From Lockdowns to Long COVID—Unraveling the Link Between Sleep, Chronotype, and Long COVID Symptoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measures
- -
- Insomnia Severity Index (ISI) [22]—results from this measure are not reported in the current publication.
- -
- Pittsburgh Sleep Quality Index (PSQI)—a PSQI score higher than 5 is widely recognized as indicative of poor sleep quality [23]. Cronbach’s α for the PSQI was 0.84 in the current study.
- -
- -
- Pre-Sleep Arousal Scale (PSAS) [26]—assesses symptoms of cognitive and somatic arousal experienced at bedtime, with clinically relevant cut-off scores of ≥14 for somatic arousal and ≥20 for cognitive arousal [27,28]. Cronbach’s α for the somatic subscale was 0.82, and for the cognitive subscale, −0.91.
2.3. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Sleep Variables
3.3. Associations Between Sleep and Long COVID Symptoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jahrami, H.; BaHammam, A.S.; Bragazzi, N.L.; Saif, Z.; Faris, M.; Vitiello, M.V. Sleep problems during the COVID-19 pandemic by population: A systematic review and meta-analysis. J. Clin. Sleep Med. 2021, 17, 299–313. [Google Scholar] [CrossRef]
- Jahrami, H.A.; Alhaj, O.A.; Humood, A.M.; Alenezi, A.F.; Fekih-Romdhane, F.; AlRasheed, M.M.; Saif, Z.Q.; Bragazzi, N.L.; Pandi-Perumal, S.R.; BaHammam, A.S.; et al. Sleep disturbances during the COVID-19 pandemic: A systematic review, meta-analysis, and meta-regression. Sleep Med. Rev. 2022, 62, 101591. [Google Scholar] [CrossRef]
- Salari, N.; Hosseinian-Far, A.; Jalali, R.; Vaisi-Raygani, A.; Rasoulpoor, S.; Mohammadi, M.; Rasoulpoor, S.; Khaledi-Paveh, B. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: A systematic review and meta-analysis. Glob. Health 2020, 16, 57. [Google Scholar] [CrossRef]
- Robinson, E.; Sutin, A.R.; Daly, M.; Jones, A. A systematic review and meta-analysis of longitudinal cohort studies comparing mental health before versus during the COVID-19 pandemic in 2020. J. Affect. Disord. 2022, 296, 567–576. [Google Scholar] [CrossRef]
- World Health Organization. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus, 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 1 January 2024).
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.-M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef]
- Kubota, T.; Kuroda, N.; Sone, D. Neuropsychiatric aspects of long COVID: A comprehensive review. Psychiatry Clin. Neurosci. 2023, 77, 84–93. [Google Scholar] [CrossRef]
- Scarpelli, S.; De Santis, A.; Alfonsi, V.; Gorgoni, M.; Morin, C.M.; Espie, C.; Merikanto, I.; Chung, F.; Penzel, T.; Bjorvatn, B.; et al. The role of sleep and dreams in long COVID. J. Sleep Res. 2023, 32, e13789. [Google Scholar] [CrossRef]
- Tedjasukmana, R.; Budikayanti, A.; Islamiyah, W.R.; Witjaksono, A.M.A.L.; Hakim, M. Sleep disturbance in post COVID-19 conditions: Prevalence and quality of life. Front. Neurol. 2023, 13, 1095606. [Google Scholar] [CrossRef]
- Schilling, C.; Nieters, A.; Schredl, M.; Peter, R.S.; Rothenbacher, D.; Brockmann, S.O.; Göpel, S.; Kindle, G.; Merle, U.; Steinacker, J.M.; et al. Pre-existing sleep problems as a predictor of post-acute sequelae of COVID-19. J. Sleep Res. 2024, 33, e13949. [Google Scholar] [CrossRef]
- Chen, S.-J.; Morin, C.M.; Ivers, H.; Wing, Y.K.; Partinen, M.; Merikanto, I.; Holzinger, B.; Espie, C.A.; De Gennaro, L.; Dauvilliers, Y.; et al. The association of insomnia with long COVID: ICOSS-II study. Sleep Med. 2023, 112, 216–222. [Google Scholar] [CrossRef]
- Salfi, F.; Amicucci, G.; Corigliano, D.; Viselli, L.; D’Atri, A.; Tempesta, D.; Ferrara, M. Poor sleep quality, insomnia, and short sleep duration before infection predict long-term symptoms after COVID-19. Brain Behav. Immun. 2023, 112, 140–151. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Silva, D.F.; Lima, M.M.; Almeida, R.M. The association between probable post-COVID-19 condition and sleep-related parameters. J. Sleep Res. 2024, 33, e14215. [Google Scholar] [CrossRef]
- Merikanto, I.; Kortesoja, L.; Benedict, C.; Chung, F.; Cedernaes, J.; Espie, C.A.; Morin, C.M.; Dauvilliers, Y.; Partinen, M.; De Gennaro, L.; et al. Evening-types show highest increase of sleep and mental health problems during the COVID-19 pandemic-multinational study on 19 267 adults. Sleep 2022, 45, zsab216. [Google Scholar] [CrossRef]
- Salfi, F.; Amicucci, G.; Corigliano, D.; Viselli, L.; D’Atri, A.; Tempesta, D.; Gorgoni, M.; Scarpelli, S.; Alfonsi, V.; Ferrara, M. Two years after lockdown: Longitudinal trajectories of sleep disturbances and mental health over the COVID-19 pandemic, and the effects of age, gender and chronotype. J. Sleep Res. 2023, 32, e13767. [Google Scholar] [CrossRef]
- Coelho, J.; Micoulaud-Franchi, J.A.; Wiet, A.S.; Nguyen, D.; Taillard, J.; Philip, P. Circadian misalignment is associated with Covid-19 infection. Sleep Med. 2022, 93, 71–74. [Google Scholar] [CrossRef]
- Quan, S.F.; Weaver, M.D.; Czeisler, M.É.; Barger, L.K.; Booker, L.A.; Howard, M.E.; Jackson, M.L.; Lane, R.I.; McDonald, C.F.; Ridgers, A.; et al. Insomnia, Poor Sleep Quality and Sleep Duration, and Risk for COVID-19 Infection and Hospitalization. Am. J. Med. 2023, 136, 780–788.e5. [Google Scholar] [CrossRef]
- Wang, S.; Huang, T.; Weisskopf, M.G.; Kang, J.H.; Chavarro, J.E.; Roberts, A.L. Multidimensional Sleep Health Prior to SARS-CoV-2 Infection and Risk of Post-COVID-19 Condition. JAMA Netw. Open 2023, 6, e2315885. [Google Scholar] [CrossRef]
- National Center for Disease Control and Public Health of Georgia. COVID-19 Response and Restrictions in Georgia, 2020–2022. Available online: https://test.ncdc.ge/Handlers/GetFile.ashx?ID=c6c26041-e123-4591-b1c6-50103eb5205f (accessed on 12 September 2024).
- Tsaava, M.; Oniani, N.; Eliozishvili, M.; Sakhelashvili, I.; Tkemaladze, N.; Aladashvili, T.; Basishvili, T.; Darchia, N. Age-Based Differences in Sleep Quality, Pre-Sleep Arousal, and Psychosocial Factors during the Second Wave Lockdown of the COVID-19 Pandemic in Georgia-A Higher Vulnerability of Younger People. Int. J. Environ. Res. Public Health 2022, 19, 16221. [Google Scholar] [CrossRef]
- Basishvili, T.; Oniani, N.; Sakhelashvili, I.; Eliozishvili, M.; Khizanashvili, M.; Arabidze, M.; Tsaava, M.; Charekishvili, T.; Tsertsvadze, N.; Darchia, N. Insomnia, Pre-Sleep Arousal, Psychosocial Factors and Changes in Sleep Pattern during the Second Wave Lockdown of the COVID-19 Pandemic in Georgia. Brain Sci. 2021, 12, 17. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Cohen, S.; Williamson, G. Perceived stress in a probability sample of the United States. Spacapan, S., Oskamp, S., Eds.; In The Social Psychology of Health; Sage publication: Thousand Oaks, CA, USA, 1988; pp. 31–67. [Google Scholar]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Nicassio, P.M.; Mendlowitz, D.R.; Fussell, J.J.; Petras, L. The development of the pre-sleep arousal scale. Behav. Res. Ther. 1985, 23, 263–271. [Google Scholar] [CrossRef]
- Puzino, K.; Amatrudo, G.; Sullivan, A.; Vgontzas, A.N.; Fernandez-Mendoza, J. Clinical Significance and Cut-Off Scores for the Pre-Sleep Arousal Scale in Chronic Insomnia Disorder: A Replication in a Clinical Sample. Behav. Sleep Med. 2020, 18, 705–718. [Google Scholar] [CrossRef]
- Gorgoni, M.; Scarpelli, S.; Mangiaruga, A.; Alfonsi, V.; Bonsignore, M.R.; Fanfulla, F.; De Gennaro, L. Pre-sleep arousal and sleep quality during lockdown. Sleep Med. 2021, 88, 46–57. [Google Scholar] [CrossRef]
- Basishvili, T.; Eliozishvili, M.; Maisuradze, L.; Lortkipanidze, N.; Nachkebia, N.; Oniani, T.; Gvilia, I.; Darchia, N. Insomnia in a displaced population is related to war-associated remembered stress. Stress Health 2012, 28, 186–192. [Google Scholar] [CrossRef]
- Sakhelashvili, I.; Eliozishvili, M.; Basishvili, T.; Datunashvili, M.; Oniani, N.; Cervena, K.; Darchia, N. Sleep-wake patterns and sleep quality in urban Georgia. Transl. Neurosci. 2016, 7, 62–70. [Google Scholar] [CrossRef]
- Sakhelashvili, I.; Eliozishvili, M.; Oniani, N.; Darchia, N.; Bruni, O. Sleep and psycho-behavioral problems in internally displaced children in Georgia. Sleep Med. 2018, 50, 42–47. [Google Scholar] [CrossRef]
- Lang, A.J.; Wilkins, K.; Roy-Byrne, P.P.; Golinelli, D.; Chavira, D.; Sherbourne, C.; Rose, R.D.; Bystritsky, A.; Sullivan, G.; Craske, M.G.; et al. Abbreviated PTSD Checklist (PCL) as a guide to clinical response. Gen. Hosp. Psychiatry 2012, 34, 332–338. [Google Scholar] [CrossRef]
- Turco, M.; Corrias, M.; Chiaromanni, F.; Bano, M.; Salamanca, M.; Caccin, L.; Merkel, C.; Amodio, P.; Romualdi, C.; De Pittà, C.; et al. The self-morningness/eveningness (Self-ME): An extremely concise and totally subjective assessment of diurnal preference. Chronobiol. Int. 2015, 32, 1192–1200. [Google Scholar] [CrossRef]
- Zakia, H.; Pradana, K.; Iskandar, S. Risk factors for psychiatric symptoms in patients with long COVID: A systematic review. PLoS ONE 2023, 18, e0284075. [Google Scholar] [CrossRef]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’HAra, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Merikanto, I.; Dauvilliers, Y.; Chung, F.; Wing, Y.K.; De Gennaro, L.; Holzinger, B.; Bjorvatn, B.; Morin, C.M.; Penzel, T.; Benedict, C.; et al. Sleep symptoms are essential features of long COVID: The ICOSS-II study. J. Sleep Res. 2023, 32, e13754. [Google Scholar] [CrossRef]
- Shao, H.; Chen, H.; Xu, K.; Gan, Q.; Chen, M.; Zhao, Y.; Yu, S.; Li, Y.K.; Chen, L.; Cai, B. Investigating associations between COVID-19, long COVID, and sleep disturbances. JMIR Public Health Surveill. 2024, 10, e53522. [Google Scholar] [CrossRef]
- Morin, C.M.; Bjorvatn, B.; Chung, F.; Holzinger, B.; Partinen, M.; Penzel, T.; Ivers, H.; Wing, Y.K.; Chan, N.Y.; Merikanto, I.; et al. Insomnia, anxiety, and depression during the COVID-19 pandemic: An international collaborative study. Sleep Med. 2021, 87, 38–45. [Google Scholar] [CrossRef]
- Salfi, F.; Lauriola, M.; D’Atri, A.; Amicucci, G.; Viselli, L.; Tempesta, D.; Ferrara, M. Predictors of sleep disturbances during the COVID-19 lockdown in Italy. Sci. Rep. 2021, 11, 11416. [Google Scholar] [CrossRef]
- Leone, M.J.; Sigman, M.; Golombek, D.A. Effects of lockdown on human sleep and chronotype. Curr. Biol. 2020, 30, R930–R931. [Google Scholar] [CrossRef]
- Blume, C.; Schmidt, M.H.; Cajochen, C. Effects of COVID-19 lockdown on human sleep and rhythms. Curr. Biol. 2020, 30, R795–R797. [Google Scholar] [CrossRef]
- Korman, M.; Tkachev, V.; Reis, C.; Komada, Y.; Kitamura, S.; Gubin, D.; Kumar, V.; Roenneberg, T. Social time pressure’s impact on sleep during COVID-19. Sci. Rep. 2020, 10, 22225. [Google Scholar] [CrossRef]
- Cellini, N.; Canale, N.; Mioni, G.; Costa, S. Changes in sleep, time perception, and digital media use. J. Sleep Res. 2020, 29, e13074. [Google Scholar] [CrossRef]
- Benham, G. Stress and sleep in college students during the COVID-19 pandemic. Stress Health 2021, 37, 504–515. [Google Scholar] [CrossRef]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef]
- Mehra, R.; Teodorescu, M. Sleep, circadian disruption, and microbial-immune interactions. Chest 2018, 154, 740–742. [Google Scholar] [CrossRef]
- Haspel, J.A.; Anafi, R.; Brown, M.K.; Cermakian, N.; Depner, C.; Desplats, P.; Gelman, A.E.; Haack, M.; Jelic, S.; Kim, B.S.; et al. Perfect timing: Circadian rhythms, sleep, and immunity—An NIH workshop summary. JCI Insight 2020, 5, e131487. [Google Scholar] [CrossRef]
- Maĭsuradze, L.; Lortkipanidze, N.; Eliozishvili, M.; Gvilia, I.; Darchia, N. Posttraumatic stress disorder and insomnia development in individuals displaced from Shida Kartli, Georgia. Georgian Med. News 2010, 180, 64–69. [Google Scholar] [PubMed]
- Morin, C.M.; Rodrigue, S.; Ivers, H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom. Med. 2003, 65, 259–267. [Google Scholar] [CrossRef]
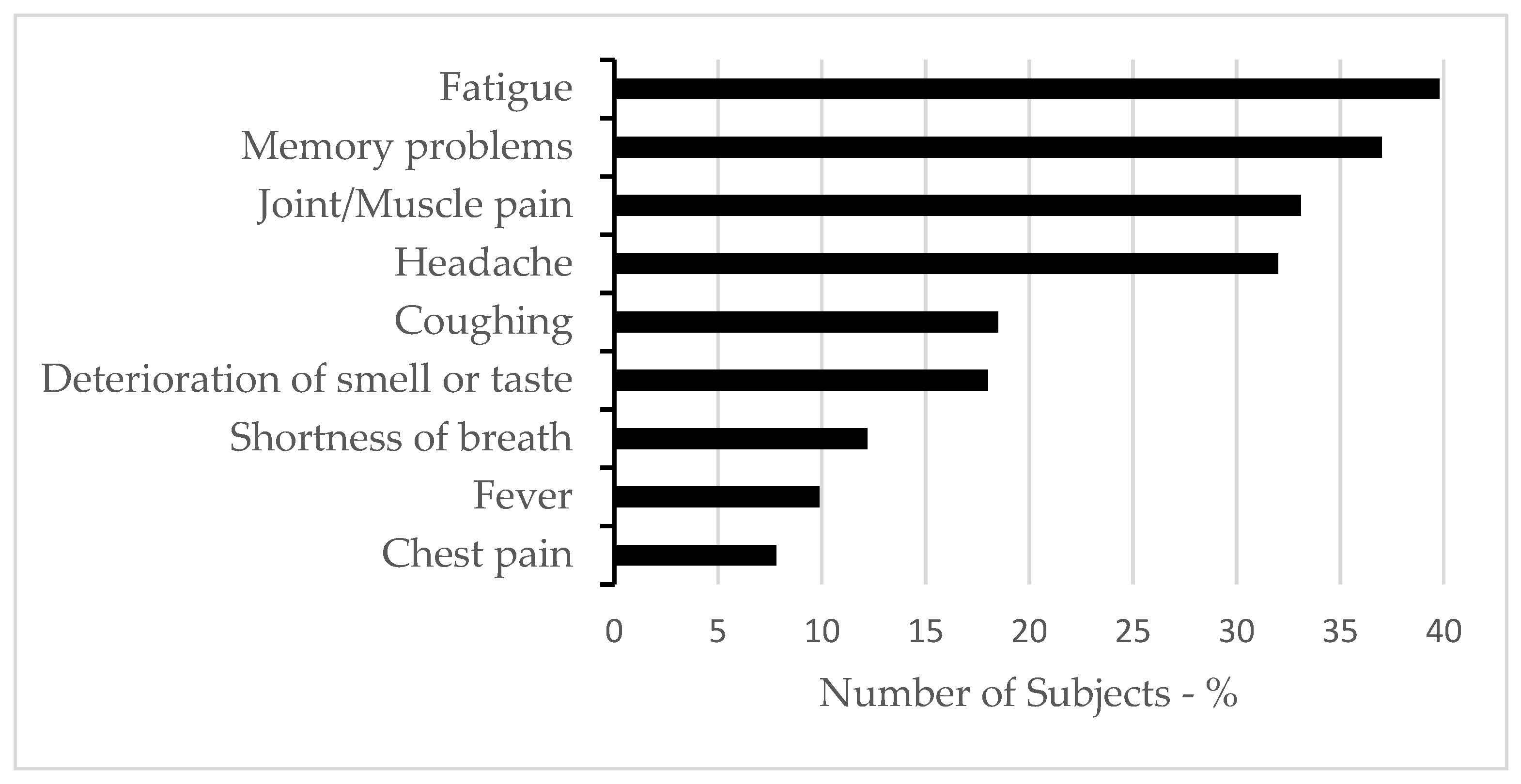
| Total Sample n = 384 | Long COVID n = 284 | Non-Long COVID n = 100 | Statistics | |
|---|---|---|---|---|
| Age | 41.76 ± 12.8 | 41.42 ± 12.66 | 42.71 ± 13.38 | t(382) = 0.864, p = 0.388 |
| Sex | ||||
| Male | 57 (14.8%) | 42 (14.8%) | 15 (15.0%) | χ2(1) = 0.003, p = 0.959 |
| Female | 327 (85.2%) | 242 (85.2%) | 85 (85.0%) | |
| Marital status | ||||
| Married/cohabiting | 225 (58.6%) | 167 (58.8%) | 58 (58.0%) | χ2(1) = 0.020, p = 0.889 |
| Single/divorced/widowed | 159 (41.4%) | 117 (41.2%) | 42 (42.0%) | |
| Education | ||||
| University | 323 (84.1%) | 237 (83.5%) | 86 (86.0%) | χ2(1) = 0.360, p = 0.549 |
| High school/student | 61 (15.9%) | 47 (16.5%) | 14 (14.0%) | |
| Employment | ||||
| Yes | 299 (77.9%) | 220 (77.5%) | 79 (79.0%) | χ2(1) = 0.101, p = 0.750 |
| No | 85 (22.1%) | 64 (22.5%) | 21 (21.0%) | |
| Chronic disease | ||||
| Yes | 70 (18.2%) | 51 (18.0%) | 19 (19.0%) | χ2(1) = 0.054, p = 0.816 |
| No | 314 (81.8%) | 233 (82.0%) | 81 (81.0%) | |
| COVID-19 Frequency | ||||
| Once | 242 (63.0%) | 165 (58.1%) | 77 (77.0%) | χ2(1) = 11.338, p = 0.001 |
| ≥1 | 142 (37.0%) | 119 (41.9%) | 23 (23.0%) | |
| COVID-19 Severity | ||||
| Mild | 170 (44.3%) | 112 (39.4%) | 58 (58.0%) | χ2(1) = 10.330, p = 0.001 |
| Severe | 214 (55.7%) | 172 (60.6%) | 42 (42.0%) | |
| COVID-19 Impact on Finances | ||||
| No impact | 114 (29.7%) | 73 (25.7%) | 41 (41.0%) | χ2(2) = 12.127, p = 0.002 |
| Slight impact | 137 (35.7%) | 100 (35.2%) | 37 (37.0%) | |
| Strong impact | 133 (34.6%) | 111 (39.1%) | 22 (22.0%) | |
| Vaccination | ||||
| Yes | 287 (74.7%) | 204 (71.8%) | 83 (83.0%) | χ2(1) = 4.887, p = 0.027 |
| No | 97 (25.3%) | 80 (28.2%) | 17 (17.0%) | |
| BMI | 25.6 ± 4.9 | 25.60 ± 4.90 | 25.67 ± 4.91 | t(382) = 0.112, p = 0.911 |
| PSS | 6.6 ± 2.5 | 6.77 ± 2.53 | 6.15 ± 2.50 | t(382) = −2.107, p < 0.036 |
| Anxiety | 2.90 ± 1.23 | 3.01 ± 1.24 | 2.57 ± 1.17 | t(382) = −3.136, p = 0.002 |
| Depression | 2.69 ± 1.34 | 2.86 ± 1.32 | 2.21 ± 1.26 | t(382) = −4.277, p < 0.001 |
| Aggression | 2.32 ± 1.14 | 2.42 ± 1.16 | 2.02 ± 1.04 | t(382) = −3.065, p = 0.002 |
| PTSS | 4.03 ± 2.14 | 1.94 ± 1.12 | 1.61 ± 0.97 | t(382) = −2.598, p = 0.010 |
| Social Isolation | 2.10 ± 1.16 | 2.18 ± 1.17 | 1.87 ± 1.08 | t(382) = −2.290, p = 0.023 |
| Chronotype | ||||
| Extreme morning | 90 (23.4%) | 69 (24.3%) | 21 (21.0%) | χ2(4) = 6.485, p = 0.166 |
| Morning | 80 (20.8%) | 57 (20.1%) | 23 (23.0%) | |
| Intermediate | 75 (19.5%) | 48 (16.9%) | 27 (27.0%) | |
| Evening | 79 (20.6%) | 62 (21.8%) | 17 (17.0%) | |
| Extreme evening | 60 (15.6%) | 48 (16.9%) | 12 (12.0%) | |
| Clinically Relevant PSAS- Somatic | ||||
| Yes | 162 (42.2%) | 139 (48.9%) | 23 (23.0%) | χ2(1) = 20.410, p < 0.001 |
| No | 222 (57.8%) | 145 (51.1%) | 77 (77.0%) | |
| Clinically Relevant PSAS- Cognitive | ||||
| Yes | 186 (48.4%) | 154 (54.2%) | 32 (32.0%) | χ2(1) = 14.627, p < 0.001 |
| No | 198 (51.6%) | 130 (45.8%) | 68 (68.0%) | |
| PSQI score | 6.2 ± 3.8 | 6.85 ± 0.2 | 4.34 ± 0.3 | t(382) = −5.876, p < 0.001 |
| PSQI categories | ||||
| Poor | 176 (45.8%) | 152 (53.5%) | 24 (24.0%) | χ2(1) = 25.962, p < 0.001 |
| Good | 208 (54.2%) | 132 (46.5%) | 76 (76.0%) | |
| Pre-Pandemic Sleep Quality | ||||
| Good | 225 (58.6%) | 159 (56.0%) | 66 (66.0%) | χ2(2) = 8.467, p = 0.015 |
| Intermediate | 113 (29.4%) | 83 (29.2%) | 30 (30.0%) | |
| Poor | 46 (12.0%) | 42 (14.8%) | 4 (4.0%) | |
| Changes in Sleep Quality During the Pandemic Peak | ||||
| No change | 233 (60.7%) | 154 (54.2%) | 79 (79.0%) | χ2(2) = 19.759, p < 0.001 |
| Worsening | 133 (34.6%) | 116 (40.9%) | 17 (17.0%) | |
| Improving | 18 (4.7%) | 14 (4.9%) | 4 (4.0%) |
| Predictor | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||
| COVID-19 Frequency | |||||||
| Once | Reference | Reference | Reference | ||||
| ≥1 | 2.42 (1.36–4.31) | 0.003 | 2.68 (1.49–4.82) | 0.001 | 2.04 (1.13–3.67) | 0.018 | |
| COVID-19 Severity | |||||||
| Mild | Reference | Reference | Reference | ||||
| Severe | 2.03 (1.20–3.44) | 0.008 | 2.18 (1.27–3.75) | 0.005 | 2.00 (1.16–3.43) | 0.012 | |
| COVID-19 Impact on Finances | |||||||
| No impact | Reference | Reference | Reference | ||||
| Slight impact | 1.39 (0.74–2.61) | 0.300 | 1.51 (0.80–2.86) | 0.204 | 1.35 (0.71–2.57) | 0.358 | |
| Strong impact | 2.43 (1.24–4.78) | 0.010 | 2.49 (1.25–4.95) | 0.009 | 2.46 (1.23–4.91) | 0.011 | |
| Vaccination | |||||||
| No | Reference | Reference | Reference | ||||
| Yes | 1.70 (0.84–3.46) | 0.143 | 1.61 (0.78–3.34) | 0.201 | 1.75 (0.84–3.63) | 0.135 | |
| Sleep Quality | |||||||
| Good | Reference | Reference | Reference | ||||
| Poor | 2.71 (1.47–4.99) | 0.001 | 3.02 (1.57–5.81) | 0.001 | 2.48 (1.34–4.60) | 0.004 | |
| Chronotype | |||||||
| Intermediate | Reference | Reference | Reference | ||||
| Extreme morning | 2.63 (1.20–5.76) | 0.016 | 2.59 (1.17–5.75) | 0.019 | 2.71 (1.22–6.04) | 0.015 | |
| Morning | 1.54 (0.70–3.35) | 0.282 | 1.57 (0.71–3.48) | 0.266 | 1.62 (0.72–3.62) | 0.244 | |
| Evening | 1.89 (0.84–4.26) | 0.124 | 1.99 (0.86–4.57) | 0.107 | 1.90 (0.83–4.35) | 0.128 | |
| Extreme evening | 2.23 (0.89–5.60) | 0.087 | 2.12 (0.83–5.39) | 0.115 | 2.82 (1.09–7.29) | 0.033 | |
| Clinically Relevant PSAS- Somatic | |||||||
| No | Reference | Reference | Reference | ||||
| Yes | 1.97 (1.01–3.87) | 0.049 | 2.44 (1.20–4.96) | 0.013 | 2.13 (1.06–4.25) | 0.033 | |
| Clinically Relevant PSAS- Cognitive | |||||||
| No | Reference | Reference | Reference | ||||
| Yes | 1.24 (0.63–2.44) | 0.535 | 1.08 (0.54–2.16) | 0.834 | 1.34 (0.67–2.70) | 0.405 | |
| Pre-Pandemic Sleep Quality | |||||||
| Good | Reference | ||||||
| Intermediate | 1.86 (0.56–6.12) | 0.308 | |||||
| Poor | 0.42 (0.21–0.82) | 0.011 | |||||
| Changes in Sleep Quality During the Pandemic Peak | |||||||
| No change | Reference | ||||||
| Worsening | 3.16 (1.64–6.09) | 0.001 | |||||
| Improving | 0.92 (0.25–3.35) | 0.901 | |||||
| Nagelkerke R2 | 0.258 | 0.290 | 0.300 | ||||
| Correct classification (%) | 77.6% | 78.1% | 78.9% | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsaava, M.; Basishvili, T.; Sakhelashvili, I.; Eliozishvili, M.; Oniani, N.; Lortkipanidze, N.; Tarielashvili, M.; Khoshtaria, L.; Darchia, N. From Lockdowns to Long COVID—Unraveling the Link Between Sleep, Chronotype, and Long COVID Symptoms. Brain Sci. 2025, 15, 800. https://doi.org/10.3390/brainsci15080800
Tsaava M, Basishvili T, Sakhelashvili I, Eliozishvili M, Oniani N, Lortkipanidze N, Tarielashvili M, Khoshtaria L, Darchia N. From Lockdowns to Long COVID—Unraveling the Link Between Sleep, Chronotype, and Long COVID Symptoms. Brain Sciences. 2025; 15(8):800. https://doi.org/10.3390/brainsci15080800
Chicago/Turabian StyleTsaava, Mariam, Tamar Basishvili, Irine Sakhelashvili, Marine Eliozishvili, Nikoloz Oniani, Nani Lortkipanidze, Maria Tarielashvili, Lali Khoshtaria, and Nato Darchia. 2025. "From Lockdowns to Long COVID—Unraveling the Link Between Sleep, Chronotype, and Long COVID Symptoms" Brain Sciences 15, no. 8: 800. https://doi.org/10.3390/brainsci15080800
APA StyleTsaava, M., Basishvili, T., Sakhelashvili, I., Eliozishvili, M., Oniani, N., Lortkipanidze, N., Tarielashvili, M., Khoshtaria, L., & Darchia, N. (2025). From Lockdowns to Long COVID—Unraveling the Link Between Sleep, Chronotype, and Long COVID Symptoms. Brain Sciences, 15(8), 800. https://doi.org/10.3390/brainsci15080800






