Delving into the Perception, Use, and Context of Duloxetine in Clinical Practice: An Analysis Based on the Experience of Healthcare Professionals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Analysis
3. Results
3.1. Familiarity and Routine Use of Duloxetine in the Context of Major Depressive Disorder
3.2. Perceived Efficacy of Duloxetine in Various Clinical Contexts
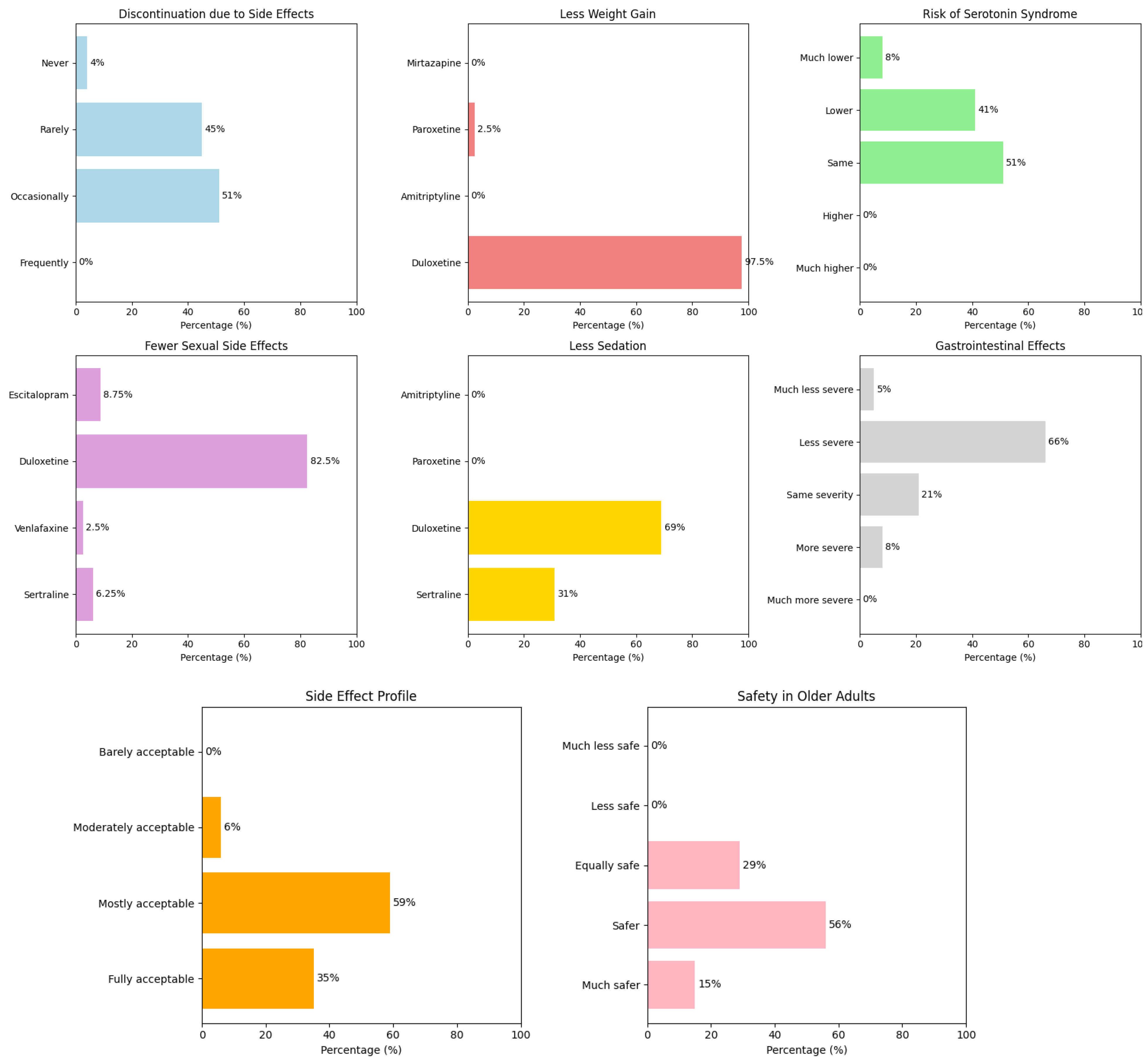
3.3. Safety and Tolerability of Duloxetine
3.4. General Perception of Duloxetine: Adherence, Combination Use, and Treatment Satisfaction
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues-Amorim, D.; Olivares, J.M.; Spuch, C.; Rivera-Baltanás, T. A Systematic Review of Efficacy, Safety, and Tolerability of Duloxetine. Front. Psychiatry 2020, 11, 554899. [Google Scholar] [CrossRef]
- Monteleone, F.; Caputo, M.; Felice Tecce, M.; Capasso, A. Duloxetine in the Treatment of Depression: An Overview. Cent. Nerv. Syst. Agents Med. Chem. 2011, 11, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Fagan, H.A.; Baldwin, D.S. Pharmacological Treatment of Generalised Anxiety Disorder: Current Practice and Future Directions. Expert Rev. Neurother. 2023, 23, 535–548. [Google Scholar] [CrossRef]
- Hossain, S.M.; Hussain, S.M.; Ekram, A.R.M.S. Duloxetine in Painful Diabetic Neuropathy: A Systematic Review. Clin. J. Pain 2016, 32, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Maffulli, N.; Eschweiler, J.; Baroncini, A.; Bell, A.; Colarossi, G. Duloxetine for Fibromyalgia Syndrome: A Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2023, 18, 504. [Google Scholar] [CrossRef] [PubMed]
- Ananías, J.; Irarrázaval, S. Is Duloxetine an Alternative in the Treatment of Osteoarthritis? Medwave 2017, 17, e7063. [Google Scholar] [CrossRef]
- Lin, J.; Sklar, G.E.; Oh, V.M.S.; Li, S.C. Factors Affecting Therapeutic Compliance: A Review from the Patient’s Perspective. Ther. Clin. Risk Manag. 2008, 4, 269. [Google Scholar] [CrossRef]
- Nieuwlaat, R.; Wilczynski, N.; Navarro, T.; Hobson, N.; Jeffery, R.; Keepanasseril, A.; Agoritsas, T.; Mistry, N.; Iorio, A.; Jack, S.; et al. Interventions for Enhancing Medication Adherence. Cochrane Database Syst. Rev. 2014, 2014, CD000011. [Google Scholar] [CrossRef]
- Montano, C.B.; Jackson, W.C.; Vanacore, D.; Weisler, R. Considerations When Selecting an Antidepressant: A Narrative Review for Primary Care Providers Treating Adults with Depression. Postgrad. Med. 2023, 135, 449–465. [Google Scholar] [CrossRef]
- Birkinshaw, H.; Friedrich, C.M.; Cole, P.; Eccleston, C.; Serfaty, M.; Stewart, G.; White, S.; Moore, R.A.; Phillippo, D.; Pincus, T. Antidepressants for Pain Management in Adults with Chronic Pain: A Network Meta-analysis. Cochrane Database Syst. Rev. 2023, 2023, CD014682. [Google Scholar] [CrossRef]
- Saha, K.; Torous, J.; Kiciman, E.; De Choudhury, M. Understanding Side Effects of Antidepressants: Large-Scale Longitudinal Study on Social Media Data. JMIR Ment. Health 2021, 8, e26589. [Google Scholar] [CrossRef]
- Ball, S.G.; Desaiah, D.; Zhang, Q.; Thase, M.E.; Perahia, D.G.S. Efficacy and Safety of Duloxetine 60 Mg Once Daily in Major Depressive Disorder: A Review with Expert Commentary. Drugs Context 2013, 2013, 212245. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Wilhelm, S.; Lledo, A.; Schacht, A.; Tölle, T.; Bouhassira, D.; Cruccu, G.; Skljarevski, V.; Freynhagen, R. Duloxetine and Pregabalin: High-Dose Monotherapy or Their Combination? The “COMBO-DN Study”—A Multinational, Randomized, Double-Blind, Parallel-Group Study in Patients with Diabetic Peripheral Neuropathic Pain. Pain 2013, 154, 2616–2625. [Google Scholar] [CrossRef] [PubMed]
- Cheon, E.J.; Lee, J.Y.; Cho, J.H.; Lee, Y.J.; Koo, B.H. Effectiveness of Duloxetine Monotherapy Compared to Combination Therapy with Other Antidepressants in Patients with Major Depressive Disorder: A Short-Term, Retrospective Study. Psychiatry Investig. 2016, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Demyttenaere, K.; Desaiah, D.; Petit, C.; Croenlein, J.; Brecht, S. Time Course of Improvement of Different Symptom Clusters in Patients with Major Depression and Pain Treated with Duloxetine or Placebo. Curr. Med. Res. Opin. 2012, 28, 41–48. [Google Scholar] [CrossRef]
- Fava, M.; Mallinckrodt, C.H.; Detke, M.J.; Watkin, J.G.; Wohlreich, M.M. The Effect of Duloxetine on Painful Physical Symptoms in Depressed Patients: Do Improvements in These Symptoms Result in Higher Remission Rates? J. Clin. Psychiatry 2004, 65, 521–530. [Google Scholar] [CrossRef]
- Kuga, A.; Tsuji, T.; Hayashi, S.; Fujikoshi, S.; Tokuoka, H.; Yoshikawa, A.; Escobar, R.; Tanaka, K.; Azekawa, T. An Observational Study of Duloxetine Versus SSRI Monotherapy in Japanese Patients with Major Depressive Disorder: Subgroup Analyses of Treatment Effectiveness for Pain, Depressive Symptoms, and Quality of Life. Neuropsychiatr. Dis. Treat. 2017, 13, 2115. [Google Scholar] [CrossRef]
- Pittman, R.D.; Sutton, S.S.; Magagnoli, J.; Cummings, T.H. A real-world analysis of antidepressant medications in US veterans aged 60 years and older: A comparative analysis. J. Comp. Eff. Res. 2025, 14, e240187. [Google Scholar] [CrossRef]
- de Heer, E.W.; Dekker, J.; Beekman, A.T.F.; van Marwijk, H.W.J.; Holwerda, T.J.; Bet, P.M.; Roth, J.; Timmerman, L.; van der Feltz-Cornelis, C.M. Comparative Effect of Collaborative Care, Pain Medication, and Duloxetine in the Treatment of Major Depressive Disorder and Comorbid (Sub)Chronic Pain: Results of an Exploratory Randomized, Placebo-Controlled, Multicenter Trial (CC:PAINDIP). Front. Psychiatry 2018, 9, 118. [Google Scholar] [CrossRef]
- Lunn, M.P.T.; Hughes, R.A.C.; Wiffen, P.J. Duloxetine for Treating Painful Neuropathy, Chronic Pain or Fibromyalgia. Cochrane Database Syst. Rev. 2014, 2014, CD007115. [Google Scholar] [CrossRef]
- Valenzuela-Fuenzalida, J.J.; López-Chaparro, M.; Barahona-Vásquez, M.; Campos-Valdes, J.; Cordero Gonzalez, J.; Nova-Baeza, P.; Orellana-Donoso, M.; Suazo-Santibañez, A.; Oyanedel-Amaro, G.; Gutiérrez Espinoza, H. Effectiveness of Duloxetine Versus Other Therapeutic Modalities in Patients with Diabetic Neuropathic Pain: A Systematic Review and Meta-Analysis. Pharmaceuticals 2024, 17, 856. [Google Scholar] [CrossRef]
- Wu, C.S.; Huang, Y.J.; Ko, Y.C.; Lee, C.H. Efficacy and safety of duloxetine in painful diabetic peripheral neuropathy: A systematic review and meta-analysis of randomized controlled trials. Syst. Rev. 2023, 12, 53. [Google Scholar] [CrossRef]
- Li, X.; Zhu, L.; Zhou, C.; Liu, J.; Du, H.; Wang, C.; Fang, S. Efficacy and Tolerability of Short-Term Duloxetine Treatment in Adults with Generalized Anxiety Disorder: A Meta-Analysis. PLoS ONE 2018, 13, e0194501. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, G.; Yang, S.; Liang, W.; Zhang, L.; Wang, C. Duloxetine in Treating Generalized Anxiety Disorder in Adults: A Meta-Analysis of Published Randomized, Double-Blind, Placebo-Controlled Trials. Asia-Pac. Psychiatry 2016, 8, 215–225. [Google Scholar] [CrossRef]
- He, H.; Xiang, Y.; Gao, F.; Bai, L.; Gao, F.; Fan, Y.; Lyu, J.; Ma, X. Comparative Efficacy and Acceptability of First-Line Drugs for the Acute Treatment of Generalized Anxiety Disorder in Adults: A Network Meta-Analysis. J. Psychiatr. Res. 2019, 118, 21–30. [Google Scholar] [CrossRef]
- Kong, W.; Deng, H.; Wan, J.; Zhou, Y.; Zhou, Y.; Song, B.; Wang, X. Comparative Remission Rates and Tolerability of Drugs for Generalised Anxiety Disorder: A Systematic Review and Network Meta-analysis of Double-Blind Randomized Controlled Trials. Front. Pharmacol. 2020, 11, 580858. [Google Scholar] [CrossRef] [PubMed]
- Muscatello, M.R.A.; Zoccali, R.A.; Pandolfo, G.; Mangano, P.; Lorusso, S.; Cedro, C.; Battaglia, F.; Spina, E.; Bruno, A. Duloxetine in Psychiatric Disorders: Expansions beyond Major Depression and Generalized Anxiety Disorder. Front. Psychiatry 2019, 10, 449672. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Schennach, R.; Riedel, M.; Möller, H.J. Duloxetine in the Treatment of Major Psychiatric and Neuropathic Disorders. Expert Rev. Neurother. 2008, 8, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Pollack, M.H.; Endicott, J.; Liebowitz, M.; Russell, J.; Detke, M.; Spann, M.; Ball, S.; Swindle, R. Examining Quality of Life in Patients with Generalized Anxiety Disorder: Clinical Relevance and Response to Duloxetine Treatment. J. Psychiatr. Res. 2008, 42, 1042–1049. [Google Scholar] [CrossRef]
- Smith, E.M.L.; Pang, H.; Cirrincione, C.; Fleishman, S.; Paskett, E.D.; Ahles, T.; Bressler, L.R.; Fadul, C.E.; Knox, C.; Le-Lindqwister, N.; et al. Effect of Duloxetine on Pain, Function, and Quality of Life among Patients with Chemotherapy-Induced Painful Peripheral Neuropathy: A Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2013, 309, 1359. [Google Scholar] [CrossRef]
- Goldstein, D.J. Duloxetine in the Treatment of Major Depressive Disorder. Neuropsychiatr. Dis. Treat. 2007, 3, 193. [Google Scholar] [CrossRef] [PubMed]
- Bitter, I.; Filipovits, D.; Czobor, P. Adverse Reactions to Duloxetine in Depression. Expert Opin. Drug Saf. 2011, 10, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.; Petersen, J.J.; Juul, S.; Kamp, C.B.; Barbateskovic, M.; Moncrieff, J.; Horowitz, M.A.; Maagaard, M.; Katakam, K.K.; Gluud, C.; et al. Beneficial and Harmful Effects of Duloxetine Versus Placebo, “Active Placebo” or No Intervention for Adults with Major Depressive Disorder: A Systematic Review with Meta-Analysis and Trial Sequential Analysis of Randomised Clinical Trials. BMJ Open 2025, 15, e082853. [Google Scholar] [CrossRef] [PubMed]
- Wise, T.N.; Perahia, D.G.S.; Pangallo, B.A.; Losin, W.G.; Wiltse, C.G. Effects of the Antidepressant Duloxetine on Body Weight: Analyses of 10 Clinical Studies. Prim. Care Companion J. Clin. Psychiatry 2006, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Mon, M.A.; García-Montero, C.; Fraile-Martinez, O.; Quintero, J.; Fernandez-Rojo, S.; Mora, F.; Gutiérrez-Rojas, L.; Molina-Ruiz, R.M.; Lahera, G.; Álvarez-Mon, M.; et al. Current Opinions about the Use of Duloxetine: Results from a Survey Aimed at Psychiatrists. Brain Sci. 2023, 13, 333. [Google Scholar] [CrossRef]
- Li, L.; Mi, Q. Serotonin Syndrome From Duloxetine Monotherapy: A Case Report. Cureus 2023, 15, e40933. [Google Scholar] [CrossRef]
- Gelener, P.; Gorgulu, U.; Kutlu, G.; Ucler, S.; Inan, L.E. Serotonin Syndrome Due to Duloxetine. Clin. Neuropharmacol. 2011, 34, 127–128. [Google Scholar] [CrossRef]
- Foong, A.L.; Grindrod, K.A.; Patel, T.; Kellar, J. Demystifying Serotonin Syndrome (or Serotonin Toxicity). Can. Fam. Physician 2018, 64, 720. [Google Scholar]
- Nelson, J.C.; Lu Pritchett, Y.; Martynov, O.; Yu, J.Y.; Mallinckrodt, C.H.; Detke, M.J. The Safety and Tolerability of Duloxetine Compared with Paroxetine and Placebo: A Pooled Analysis of 4 Clinical Trials. Prim. Care Companion J. Clin. Psychiatry 2006, 8, 212. [Google Scholar] [CrossRef]
- Clayton, A.; Kornstein, S.; Prakash, A.; Mallinckrodt, C.; Wohlreich, M. ORIGINAL RESEARCH—PSYCHOLOGY: Changes in Sexual Functioning Associated with Duloxetine, Escitalopram, and Placebo in the Treatment of Patients with Major Depressive Disorder. J. Sex. Med. 2007, 4, 917–929. [Google Scholar] [CrossRef]
- Perahia, D.G.S.; Kajdasz, D.K.; Walker, D.J.; Raskin, J.; Tylee, A. Duloxetine 60 Mg Once Daily in the Treatment of Milder Major Depressive Disorder. Int. J. Clin. Pract. 2006, 60, 613. [Google Scholar] [CrossRef]
- Oliva, V.; Lippi, M.; Paci, R.; Del Fabro, L.; Delvecchio, G.; Brambilla, P.; De Ronchi, D.; Fanelli, G.; Serretti, A. Gastrointestinal Side Effects Associated with Antidepressant Treatments in Patients with Major Depressive Disorder: A Systematic Review and Meta-Analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110266. [Google Scholar] [CrossRef]
- Raskin, J.; Wiltse, C.G.; Dinkel, J.J.; Walker, D.J.; Desaiah, D.; Katona, C. Safety and Tolerability of Duloxetine at 60 Mg Once Daily in Elderly Patients with Major Depressive Disorder. J. Clin. Psychopharmacol. 2008, 28, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Wise, T.N.; Wiltse, C.G.; Iosifescu, D.V.; Sheridan, M.; Xu, J.Y.; Raskin, J. The Safety and Tolerability of Duloxetine in Depressed Elderly Patients with and without Medical Comorbidity. Int. J. Clin. Pract. 2007, 61, 1283. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Mullins, C.D. Treatment Adherence and Persistence with Duloxetine, Venlafaxine XR, and Escitalopram among Patients with Major Depressive Disorder and Chronic Pain-Related Diseases. Curr. Med. Res. Opin. 2011, 27, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, G.; Rozsa, S.; Dome, P.; Barsony, G.; Gonda, X. A Real-World, Prospective, Multicenter, Single-Arm Observational Study of Duloxetine in Patients with Major Depressive Disorder or Generalized Anxiety Disorder. Front. Psychiatry 2021, 12, 689143. [Google Scholar] [CrossRef]
- Able, S.L.; Cui, Z.; Shen, W. Duloxetine Treatment Adherence across Mental Health and Chronic Pain Conditions. ClinicoEcon Outcomes Res. 2014, 6, 75–81. [Google Scholar] [CrossRef][Green Version]
- Menchetti, M.; Ferrari Gozzi, B.; Saracino, M.A.; Mercolini, L.; Petio, C.; Raggi, M.A. Non-Fatal Overdose of Duloxetine in Combination with Other Antidepressants and Benzodiazepines. World J. Biol. Psychiatry 2009, 10, 385–389. [Google Scholar] [CrossRef]
- Ball, S.G.; Desaiah, D.; Spann, M.E.; Zhang, Q.; Russell, J.M.; Robinson, M.J.; Demyttenaere, K. Efficacy of duloxetine on painful physical symptoms in major depressive disorder for patients with clinically significant painful physical symptoms at baseline: A meta-analysis of 11 double-blind, placebo-controlled clinical trials. Prim. Care Companion CNS Disord. 2011, 13, PCC.11r01181. [Google Scholar] [CrossRef]
- Baldaçara, L. Duloxetine: An Update. Res. Soc. Dev. 2024, 13, e7313345331. [Google Scholar] [CrossRef]
- Bhattacharyya, U.; John, J.; Lam, M.; Fisher, J.; Sun, B.; Baird, D.; Burgess, S.; Chen, C.-Y.; Lencz, T. Circulating Blood-Based Proteins in Psychopathology and Cognition: A Mendelian Randomization Study. JAMA Psychiatry 2025, 82, 481–491. [Google Scholar] [CrossRef]
- Bokhari, S.A.; Nasr, M.H.; Alawadhi, Y.T. Efficacy of Supratherapeutic Duloxetine Combined with Cognitive-Behavioral Therapy in Severe Treatment-Resistant Obsessive-Compulsive Disorder with Comorbid Depression: A Case Report. Cureus 2024, 16, e74541. [Google Scholar] [CrossRef]
- Cui, Y.; Abdi, S.A.H.; Wei, J.; Azhar, G. The Long-Term Cardiovascular Risks of Duloxetine Use in Older Adults: A Retrospective Medical Record-Based Adverse Drug Reaction Assessment. J. Clin. Med. 2024, 13, 7595. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraile-Martinez, O.; Garcia-Montero, C.; Alvarez-Mon, M.A.; Ortega, M.A.; Alvarez-Mon, M.; Quintero, J. Delving into the Perception, Use, and Context of Duloxetine in Clinical Practice: An Analysis Based on the Experience of Healthcare Professionals. Brain Sci. 2025, 15, 757. https://doi.org/10.3390/brainsci15070757
Fraile-Martinez O, Garcia-Montero C, Alvarez-Mon MA, Ortega MA, Alvarez-Mon M, Quintero J. Delving into the Perception, Use, and Context of Duloxetine in Clinical Practice: An Analysis Based on the Experience of Healthcare Professionals. Brain Sciences. 2025; 15(7):757. https://doi.org/10.3390/brainsci15070757
Chicago/Turabian StyleFraile-Martinez, Oscar, Cielo Garcia-Montero, Miguel Angel Alvarez-Mon, Miguel A. Ortega, Melchor Alvarez-Mon, and Javier Quintero. 2025. "Delving into the Perception, Use, and Context of Duloxetine in Clinical Practice: An Analysis Based on the Experience of Healthcare Professionals" Brain Sciences 15, no. 7: 757. https://doi.org/10.3390/brainsci15070757
APA StyleFraile-Martinez, O., Garcia-Montero, C., Alvarez-Mon, M. A., Ortega, M. A., Alvarez-Mon, M., & Quintero, J. (2025). Delving into the Perception, Use, and Context of Duloxetine in Clinical Practice: An Analysis Based on the Experience of Healthcare Professionals. Brain Sciences, 15(7), 757. https://doi.org/10.3390/brainsci15070757





