]
- Stasny, B.M.; Newton, R.A.; Viggiano LoCascio, L.; Bedio, N.; Lauke, C.; Conroy, M.; Thompson, A.; Vakhnenko, L.; Polidoro, C. The ABC Scale and Fall Risk: A Systematic Review. Phys. Occup. Ther. Geriatr. 2011, 29, 233–242. [Google Scholar] [CrossRef]
- Lord, S.; Galna, B.; Verghese, J.; Coleman, S.; Burn, D.; Rochester, L. Independent Domains of Gait in Older Adults and Associated Motor and Nonmotor Attributes: Validation of a Factor Analysis Approach. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 820–827. [Google Scholar] [CrossRef]
- Katz, M.J.; Wang, C.; Nester, C.O.; Derby, C.A.; Zimmerman, M.E.; Lipton, R.B.; Sliwinski, M.J.; Rabin, L.A. T-MoCA: A Valid Phone Screen for Cognitive Impairment in Diverse Community Samples. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2021, 13, e12144. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Ghai, I.; Schmitz, G.; Effenberg, A.O. Effect of Rhythmic Auditory Cueing on Parkinsonian Gait: A Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 506. [Google Scholar] [CrossRef]
- Wittwer, J.E.; Webster, K.E.; Hill, K. Rhythmic Auditory Cueing to Improve Walking in Patients with Neurological Conditions Other than Parkinson’s Disease—What Is the Evidence? Disabil. Rehabil. 2013, 35, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Alexi, T. Neuroprotective Strategies for Basal Ganglia Degeneration: Parkinson’s and Huntington’s Diseases. Prog. Neurobiol. 2000, 60, 409–470. [Google Scholar] [CrossRef]
- Aylward, E.H.; Li, Q.; Stine, O.C.; Ranen, N.; Sherr, M.; Barta, P.E.; Bylsma, F.W.; Pearlson, G.D.; Ross, C.A. Longitudinal Change in Basal Ganglia Volume in Patients with Huntington’s Disease. Neurology 1997, 48, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K. Neurophysiology of Gait: From the Spinal Cord to the Frontal Lobe. Mov. Disord. 2013, 28, 1483–1491. [Google Scholar] [CrossRef]
- Takakusaki, K.; Tomita, N.; Yano, M. Substrates for Normal Gait and Pathophysiology of Gait Disturbances with Respect to the Basal Ganglia Dysfunction. J. Neurol. 2008, 255, 19–29. [Google Scholar] [CrossRef]
- Mirelman, A.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelosin, E.; Almeida, Q.J. Gait Impairments in Parkinson’s Disease. Lancet Neurol. 2019, 18, 697–708. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Cudkowicz, M.E.; Firtion, R.; Wei, J.Y.; Goldberger, A.L. Gait Variability and Basal Ganglia Disorders: Stride-to-Stride Variations of Gait Cycle Timing in Parkinson’s Disease and Huntington’s Disease. Mov. Disord. 1998, 13, 428–437. [Google Scholar] [CrossRef]
- Harrison, E.C.; Grossen, S.; Tueth, L.E.; Haussler, A.M.; Rawson, K.S.; Campbell, M.C.; Earhart, G.M. Neural Mechanisms Underlying Synchronization of Movement to Musical Cues in Parkinson Disease and Aging. Front. Neurosci. 2025, 19, 1550802. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, S.L.; Ullén, F.; Henrik Ehrsson, H.; Hashimoto, T.; Kito, T.; Naito, E.; Forssberg, H.; Sadato, N. Listening to Rhythms Activates Motor and Premotor Cortices. Cortex 2009, 45, 62–71. [Google Scholar] [CrossRef]
- Chen, J.L.; Zatorre, R.J.; Penhune, V.B. Interactions between Auditory and Dorsal Premotor Cortex during Synchronization to Musical Rhythms. NeuroImage 2006, 32, 1771–1781. [Google Scholar] [CrossRef]
- Grahn, J.A.; Brett, M. Rhythm and Beat Perception in Motor Areas of the Brain. J. Cogn. Neurosci. 2007, 19, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Bijsterbosch, J.D.; Lee, K.-H.; Hunter, M.D.; Tsoi, D.T.; Lankappa, S.; Wilkinson, I.D.; Barker, A.T.; Woodruff, P.W.R. The Role of the Cerebellum in Sub- and Supraliminal Error Correction during Sensorimotor Synchronization: Evidence from fMRI and TMS. J. Cogn. Neurosci. 2011, 23, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Thaut, M.H.; Stephan, K.M.; Wunderlich, G.; Schicks, W.; Tellmann, L.; Herzog, H.; McIntosh, G.C.; Seitz, R.J.; Hömberg, V. Distinct Cortico-Cerebellar Activations in Rhythmic Auditory Motor Synchronization. Cortex 2009, 45, 44–53. [Google Scholar] [CrossRef]
- Martinu, K.; Monchi, O. Cortico-Basal Ganglia and Cortico-Cerebellar Circuits in Parkinson’s Disease: Pathophysiology or Compensation? Behav. Neurosci. 2013, 127, 222–236. [Google Scholar] [CrossRef]
- Torres, E.B.; Heilman, K.M.; Poizner, H. Impaired Endogenously Evoked Automated Reaching in Parkinson’s Disease. J. Neurosci. 2011, 31, 17848–17863. [Google Scholar] [CrossRef]
- Mirdamadi, J.L. Cerebellar Role in Parkinson’s Disease. J. Neurophysiol. 2016, 116, 917–919. [Google Scholar] [CrossRef]
- Tereshchenko, A.V.; Schultz, J.L.; Bruss, J.E.; Magnotta, V.A.; Epping, E.A.; Nopoulos, P.C. Abnormal Development of Cerebellar-Striatal Circuitry in Huntington Disease. Neurology 2020, 94, e1908–e1915. [Google Scholar] [CrossRef]
- Feigin, A.; Tang, C.; Ma, Y.; Mattis, P.; Zgaljardic, D.; Guttman, M.; Paulsen, J.S.; Dhawan, V.; Eidelberg, D. Thalamic Metabolism and Symptom Onset in Preclinical Huntington’s Disease. Brain 2007, 130, 2858–2867. [Google Scholar] [CrossRef]
- Yu, H.; Sternad, D.; Corcos, D.M.; Vaillancourt, D.E. Role of Hyperactive Cerebellum and Motor Cortex in Parkinson’s Disease. NeuroImage 2007, 35, 222–233. [Google Scholar] [CrossRef]
- Rüb, U.; Hoche, F.; Brunt, E.R.; Heinsen, H.; Seidel, K.; Del Turco, D.; Paulson, H.L.; Bohl, J.; Von Gall, C.; Vonsattel, J.; et al. Degeneration of the Cerebellum in Huntington’s Disease (HD): Possible Relevance for the Clinical Picture and Potential Gateway to Pathological Mechanisms of the Disease Process. Brain Pathol. 2013, 23, 165–177. [Google Scholar] [CrossRef]
- Franklin, G.L.; Camargo, C.H.F.; Meira, A.T.; Lima, N.S.C.; Teive, H.A.G. The Role of the Cerebellum in Huntington’s Disease: A Systematic Review. Cerebellum 2021, 20, 254–265. [Google Scholar] [CrossRef]
- Rees, E.M.; Farmer, R.; Cole, J.H.; Haider, S.; Durr, A.; Landwehrmeyer, B.; Scahill, R.I.; Tabrizi, S.J.; Hobbs, N.Z. Cerebellar Abnormalities in Huntington’s Disease: A Role in Motor and Psychiatric Impairment? Mov. Disord. 2014, 29, 1648–1654. [Google Scholar] [CrossRef]
- Singh-Bains, M.K.; Mehrabi, N.F.; Sehji, T.; Austria, M.D.; Tan, A.Y.; Tippett, L.J.; Dragunow, M.; Waldvogel, H.J.; Faull, R.L. Cerebellar Degeneration Correlates with Motor Symptoms in Huntington Disease. Ann. Neurol. 2019, 85, 396–405. [Google Scholar] [CrossRef]
- Solstrand Dahlberg, L.; Lungu, O.; Doyon, J. Cerebellar Contribution to Motor and Non-Motor Functions in Parkinson’s Disease: A Meta-Analysis of fMRI Findings. Front. Neurol. 2020, 11, 127. [Google Scholar] [CrossRef]
- Hannaway, N.; Lao-Kaim, N.P.; Martín-Bastida, A.; Roussakis, A.-A.; Howard, J.; Wall, M.B.; Loane, C.; Barker, R.A.; Piccini, P. Longitudinal Changes in Movement-Related Functional MRI Activity in Parkinson’s Disease Patients. Park. Relat. Disord. 2021, 87, 61–69. [Google Scholar] [CrossRef]
- Hausdorff, J.M. Gait Dynamics in Parkinson’s Disease: Common and Distinct Behavior among Stride Length, Gait Variability, and Fractal-like Scaling. Chaos 2009, 19, 026113. [Google Scholar] [CrossRef]
- Brach, J.S.; Berlin, J.E.; VanSwearingen, J.M.; Newman, A.B.; Studenski, S.A. Too Much or Too Little Step Width Variability Is Associated with a Fall History in Older Persons Who Walk at or near Normal Gait Speed. J. Neuroeng. Rehabil. 2005, 2, 21. [Google Scholar] [CrossRef]
- Brach, J.S.; Studenski, S.; Perera, S.; VanSwearingen, J.M.; Newman, A.B. Stance Time and Step Width Variability Have Unique Contributing Impairments in Older Persons. Gait Posture 2008, 27, 431–439. [Google Scholar] [CrossRef]
- Beauchet, O.; Allali, G.; Annweiler, C.; Bridenbaugh, S.; Assal, F.; Kressig, R.W.; Herrmann, F.R. Gait Variability among Healthy Adults: Low and High Stride-to-Stride Variability Are Both a Reflection of Gait Stability. Gerontology 2009, 55, 702–706. [Google Scholar] [CrossRef]
- Muratori, L.M.; Quinn, L.; Li, X.; Youdan, G.; Busse, M.; Fritz, N.E. Measures of Postural Control and Mobility during Dual-Tasking as Candidate Markers of Instability in Huntington’s Disease. Hum. Mov. Sci. 2021, 80, 102881. [Google Scholar] [CrossRef]
- Purcell, N.L.; Goldman, J.G.; Ouyang, B.; Liu, Y.; Bernard, B.; O’Keefe, J.A. The Effects of Dual-Task Cognitive Interference on Gait and Turning in Huntington’s Disease. PLoS ONE 2020, 15, e0226827. [Google Scholar] [CrossRef]
- Fritz, N.E.; Hamana, K.; Kelson, M.; Rosser, A.; Busse, M.; Quinn, L. Motor-Cognitive Dual-Task Deficits in Individuals with Early-Mid Stage Huntington Disease. Gait Posture 2016, 49, 283–289. [Google Scholar] [CrossRef]
- Delval, A.; Krystkowiak, P.; Delliaux, M.; Dujardin, K.; Blatt, J.; Destée, A.; Derambure, P.; Defebvre, L. Role of Attentional Resources on Gait Performance in Huntington’s Disease. Mov. Disord. 2008, 23, 684–689. [Google Scholar] [CrossRef]
- Radovanović, S.; Vodopić, S.; Stanković, I.; Dragašević-Mišković, N.; Kostić, V. Spatiotemporal Gait Characteristics of Huntington’s Disease during Dual-Task Walking. Int. J. Neurosci. 2020, 130, 136–143. [Google Scholar] [CrossRef]
- Kloos, A.D.; Kegelmeyer, D.A.; Fritz, N.E.; Daley, A.M.; Young, G.S.; Kostyk, S.K. Cognitive Dysfunction Contributes to Mobility Impairments in Huntington’s Disease. J. Huntington’s Dis. 2017, 6, 363–370. [Google Scholar] [CrossRef]
- Vaportzis, E.; Georgiou-Karistianis, N.; Churchyard, A.; Stout, J.C. Dual Task Performance May Be a Better Measure of Cognitive Processing in Huntington’s Disease than Traditional Attention Tests. J. Huntingt. Dis. 2015, 4, 119–130. [Google Scholar] [CrossRef]
- Sprengelmeyer, R.; Lange, H.; Hömberg, V. The Pattern of Attentional Deficits in Huntington’s Disease. Brain 1995, 118, 145–152. [Google Scholar] [CrossRef]
- Georgiou-Karistianis, N.; Farrow, M.; Wilson-Ching, M.; Churchyard, A.; Bradshaw, J.L.; Sheppard, D.M. Deficits in Selective Attention in Symptomatic Huntington Disease: Assessment Using an Attentional Blink Paradigm. Cogn. Behav. Neurol. 2012, 25, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.R.; Watkins, L.; Sahakian, B.J.; Monsell, S.; Barker, R.A.; Robbins, T.W. Task-Set Switching Deficits in Early-Stage Huntington’s Disease: Implications for Basal Ganglia Function. J. Cogn. Neurosci. 2003, 15, 629–642. [Google Scholar] [CrossRef]
- Migliore, S.; D’Aurizio, G.; Curcio, G.; Squitieri, F. Task-Switching Abilities in Pre-Manifest Huntington’s Disease Subjects. Park. Relat. Disord. 2019, 60, 111–117. [Google Scholar] [CrossRef]
- Baker, K.; Rochester, L.; Nieuwboer, A. The Immediate Effect of Attentional, Auditory, and a Combined Cue Strategy on Gait During Single and Dual Tasks in Parkinson’s Disease. Arch. Phys. Med. Rehabil. 2007, 88, 1593–1600. [Google Scholar] [CrossRef]
- Lohnes, C.A.; Earhart, G.M. The Impact of Attentional, Auditory, and Combined Cues on Walking during Single and Cognitive Dual Tasks in Parkinson Disease. Gait Posture 2011, 33, 478–483. [Google Scholar] [CrossRef]
- Rochester, L.; Rafferty, D.; Dotchin, C.; Msuya, O.; Minde, V.; Walker, R.W. The Effect of Cueing Therapy on Single and Dual-Task Gait in a Drug Naïve Population of People with Parkinson’s Disease in Northern Tanzania. Mov. Disord. 2010, 25, 906–911. [Google Scholar] [CrossRef]
- Rochester, L.; Nieuwboer, A.; Baker, K.; Hetherington, V.; Willems, A.-M.; Chavret, F.; Kwakkel, G.; Van Wegen, E.; Lim, I.; Jones, D. The Attentional Cost of External Rhythmical Cues and Their Impact on Gait in Parkinson’s Disease: Effect of Cue Modality and Task Complexity. J. Neural Transm. 2007, 114, 1243–1248. [Google Scholar] [CrossRef]
- Danoudis, M.; Iansek, R. Gait in Huntington’s Disease and the Stride Length-Cadence Relationship. BMC Neurol. 2014, 14, 161. [Google Scholar] [CrossRef]
- Pacchetti, C.; Mancini, F.; Aglieri, R.; Fundarò, C.; Martignoni, E.; Nappi, G. Active Music Therapy in Parkinson’s Disease: An Integrative Method for Motor and Emotional Rehabilitation. Psychosom. Med. 2000, 62, 386–393. [Google Scholar] [CrossRef]
- Leman, M.; Moelants, D.; Varewyck, M.; Styns, F.; Van Noorden, L.; Martens, J.-P. Activating and Relaxing Music Entrains the Speed of Beat Synchronized Walking. PLoS ONE 2013, 8, e67932. [Google Scholar] [CrossRef]
- Agus, T.R.; Thorpe, S.J.; Pressnitzer, D. Rapid Formation of Robust Auditory Memories: Insights from Noise. Neuron 2010, 66, 610–618. [Google Scholar] [CrossRef]
- Dunbar, R.I.M.; Kaskatis, K.; MacDonald, I.; Barra, V. Performance of Music Elevates Pain Threshold and Positive Affect: Implications for the Evolutionary Function of Music. Evol. Psychol. 2012, 10, 688–702. [Google Scholar] [CrossRef]
- Cruickshank, T.; Reyes, A.; Peñailillo, L.; Thompson, J.; Ziman, M. Factors That Contribute to Balance and Mobility Impairments in Individuals with Huntington’s Disease. Basal Ganglia 2014, 4, 67–70. [Google Scholar] [CrossRef]
- Kloos, A.D.; Fritz, N.E.; Kostyk, S.K.; Young, G.S.; Kegelmeyer, D.A. Clinimetric Properties of the Tinetti Mobility Test, Four Square Step Test, Activities-Specific Balance Confidence Scale, and Spatiotemporal Gait Measures in Individuals with Huntington’s Disease. Gait Posture 2014, 40, 647–651. [Google Scholar] [CrossRef]
- Tueth, L.E.; Haussler, A.M.; Lohse, K.R.; Rawson, K.S.; Earhart, G.M.; Harrison, E.C. Effect of Musical Cues on Gait in Individuals with Parkinson Disease with Comorbid Dementia. Gait Posture 2024, 107, 275–280. [Google Scholar] [CrossRef]
- Heindel, W.C.; Butters, N.; Salmon, D.P. Impaired Learning of a Motor Skill in Patients with Huntington’s Disease. Behav. Neurosci. 1988, 102, 141. [Google Scholar] [CrossRef]
- Smith, M.A.; Shadmehr, R. Intact Ability to Learn Internal Models of Arm Dynamics in Huntington’s Disease But Not Cerebellar Degeneration. J. Neurophysiol. 2005, 93, 2809–2821. [Google Scholar] [CrossRef]
- Smith, M.A.; Brandt, J.; Shadmehr, R. Motor Disorder in Huntington’s Disease Begins as a Dysfunction in Error Feedback Control. Nature 2000, 403, 544–549. [Google Scholar] [CrossRef]
- Holtbernd, F.; Tang, C.C.; Feigin, A.; Dhawan, V.; Ghilardi, M.F.; Paulsen, J.S.; Guttman, M.; Eidelberg, D. Longitudinal Changes in the Motor Learning-Related Brain Activation Response in Presymptomatic Huntington’s Disease. PLoS ONE 2016, 11, e0154742. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Cui, Y.; He, C.; Yin, P.; Bai, R.; Zhu, J.; Lam, J.S.T.; Zhang, J.; Yan, R.; Zheng, X.; et al. Projections for Prevalence of Parkinson’s Disease and Its Driving Factors in 195 Countries and Territories to 2050: Modelling Study of Global Burden of Disease Study 2021. BMJ 2025, 388, e080952. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cui, Y.; Zhang, J.; Yan, R.; Su, D.; Zhao, D.; Wang, A.; Feng, T. Temporal Trends in the Prevalence of Parkinson’s Disease from 1980 to 2023: A Systematic Review and Meta-Analysis. Lancet Healthy Longev. 2024, 5, e464–e479. [Google Scholar] [CrossRef]
- De Bartolo, D.; Morone, G.; Giordani, G.; Antonucci, G.; Russo, V.; Fusco, A.; Marinozzi, F.; Bini, F.; Spitoni, G.F.; Paolucci, S.; et al. Effect of Different Music Genres on Gait Patterns in Parkinson’s Disease. Neurol. Sci. 2020, 41, 575–582. [Google Scholar] [CrossRef]
- Park, K.S. Decomposing the Effects of Familiarity with Music Cues on Stride Length and Variability in Persons with Parkinson’s Disease: On the Role of Covariates. IJERPH 2022, 19, 10793. [Google Scholar] [CrossRef]
- Leow, L.; Rinchon, C.; Grahn, J. Familiarity with Music Increases Walking Speed in Rhythmic Auditory Cuing. Ann. New York Acad. Sci. 2015, 1337, 53–61. [Google Scholar] [CrossRef]
- Mazzoni, P.; Hristova, A.; Krakauer, J.W. Why Don’t We Move Faster? Parkinson’s Disease, Movement Vigor, and Implicit Motivation. J. Neurosci. 2007, 27, 7105–7116. [Google Scholar] [CrossRef] [PubMed]
- Newell, K.M.; McDonald, P.V. Searching for Solutions to the Coordination Function: Learning as Exploratory Behavior. In Tutorials in Motor Behavior; North-Holland Publishing, Amsterdam, The Netherlands, 1992.
- Harbourne, R.T.; Stergiou, N. Movement Variability and the Use of Nonlinear Tools: Principles to Guide Physical Therapist Practice. Phys. Ther. 2009, 89, 267–282. [Google Scholar] [CrossRef]
- Cardis, M.; Casadio, M.; Ranganathan, R. High Variability Impairs Motor Learning Regardless of Whether It Affects Task Performance. J. Neurophysiol. 2018, 119, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.C.; Horin, A.P.; Myers, P.S.; Rawson, K.S.; Earhart, G.M. Changes in Parkinsonian Gait Kinematics with Self-Generated and Externally-Generated Cues: A Comparison of Responders and Non-Responders. Somatosens. Mot. Res. 2020, 37, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Baudendistel, S.T.; Earhart, G.M. Characteristics of Responders to Interventions for Parkinson Disease: A Scoping Systematic Review. Neurodegener. Dis. Manag. 2025, 15, 173–186. [Google Scholar] [CrossRef] [PubMed]
| Variable | Mean ± SD | Range |
|---|---|---|
| Age (years) | 54 ± 12 | [31–74] |
| Age of Symptom Onset (years) | 44 ± 10 | [28–61] |
| Male, Female (count) | n = 10, n = 12 | NA |
| Falls in the Previous Month | 1 ± 2 | [0–10] |
| MoCA (points) | 22 ± 5 | [10–29] |
| UHDRS-TMS (points) | 22 ± 16 | [2–54] |
| BESTest (points) | 72 ± 19 | [42–101] |
| ABC (%) | 75% ± 25% | [23–100] |
| Gait Variable | PRE | DT | MUSIC | SING |
|---|---|---|---|---|
| Cadence (steps/min) | 109.75 ± 11.52 | 104.06 ± 12.97 | 111.22 ± 13.7 | 106.48 ± 14.61 |
| Stride length (m) | 1.19 ± 0.19 | 1.11 ± 0.17 | 1.13 ± 0.24 | 1.18 ± 0.22 |
| Velocity (m/s) | 1.08 ± 0.2 | 0.96 ± 0.17 | 1.06 ± 0.27 | 1.05 ± 0.26 |
| Stride length CV (%) | 2.58 ± 2.28 | 3.30 ± 2.43 | 3.73 ± 3.28 | 3.04 ± 2.31 |
| Stride time CV (%) | 1.85 ± 1.07 | 2.98 ± 2.21 | 2.60 ± 1.54 | 2.54 ± 1.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baudendistel, S.T.; Tueth, L.E.; Haussler, A.M.; Earhart, G.M. Musical Distractions: Music-Based Rhythmic Auditory Stimulation Fails to Improve Gait in Huntington’s Disease. Brain Sci. 2025, 15, 820. https://doi.org/10.3390/brainsci15080820
Baudendistel ST, Tueth LE, Haussler AM, Earhart GM. Musical Distractions: Music-Based Rhythmic Auditory Stimulation Fails to Improve Gait in Huntington’s Disease. Brain Sciences. 2025; 15(8):820. https://doi.org/10.3390/brainsci15080820
Chicago/Turabian StyleBaudendistel, Sidney T., Lauren E. Tueth, Allison M. Haussler, and Gammon M. Earhart. 2025. "Musical Distractions: Music-Based Rhythmic Auditory Stimulation Fails to Improve Gait in Huntington’s Disease" Brain Sciences 15, no. 8: 820. https://doi.org/10.3390/brainsci15080820
APA StyleBaudendistel, S. T., Tueth, L. E., Haussler, A. M., & Earhart, G. M. (2025). Musical Distractions: Music-Based Rhythmic Auditory Stimulation Fails to Improve Gait in Huntington’s Disease. Brain Sciences, 15(8), 820. https://doi.org/10.3390/brainsci15080820






