The Efficacy and Central Remodeling Mechanism of a Composite TMS Pattern in First-Episode and Recurrent Depressive Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Clinical and Psychological Evaluation
2.3. Event-Related Potentials (ERPs)
2.4. Blinding
2.5. Interventions
2.6. Outcome Measures
2.7. Safety Measures
2.8. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. Efficacy Outcomes
3.2.1. Primary Outcome
3.2.2. Secondary Outcomes
3.3. Correlation Between Changes of Pittsburgh Sleep Quality Index and Hamilton Depression Rating Scale in the First-Episode Group
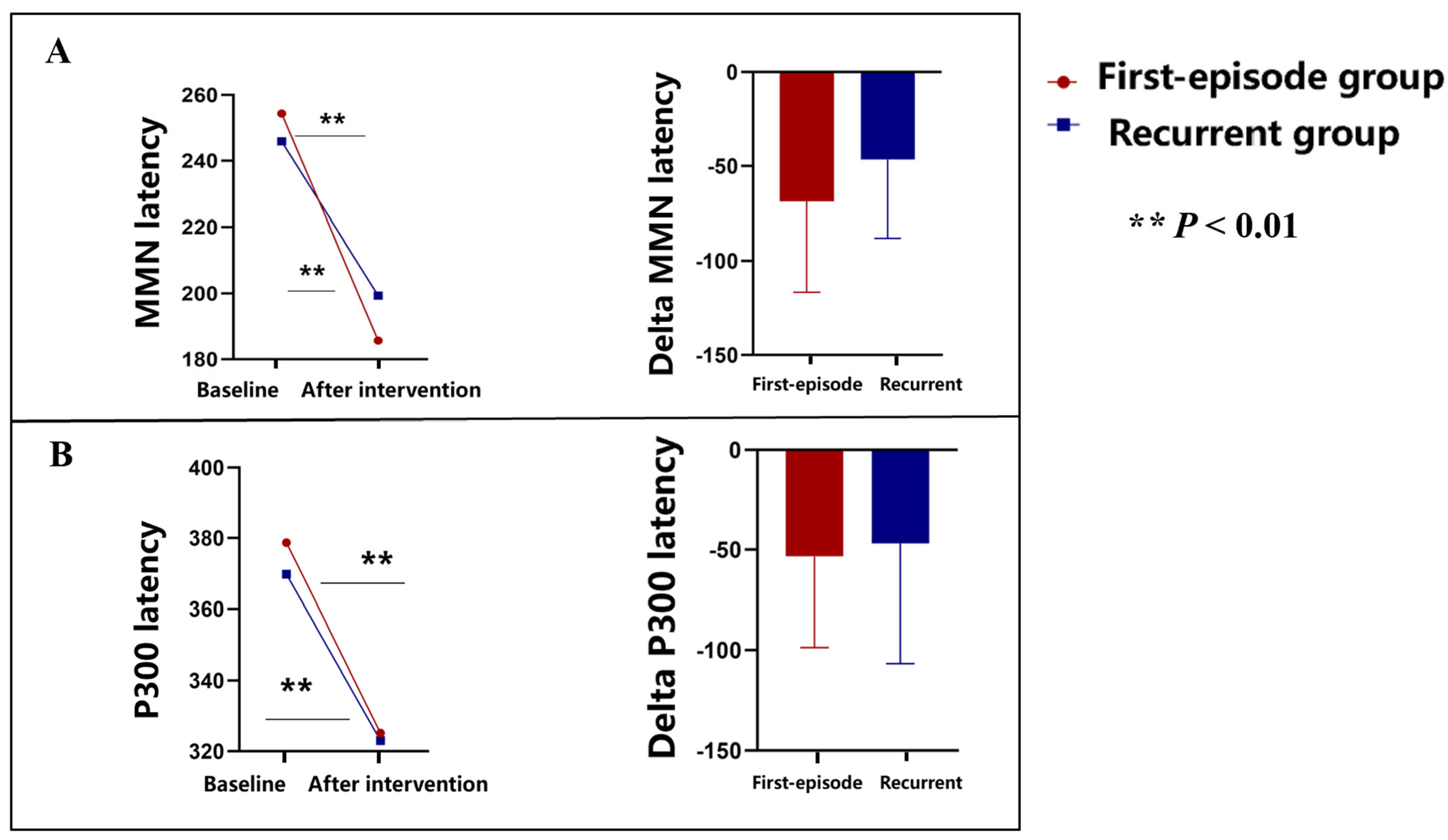
3.4. Event-Related Potentials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bech, P.; Paykel, E.; Sireling, L.; Yiend, J. Rating scales in general practice depression: Psychometric analyses of the clinical interview for depression and the Hamilton rating scale. J. Affect. Disord. 2015, 171, 68–73. [Google Scholar] [CrossRef]
- Lai, C.J.; Fan, Y.; Man, H.Y.; Huang, Y. Childhood adversity and depression in Chinese populations: A multilevel meta-analysis of studies using the Childhood Trauma Questionnaire (CTQ). Asian J. Psychiatry 2023, 84, 103582. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, C.; Zhao, M.; Ye, Y.; Yu, L.; Li, Y.; Luan, H.; Zhang, S.; Xu, P.; Chen, X.; et al. Comparisons of Accelerated Continuous and Intermittent Theta Burst Stimulation for Treatment-Resistant Depression and Suicidal Ideation. Biol. Psychiatry 2023, 96, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.T.; Zeng, B.Y.; Wang, H.Y.; Zeng, B.S.; Liang, C.S.; Chen, Y.C.; Stubbs, B.; Carvalho, A.F.; Brunoni, A.R.; Su, K.P.; et al. Efficacy and acceptability of noninvasive brain stimulation for treating posttraumatic stress disorder symptoms: A network meta-analysis of randomized controlled trials. Acta Psychiatr. Scand. 2024, 150, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.L.M.; Lee, G.T.H.; Lam, Y.H.; Chan, R.C.C.; Wu, J.Y.M. The use of the Digit Span Test in screening for cognitive impairment in acute medical inpatients. Int. Psychogeriatr. 2011, 23, 1569–1574. [Google Scholar] [CrossRef]
- Strumila, R.; Lengvenyte, A.; Zdanavicius, L.; Badaras, R.; Dlugauskas, E.; Lesinskiene, S.; Matiekus, E.; Marcinkevicius, M.; Venceviciene, L.; Utkus, A.; et al. Higher levels of plasma Adrenocorticotropic hormone (ACTH) are associated with lower suicidal ideation in depressed patients compared to controls and suicide attempters, independently from depression severity. Compr. Psychoneuroendocrinol. 2024, 19, 100235. [Google Scholar] [CrossRef] [PubMed]
- Tolentino, J.C.; Schmidt, S.L. DSM-5 Criteria and Depression Severity: Implications for Clinical Practice. Front. Psychiatry 2018, 9, 450. [Google Scholar] [CrossRef]
- Chang, Q.; Xia, Y.; Bai, S.; Zhang, X.; Liu, Y.; Yao, D.; Xu, X.; Zhao, Y. Association Between Pittsburgh Sleep Quality Index and Depressive Symptoms in Chinese Resident Physicians. Front. Psychiatry 2021, 12, 564815. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, Q.; Li, F.; Zhou, Y.; Wang, B.; Hong, Z. The Shape Trail Test: Application of a new variant of the Trail making test. PLoS ONE 2013, 8, e57333. [Google Scholar] [CrossRef]
- Maier, W.; Buller, R.; Philipp, M.; Heuser, I. The Hamilton Anxiety Scale: Reliability, validity and sensitivity to change in anxiety and depressive disorders. J. Affect. Disord. 1988, 14, 61–68. [Google Scholar] [CrossRef]
- Kong, J.; Liang, C.; Zhao, Y.; Chen, Q.; Xv, H.; Yan, X.; Zhang, H.; Zhang, H. Relationship between social support and self-perceived burden in patients with obstructive sleep apnea: An analysis of chain-mediated effects. Sleep Med. 2024, 119, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Le, G.H.; Phan, L.; Rhee, T.G.; Ho, R.; Meshkat, S.; Teopiz, K.M.; Kwan, A.T.; Mansur, R.B.; Rosenblat, J.D.; et al. Effects of anhedonia on health-related quality of life and functional outcomes in major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2024, 356, 684–698. [Google Scholar] [CrossRef]
- Dunstan, D.A.; Scott, N. Norms for Zung’s Self-rating Anxiety Scale. BMC Psychiatry 2020, 20, 90. [Google Scholar] [CrossRef]
- Zung, W.W. A Self-Rating Depression Scale. Arch. Gen. Psychiatry 1965, 12, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Ji, Y.; Lou, Z.; Hou, Y.; Ruan, L. Left intermittent theta burst stimulation combined with right low-frequency rTMS as an additional treatment for major depression: A retrospective study. Indian J. Psychiatry 2022, 64, 364–369. [Google Scholar] [CrossRef]
- Diana, M.; Raij, T.; Melis, M.; Nummenmaa, A.; Leggio, L.; Bonci, A. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat. Rev. Neurosci. 2017, 18, 685–693. [Google Scholar] [CrossRef]
- Nashida, T.; Yabe, H.; Sato, Y.; Hiruma, T.; Sutoh, T.; Shinozaki, N.; Kaneko, S. Automatic auditory information processing in sleep. Sleep 2000, 23, 821–828. [Google Scholar] [CrossRef]
- Demirayak, P.; Kiyi, I.; Isbitiren, Y.O.; Yener, G. Cognitive load associates prolonged P300 latency during target stimulus processing in individuals with mild cognitive impairment. Sci. Rep. 2023, 13, 15956. [Google Scholar] [CrossRef] [PubMed]
- Gmaj, B.; Januszko, P.; Kaminski, J.; Drozdowicz, E.; Kopera, M.; Wolynczyk-Gmaj, D.; Szelenberger, W.; Wojnar, M. EEG source activity during processing of neutral stimuli in subjects with anxiety disorders. Acta Neurobiol. Exp. 2016, 76, 75–85. [Google Scholar] [CrossRef]
- Nabavi, S.; Fox, R.; Proulx, C.D.; Lin, J.Y.; Tsien, R.Y.; Malinow, R. Engineering a memory with LTD and LTP. Nature 2014, 511, 348–352. [Google Scholar] [CrossRef]
- Torrecillos, F.; Falato, E.; Pogosyan, A.; West, T.; Di Lazzaro, V.; Brown, P. Motor Cortex Inputs at the Optimum Phase of Beta Cortical Oscillations Undergo More Rapid and Less Variable Corticospinal Propagation. J. Neurosci. 2019, 40, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Curtin, A.; Ayaz, H.; Tang, Y.; Sun, J.; Wang, J.; Tong, S. Enhancing neural efficiency of cognitive processing speed via training and neurostimulation: An fNIRS and TMS study. NeuroImage 2019, 198, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Fricke, C.; Gentner, R.; Alizadeh, J.; Classen, J. Linking Individual Movements to a Skilled Repertoire: Fast Modulation of Motor Synergies by Repetition of Stereotyped Movements. Cereb. Cortex 2019, 30, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Roebuck, H.; Bourke, P.; Guo, K. Role of lateral and feedback connections in primary visual cortex in the processing of spatiotemporal regularity—A TMS study. Neuroscience 2014, 263, 231–239. [Google Scholar] [CrossRef] [PubMed]


| MDD Group (n = 42) | MDD Group | p Value | ||
|---|---|---|---|---|
| First-Episode Group (n = 21) | Recurrent Group (n = 21) | |||
| Gender (Female, %) | 28 (66.67) | 15 (71.43) | 13 (61.91) | 0.74 |
| Age (years) | 31.07 (9.20) | 26.1 (6.07) | 36.05 (9.21) | <0.001 |
| Height (cm) | 163.71 (8.47) | 164.33 (8.14) | 163.10 (8.95) | 0.64 |
| Weight (kg) | 59.27 (12.93) | 57.95 (11.49) | 60.60 (14.39) | 0.52 |
| Smoking (%) | 12 (28.57) | 6 (28.57) | 6 (28.57) | 0.48 |
| Alcohol consumption (%) | 19 (45.24) | 11 (52.39) | 8 (38.10) | 0.54 |
| Family history of psychiatric disorders (%) | 1 (2.38) | 1 (4.76) | 0 (0) | 1.00 |
| Pittsburgh sleep quality index | 12.55 (4.37) | 12.43 (4.06) | 12.67 (4.77) | 0.86 |
| Childhood trauma questionnaire | 54.48 (6.77) | 55.57 (5.29) | 53.38 (7.97) | 0.30 |
| Trail making test-A | 31.58 (11.96) | 31.39 (11.94) | 31.78 (11.94) | 0.92 |
| Trail making test-B | 86.84 (32.16) | 80.68 (31.85) | 93.01 (32.04) | 0.22 |
| Digit span test-forward | 7.64 (1.38) | 8.05 (1.43) | 7.24 (1.22) | 0.06 |
| Digit span test—backward | 4.55 (1.71) | 4.86 (1.71) | 4.24 (1.70) | 0.25 |
| Hamilton anxiety rating scale | 15.81 (5.61) | 14.71 (5.51) | 16.90 (5.64) | 0.21 |
| Hamilton depression rating scale | 21.17 (3.89) | 21.10 (4.32) | 21.24 (3.52) | 0.91 |
| Social support rating scale—objective support | 7.14 (2.86) | 7.19 (2.50) | 7.10 (3.24) | 0.92 |
| Social support rating scale—subjective support | 16.10 (4.84) | 16.14 (4.40) | 16.05 (5.35) | 0.95 |
| Social support rating scale—utilization of Support | 6.43 (1.47) | 6.81 (1.57) | 6.05 (1.28) | 0.09 |
| Beck Scale for suicide ideation in the past week | 7.69 (8.16) | 9.05 (8.98) | 6.33 (7.21) | 0.17 |
| Snaith–Hamilton pleasure scale | 33.21 (7.26) | 32.33 (6.80) | 34.10 (7.77) | 0.44 |
| Emotion regulation score | 42.45 (9.25) | 42.71 (8.71) | 42.19 (9.96) | 0.86 |
| Self-rating anxiety scale | 46.64 (9.97) | 48.05 (9.04) | 45.24 (10.86) | 0.37 |
| Self-rating depression scale | 55.10 (7.92) | 55.67 (7.80) | 54.52 (8.18) | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, L.; Wu, J.; Huang, S.; Liu, D.; Tan, X.; Yan, H.; Yan, G.; Yao, D. The Efficacy and Central Remodeling Mechanism of a Composite TMS Pattern in First-Episode and Recurrent Depressive Disorders. Brain Sci. 2025, 15, 801. https://doi.org/10.3390/brainsci15080801
Pu L, Wu J, Huang S, Liu D, Tan X, Yan H, Yan G, Yao D. The Efficacy and Central Remodeling Mechanism of a Composite TMS Pattern in First-Episode and Recurrent Depressive Disorders. Brain Sciences. 2025; 15(8):801. https://doi.org/10.3390/brainsci15080801
Chicago/Turabian StylePu, Li, Jiang Wu, Shan Huang, Dandan Liu, Xi Tan, Hongmei Yan, Guojian Yan, and Dezhong Yao. 2025. "The Efficacy and Central Remodeling Mechanism of a Composite TMS Pattern in First-Episode and Recurrent Depressive Disorders" Brain Sciences 15, no. 8: 801. https://doi.org/10.3390/brainsci15080801
APA StylePu, L., Wu, J., Huang, S., Liu, D., Tan, X., Yan, H., Yan, G., & Yao, D. (2025). The Efficacy and Central Remodeling Mechanism of a Composite TMS Pattern in First-Episode and Recurrent Depressive Disorders. Brain Sciences, 15(8), 801. https://doi.org/10.3390/brainsci15080801






