Abstract
Background/Objectives: Depression, anxiety and apathy are often associated with subjective cognitive complaints (SCCs) in people with Parkinson’s disease (PwPD) without cognitive impairment. Cognitive reserve (CR) enhances emotional resilience, allowing people to better cope with stress and emotional challenges, factors affecting quality of life. We aimed to explore the relationship between CR and mood/anxiety in cognitively intact PwPD with and without SCCs. Methods: In this cross-sectional study we enrolled 133 PwPD and normal cognitive function (age 59.8 ± 6.7 years; disease duration 9.0 ± 5.5 years; male/female 84/49). We assessed cognitive reserve (CR scale), subjective cognitive complaints (with PD-CFRS), QoL (PDQ8), mood, anxiety and apathy (BDI-II; STAI, PAS, Apathy scales). We used a t-test to compare groups (with/without SCC; M/F); correlations and moderation analysis to evaluate the relation between CR and behavioral features and the interplay between CR, behavioral discomfort and QoL. Results: The group with SCCs had significantly (p < 0.05) higher scores in PDQ8, Apathy, STAI, PAS-C and BDI-II scales than those with no SCCs. Males with SCCs had higher scores in PDQ8, Apathy scale and BDI-II while females differed in PDQ8 and Apathy scale scores. In the SCC group, late-life CR was negatively correlated with PAS-C (avoidance behavior) and BDI-II; correlations were confirmed in the male group where CR also correlated with PDQ-8 and PAS persistent anxiety. Conclusions: PwPD and SCCs are more depressed and anxious compared to people without SCCs. Furthermore, we found a relationship between depressive symptoms, anxiety and CR: PwPD with SCCs may rely on cognitive reserve to better cope with the feeling of anxiety and depression, especially in male gender.
1. Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by motor and nonmotor symptoms. Rigidity, tremor, bradykinesia and instability are the main motor symptoms causing movement impairment; cognitive and behavioral alterations are common among the nonmotor symptoms. Cognitive alterations have been extensively studied: mild cognitive impairment (MCI) has been reported in up to 40% of patients, and dementia in 30–40% of patients, with the incidence increasing with disease duration and age, and MCI as a risk factor [1,2,3,4]. Behavioral and neuropsychiatric symptoms such as anxiety, depression, apathy and impulsive compulsive behaviors have been described and can be common (see [5] for a review): depression symptoms occur in up to 37% of the patients (17% meet criteria for major depression), significant anxiety symptoms were reported in about 25% of patients, mean apathy prevalence was 35% and impulse control disorders occurred in about 10–20% of the patients, with different prevalence rates for each disorder. Motor, neuropsychiatric and cognitive symptoms have been shown to impact the quality of life (QoL) [6,7].
The estimated prevalence of PD in high-Human-Development-Index European countries, such as Italy, is 3.58 cases per 1000, and projections predict a 3% increase in Italy by 2050 [8,9,10,11,12]. The growing prevalence and the disability associated with PD make it extremely important to better understand the nature of motor and nonmotor symptoms to help people with Parkinson’s disease (PwPD) and their caregivers cope with the deterioration in QoL [13].
Subjective cognitive complaints (SCCs) consist of the self-experienced difficulties in memory or other cognitive abilities despite achieving normal results at formal assessment. In PD, SCCs are two times more common than in the healthy population [14], may precede MCI [15] and can be present in up to 83% of PwPD with normal cognitive function [16]. Depression, anxiety and apathy have been associated with SCCs in PD [17], but the relationship between psychiatric disorders and SCCs is not clear yet.
Cognitive reserve (CR) is the brain’s ability to cope with brain damage using pre-existing cognitive procedures or enlisting compensatory processes [18]. Therefore, CR consists of individual differences in cerebral processes that allow some to cope better than others with brain pathology and to maintain brain functions.
Years of education have been used as a proxy to quantify CR, but a multidimensional conceptualization of the construct is more appropriate. Most common proxies’ indicators are education, occupation, physical and leisure activity, creativity and/or premorbid intelligence [19,20]. To date, many questionnaires have been developed to measure CR, and some have been used in PD, mainly to examine the relationship between CR and cognitive decline in PwPD [21,22,23].
Studies on CR in PD focused on its relationship with cognitive impairment and explored CR’s protective role, especially on executive functions. They confirmed that cognitive decline is delayed in PwPD who have higher CR, even if cognitive impairment progression is faster once started [24,25,26].However, to the best of our knowledge, no studies have examined whether CR has a role in mediating emotional responses to stressful situations, such as experiencing cognitive loss, albeit subjective, and deterioration in quality of life (QoL).
In this study, we want to evaluate whether CR modulates emotional responses in addition to preserving cognitive abilities. Therefore, this study aimed to explore the relationship between CR and the ability to cope with neuropsychiatric symptoms (i.e., anxiety, depression, apathy and impulsive compulsive behaviors) associated with SCCs in PwPD with normal cognition.
Furthermore, considering that mood and anxiety disorders are more common in the female gender in healthy populations [27,28,29,30], we wanted to explore eventual gender differences in how CR interacts with these psychiatric symptoms.
2. Materials and Methods
This cross-sectional study is part of a larger longitudinal and multicenter (Milano, Salerno and Venice) Italian study “Validation of Mild Cognitive Impairment criteria in Italian Parkinson’s disease patients” (GR-2016-02361986) which aims to explore PD’s cognitive and behavioral profile by improving cognitive and behavioral assessment instruments.
It includes 133 PwPD recruited in Milano (ASST-Azienda Socio Sanitaria Territoriale-Gaetano Pini-CTO) and Venezia (IRCCS, San Camillo Hospital) from August 2018 to December 2024.
Inclusion criteria were as follows: aged between 40 and 70 years; clinically confirmed diagnosis based on UK Brain Bank criteria (PD with at least 5 years of disease duration) and normal cognitive abilities as per cognitive assessment allowing for Level II criteria for PD MCI to be applied.
Exclusion criteria were as follows: linguistic comprehension deficit; an education level of less than 5 years; presence of severe sensory deficits; severe psychiatric, neurological and cardiovascular pathologies; and previous neurosurgical procedures.
The present study was approved by the Ethics Committees of the enrolment centers. Written informed consent was obtained from all study subjects after full explanation of the procedure involved. The research was completed in accordance with the Declaration of Helsinki [31].
2.1. Assessment
PD cognitive abilities were evaluated in the ON state by means of a comprehensive neuropsychological battery common to both recruitment sites, which allowed for the evaluation of PD-MCI as per Level II criteria [32]. Moreover, we assessed cognitive reserve (QCR [21]), SCCs (with PD-CFRS [33]), QoL (with PDQ8, [34]), mood, anxiety and apathy (by administering BDI-II, STAI scales, PAS scale and Apathy scale).
We collected data from the following scales/tests/questionnaires:
The Mini-Mental State Examination (MMSE) [35] and Montreal Cognitive Assessment (MoCA) [36] for global cognitive abilities.
The PD-Cognitive Functional Rating Scale (PD-CFRS) (Italian validation [33]): A brief questionnaire developed for PD which explores various functional aspects that may be affected by cognitive impairment. It contains 12 questions, each addressing different functional areas related to this cognitive decline.
Cognitive reserve questionnaire (QRC): To evaluate the cognitive reserve, we adapted the criteria for proxies proposed by Hindle and colleagues [24]; the scale evaluates early life (education), middle years (work activity) and late life (socialization/significant relationship).
Parkinson’s Disease Questionnaire PDQ-8 [34] was used to measure quality of life. This is a shortened version of the 39-item PDQ-39, a scale used to quantify the quality of life for individuals with Parkinson’s disease.
Parkinson Anxiety scale (PAS-A, PAS-B, PAS-C [37]) was used to evaluate anxiety symptoms. It includes three subscales evaluating persistent anxiety, episodic anxiety and avoidance behaviors and it has been specifically developed for the PD population.
The State–Trait Anxiety Inventory (STAI scales, trait or state) [38] is a 40-item self-report measure of anxiety using a 4-point Likert-type scale (from 0 to 3 points) for each item.
The Apathy scale [39] to measure apathy. This 14-item questionnaire has been proved to be a reliable and valid measure of apathy in PD.
Beck Depression Inventory-II (BDI-II) [40], a 21-item self-report inventory measuring the severity of depression in adolescents and adults and widely used in PwPD.
The Barratt Impulsiveness Scale (BIS-11) [41] was used to evaluate impulsivity. This is a 30-question self-report measure of impulsiveness which has been used in PwPD in many studies.
Questionnaire for Impulsive Compulsive Disorders in Parkinson’s Disease-Rating Scale (QUIP-RS) [42]: This questionnaire was used to evaluate behavioral addictions and compulsive behavior in PwPD and was used to quantify these symptoms.
2.2. Statistical Analysis
To explore the interplay between CR and SCCs in PwPD, we defined two subgroups based on the presence of subjective complaints. These subgroups are as follows: (a) PD-SCC, patients with a PD-CFRS score of 0 (no subjective complaint); and (b) PD-SCC+, patients with a PD-CFRS score of 1 or higher.
Categorical variables were reported as numbers and percentages, while continuous variables were reported as mean and Standard Deviation, or median and inter-quartile range, depending on their distribution (Gaussian or not), as assessed by the Shapiro–Wilk Test.
Between-group comparisons of demographic and clinical characteristics were conducted using the Chi-square test or the Fisher exact test for categorical and unpaired t-tests, or the Mann–Whitney U test for continuous variables, as appropriate; for all the analyses, p-values < 0.05 were considered to be statistically significant.
Differences between groups differentiated by the presence of SCCs overall and within gender-based groups were explored. Correlation analyses were applied using Spearman rank correlation to comprehensively evaluate the relationship between cognitive reserve and relevant behavioral characteristics with a comparison between males and females; we used moderation analysis to evaluate the extent to which cognitive reserve influences the symptoms and overall quality of life of patients with Parkinson’s disease and to explore eventual gender differences in how CR interacts with these psychiatric symptoms. We explore this possible modulation in the overall sample and with focus on the PD-SCC+ group.
We created dichotomous variables (Yes/No) for depression, general, state and trait anxiety, apathy and impulsivity, based on the cut-off scores. Additionally, we dichotomized the late life subscale of QCR (Low/High; cut-off median value =8) and assessed differences in percentages among groups and by gender with the Chi-square test. The analyses were conducted with Jamovi software version 2.3.8.0.
3. Results
Participants had a mean age of 60.3 years (±6.8) and a disease duration of 8.99 years (±5.5), with a distribution of 84 males and 49 females (see Table 1 for clinical and demographic characteristics).

Table 1.
Clinical and demographic characteristics of Parkinson’s patients with and without subjective cognitive complaints.
3.1. Subjective Cognitive Complaints
Eighty-one PwPD reported SCCs (PD-CFRS ≥ 1; PD-SCC+ group) while 52 did not report SCCs (PD-CFRS = 0; SCC− group).
No differences were found when comparing the two groups in demographic and clinical data; the group PD-SCC+ had significantly higher scores in the PDQ8 t (117) (p < 0.001, d = 0.79), Apathy scale t (119) (p < 0.001, d = 0.71), STAI scales t (106) (p < 0.05, d = 0.42), PAS-C scale t (108) (p < 0.05, d = 0.42), BDI-II t (108) (p < 0.01, d = 0.48) and BIS11 t (105) (p < 0.01, d = 0.55) scale compared to the group SCC− (see Table 2). Only apathy and impulsivity were considered in terms of their presence/absence, and they resulted to be more frequent in PD-SCC+ at the Chi-square comparison.

Table 2.
Significant differences in behavioral characteristics and Qol in PwPD with (PD-SCC+) and without (PD-SCC−) subjective cognitive complaints.
We did not find any significant difference in clinical and demographic characteristics between people with higher CR late life apart from age, which was higher compared to PwPD and low CR late life (mean years: 61.2 vs. 58.7, p < 0.05).
3.2. Gender Differences
No differences emerge in male and females for behavioral characteristics and quality of life (Table S1). However, when separately considering gender, we observed that males with SCC+ had higher scores in the PDQ8 (p < 0.01), Apathy scale (p < 0.01) and BDI-II (p < 0.05) than SCC−, while females differed in the PDQ8 scores (p < 0.05) and Apathy (p < 0.01) scale but not in BDI-II scores (Tables S2 and S3). The difference in pathological scores was not significant as per the Chi-square test.
In males, we found significative negative correlations between CR late life and the apathy (r = −0.282, p < 0.05), QUIP RS (r = −0.448, p < 0.001), persistent anxiety (r = −0.261, p < 0.05) and avoidance behavior (r = −0.311, p < 0.010) subscales of PAS and PDQ8 (r = −0.229, p < 0.010); in females, we found CR late life to negatively correlate only with PAS-C (r = −0.394, p < 0.05).
3.3. Cognitive Reserve
CR (early-, middle- and late-life proxies) did not differ between the PD-SCC+ and PD-SCC− groups.
In the PD-SCC+ group, late-life CR was significantly correlated with PAS, avoidance behavior (r = −0.418, p < 0.001) and BDI-II (r = −0.248, p < 0.05); correlations that were confirmed and found to be stronger in the male group where late-life CR also correlated with the PDQ-8 (r = −0.475, p < 0.01) and persistent anxiety subtest of PAS (r = −0.348, p < 0.05).
Moderation analyses were conducted both in the all-sample and, in particular, in the PD-SCC+ group (Table 3; Tables S4 and S5).

Table 3.
QRC effect on behavioral symptoms and PDQ8 in PwPD in SCC+.
Considering the total sample (PD-SCC+ and PD-SCC−), the interaction term between PAS-A and QRC late life is significant (b = −0.162, p < 0.05), suggesting that the effect of PAS-A on PDQ8 varies with late-life cognitive reserve (Table 3 and Table 4, and Figure 1).

Table 4.
Relation between PAS-A and PDQ8 moderated by QRC total in total sample of PwPD.
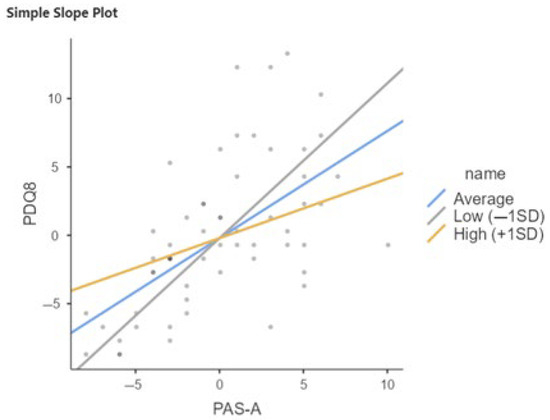
Figure 1.
Relation between PAS-A and PDQ8 moderated by QRC total in total sample of PwPD. SD, Standard Deviation; Low = Average (Mean) QRC −1SD; High = Average (Mean) QRC +1SD.
Late-life cognitive reserve is also a moderator in the relationship between PAS-C and PDQ8; in particular, the interaction term is significant (b = 269, p < 0.001), indicating that the effect of PAS-C on PDQ8 increases with increasing late-life CR (Figure 2; Table 5).
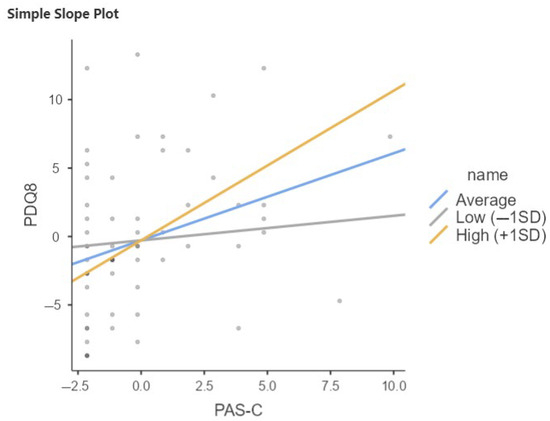
Figure 2.
Relation between PAS-C and PDQ8 moderated by QRC total in total sample of PwPD. SD, Standard Deviation; Low = Average (mean) −1SD; High = Average (mean) +1SD.

Table 5.
Relation between PAS-C and PDQ8 moderated by QRC total in total sample of PwPD.
The same results emerge in the PD-SCC+ group, where CR influences how anxiety is perceived in relation to scores on quality of life questionnaires. Notably, it is the late-life cognitive reserve—particularly concerning relationships with others—that shows significant scores in this relationship. In this group, the moderation analysis shows that the interaction term between PAS-A and QRC late life is significant (b = −0.212, p < 0.05 (Figure 3, Table 6)). Specifically, the association between anxiety and quality of life decreases with increasing late-life cognitive reserve. When late-life CR is low, the effect of PAS-A on PDQ8 is stronger; the higher the late-life CR, the weaker the effect. This moderation does not emerge in the SCC− group.
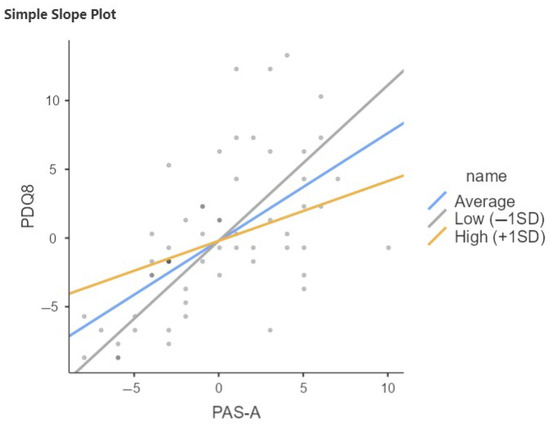
Figure 3.
Relation between PAS-A and PDQ8 moderated by QRC late life in PD-SCC+. SD, Standard Deviation; Low = Average (mean) −1SD; High = Average (mean) +1SD.

Table 6.
Relation between PAS-A and PDQ8 moderated by QRC late life in PD-SCC+.
The same trend emerges in the PD-SCC+ sample as in the total sample with respect to moderation of CR late life in the relationship between PAS-C and PDQ8 (Table 7, Figure 4). Notably, the interaction term is significant (b = 0.278, p < 0.001), indicating that again, the effect of PAS-C on PDQ8 increases with increasing late-life CR.

Table 7.
Relation between PAS-C and PDQ8 moderated by QRC late life in PD-SCC+.
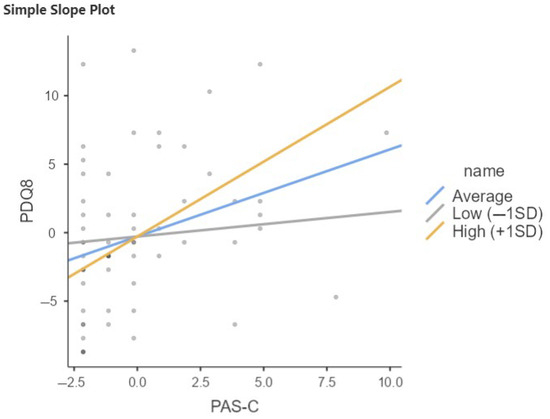
Figure 4.
Relation between PAS-C and PDQ8 moderated by QRC late life in PD-SCC+. SD, Standard Deviation; Low = Average (mean) −1SD; High = Average (mean) +1SD.
4. Discussion and Conclusions
Our study aimed to explore the effect of cognitive reserve on coping with the neuropsychiatric symptoms (i.e., anxiety, depression, apathy and impulsive compulsive behaviors) associated with SCCs in PwPD. We studied patients with unimpaired cognitive functions with and without cognitive complaints and explored the relationship between CR and neuropsychiatric symptoms in those populations.
In PwPD, both SCCs and CR have been studied regarding cognition [14,15,16,17,20,21,22,23,43,44,45,46]. However, this is the first study exploring how CR can help cope with neuropsychiatric symptoms and alterations in quality of life in PwPD with normal cognitive functions and SCCs.
Being an original topic, it is important to underline that it has been studied in a large and well-selected sample, since we included PwPD enrolled consecutively who were screened using a common cognitive assessment battery built to fit the Level II criteria for PD-MCI.
4.1. Subjective Cognitive Complaints
In agreement with the literature about the prevalence of SCCs in cognitively intact PwPD [17], about 60% of our sample reported SCCs. However, this rate is quite high compared to the median reported in the systematic review, and this may be due to the strict criterion we used to attribute a participant to the group without SCCs (PD-CFRS = 0), while other authors [44] used a different cut-off with the same instrument or applied different instruments [17,43].
Without a specific instrument or established criteria to evaluate SCCs, using the PD-CFRS allows for the detection of eventual subjective cognitive complaints. The 12 questions explore functional aspects sensitive to cognitive impairment, allowing the patient to better reflect on their abilities and to report eventual difficulties. Moreover, unlike other questionnaires, it minimizes the effect of motor bias when reporting subtle cognitive changes.
Furthermore, we did not find any difference between the two groups in demographic or clinical variables or general cognition as measured by MoCA and MMSE; those aspects did not even correlate with the PD-CFRS score, allowing for a better interpretation of the results. Indeed, a recent meta-analysis has shown that the relationship between poor performance in general cognitive tests and SCCs is weak [43]. Similarly, motor symptoms and dopamine replacement therapy were not found to be associated with SCCs.
We found that people with SCCs had higher scores in scales measuring quality of life, mood, anxiety, apathy and impulsivity, as expected considering previous studies [17,45,46]. We also found a higher prevalence of pathological scores in apathy and impulsivity in the PD-SCC+ group.
4.2. Gender Differences
When exploring the gender differences, we observed that males with SCCs have worse quality of life, are more apathetic and are more depressed than females with SCCs, while females differ in QoL and Apathy scale scores as well, but not in BDI-II scores. Considering the recent observation [47] of worse anxious–depressive symptoms with increasing age in women compared to men in a large population of 1509 PwPD, our finding may seem contradictory. However, it might be possible that from a “gender perspective”, intended as the role of norms, rules, stereotypes and cultural expectations based on biological gender, men could pay a higher toll than women when feeling to be less cognitively performant.
4.3. The Role of Cognitive Reserve
To our knowledge, this is the first study assessing the possible role of cognitive reserve in modulating strategies to cope with neuropsychiatric symptoms related to subjective cognitive complaints in Parkinson’s disease.
Cognitive reserve was considered separately in its three proxies (early, middle and late life), with early life considering education, middle life working experiences and late life social relationships. We found that in people experiencing SCCs, the late-life cognitive reserve proxy was the one most related to anxiety and depression, particularly in men, where it was correlated with quality of life too.
Interestingly, with the moderation analysis, we showed that late-life CR influences how anxiety is perceived in relation to scores on quality of life questionnaires, specifically in PD-SCC+. When quality of life is worse, persistent anxiety scores increase; however, this pattern is modulated by late-life CR, as the increase in anxiety is weaker in people with higher late-life CR. An opposite effect is noted when considering the effect of worse QoL on avoidance behavior, where people with higher late-life CR tend to have a greater increase in avoidance behavior. From a psychological point of view, this apparent contradiction may be explained as it is a way to cope with persistent anxiety arising from a poor quality of life: by using avoidance behaviors, people with higher CR can manage their anxiety. This may mean that, being more aware of their difficulties with social networking or social moments, patients may decide not to expose themselves to social situations unless they have a significant relationship with the attendees. Alternatively, they may rely more on phone video calls to maintain their relations.
Aside from this specific observation, our data show that late-life CR is an important factor correlating with apathy, anxiety and depressive symptoms in PwPD and subjective cognitive complaints. Frequent telephone calls and maintenance of relationships may be a feature of an active cognitive lifestyle, which is associated with a positive cognitive trajectory as people get older [6], and our data show it is related to better coping with SCCs and the neuropsychiatric symptoms associated with them.
Alongside cognitive deficits, neuropsychiatric symptoms have been shown to have a significant impact on QoL [48], and it is worth exploring ways to improve these aspects. While education and work life are scarcely modifiable in adult–elderly people, social interactions can be augmented, and this would lead to benefits for both patients and caregivers since it has been shown that higher perceived social contact is associated with a lower caregiver burden [49,50]. Furthermore, since we did not find any significant difference between people with high and low late-life CR apart from older age in the former, an intervention to increase social interactions is likely beneficial for all patients. Music, dance, yoga and other group activities have been shown to improve neuropsychiatric symptoms in these patients [51,52,53] and can be a way to implement sociality.
One limitation of the study is the definition of SCCs. Since there are no established criteria or scales to evaluate SCCs, we chose a criterion to define the presence of an SCC, which is reasonable but may have been too strict compared to the prevalence found in other samples. Generally, the lack of standardized measures for SCCs impedes comparing results. Furthermore, we considered only cognitively intact PwPD in our study, but cognitive complaints are present in MCI and PDD; therefore, our results cannot be generalized to the whole PD population.
In conclusion, our data confirmed the presence of higher neuropsychiatric symptoms in cognitively unimpaired PwPD and subjective cognitive complaints. They showed a relation with cognitive reserve being associated with social interactions in late life, especially in males. On one hand, we confirm previous findings showing higher anxiety, depression and apathy in PwPD and with SCCs; on the other hand, for the first time, the roles of cognitive reserve and social interaction in particular on these psychological and behavioral features are highlighted.
Further studies are needed to confirm our findings, explore the effect of other cognitive reserve proxies (such as premorbid intelligence, leisure activities and creativity) and evaluate programs that help patients and their caregivers implement social relationships.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/brainsci15080795/s1, Table S1: Comparison between males and females in behavioral characteristics and Qol in PwPD; Table S2: Comparison of behavioral characteristics and Qol between male PwPD with (PD-SCC+) and without (PD-SCC−) subjective complaints; Table S3: Comparison of behavioral characteristics and Qol between female PwPD with (PD-SCC+) and without (PD-SCC−) subjective cognitive complaints; Table S4: QRC effect on behavioral symptoms and PDQ8 in PwPD (total sample); Table S5: QRC effect on behavioral symptoms and PDQ8 in PwPD in SCC−.
Author Contributions
Conceptualization, C.S., A.C., R.B., L.W., M.L.R. and M.C. (Margherita Canesi); Data curation, L.W.; Formal analysis, M.C. (Maura Crepaldi); Methodology, C.S., R.B., M.C. (Maura Crepaldi) and L.W.; Supervision, M.L.R. and M.C. (Margherita Canesi); Writing—original draft, C.S. and M.C. (Maura Crepaldi); Writing—review and editing, A.C., R.B., L.W., I.U.I., A.A., M.L.R. and M.C. (Margherita Canesi). All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Italian Ministry of Health under Grant Number GR-2016-02361986.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of IRCCS San Camillo (2018.03-IVA-PD-MCI; approved on the 23 March 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PD | Parkinson’s disease |
| PwPD | People with Parkinson’s disease |
| LEDD | Levodopa equivalent daily dose |
| DAED | Dopamine agonist equivalent daily dose |
References
- Weintraub, D.; Tröster, A.I.; Marras, C.; Stebbins, G. Initial cognitive changes in Parkinson’s disease. Mov. Disord. 2018, 33, 511–519. [Google Scholar] [CrossRef]
- Aarsland, D.; Andersen, K.; Larsen, J.P.; Lolk, A. Prevalence and characteristics of dementia in Parkinson disease: An 8-year prospective study. Arch. Neurol. 2003, 60, 387–392. [Google Scholar] [CrossRef]
- Wallace, E.R.; Segerstrom, S.C.; van Horne, C.G.; Schmitt, F.A.; Koehl, L.M. Meta-Analysis of Cognition in Parkinson’s Disease Mild Cognitive Impairment and Dementia Progression. Neuropsychol. Rev. 2022, 32, 149–160. [Google Scholar] [CrossRef]
- Gonzalez-Latapi, P.; Bayram, E.; Litvan, I.; Marras, C. Cognitive impairment in Parkinson’s disease: Epidemiology, clinical profile, protective and risk factors. Behav. Sci. 2021, 11, 74. [Google Scholar] [CrossRef]
- Nagy, A.; Schrag, A. Neuropsychiatric aspects of Parkinson’s disease. J. Neural Transm. 2019, 126, 889–896. [Google Scholar] [CrossRef]
- Marioni, R.E.; Valenzuela, M.J.; van den Hout, A.; Brayne, C.; Matthews, F.E. MRC Cognitive Function and Ageing Study. Active cognitive lifestyle is associated with positive cognitive health transitions and compression of morbidity from age sixty-five. PLoS ONE 2012, 7, e50940. [Google Scholar] [CrossRef]
- Lo Buono, V.; Palmeri, R.; De Salvo, S.; Berenati, M.; Greco, A.; Ciurleo, R.; Sorbera, C.; Cimino, V.; Corallo, F.; Bramanti, P.; et al. Anxiety, depression, and quality of life in Parkinson’s disease: The implications of multidisciplinary treatment. Neural Regen. Res. 2021, 16, 587–590. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, J.; Cui, Y.; Zhang, J.; Yan, R.; Su, D.; Zhao, D.; Wang, A.; Feng, T. Temporal trends in the prevalence of Parkinson’s disease from 1980 to 2023: A systematic review and meta-analysis. Lancet Healthy Longev. 2024, 5, e464–e479. [Google Scholar] [CrossRef] [PubMed]
- Cicero, C.E.; Scondotto, S.; Allotta, A.V.; De Luca, G.; Murolo, G.; Nicoletti, A.; Zappia, M. Burden of Parkinson’s disease in Sicily: A health administrative database study. Neurol. Sci. 2022, 43, 1043–1046. [Google Scholar] [CrossRef]
- Valeikiene, V.; Ceremnych, J.; Mieliauskaite, D.; Alekna, V. The prevalence of Parkinson’s disease among Vilnius inhabitants. Cent. Eur. J. Med. 2008, 3, 195–198. [Google Scholar] [CrossRef]
- Valent, F.; Devigili, G.; Rinaldo, S.; Del Zotto, S.; Tullio, A.; Eleopra, R. The epidemiology of Parkinson’s disease in the Italian region Friuli Venezia Giulia: A population-based study with administrative data. Neurol. Sci. 2018, 39, 699–704. [Google Scholar] [CrossRef]
- Su, D.; Cui, Y.; He, C.; Yin, P.; Bai, R.; Zhu, J.; Lam, J.S.T.; Zhang, J.; Yan, R.; Zheng, X.; et al. Projections for prevalence of Parkinson’s disease and its driving factors in 195 countries and territories to 2050: Modelling study of Global Burden of Disease Study 2021. BMJ 2025, 388, e080952. [Google Scholar] [CrossRef]
- Al-Khammash, N.; Al-Jabri, N.; Albishi, A.; Al-Onazi, A.; Aseeri, S.; Alotaibi, F.; Almazroua, Y.; Albloushi, M. Quality of Life in Patients With Parkinson’s Disease: A Cross-Sectional Study. Cureus 2023, 15, e33989. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lehrner, J.; Moser, D.; Klug, S.; Gleiß, A.; Auff, E.; Pirker, W.; Pusswald, G. Subjective memory complaints, depressive symptoms and cognition in Parkinson’s disease patients. Eur. J. Neurol. 2014, 21, 1276–1277. [Google Scholar] [CrossRef]
- Purri, R.; Brennan, L.; Rick, J.; Xie, S.X.; Deck, B.L.; Chahine, L.M.; Dahodwala, N.; Chen-Plotkin, A.; Duda, J.E.; Morley, J.F.; et al. Subjective Cognitive Complaint in Parkinson’s Disease Patients With Normal Cognition: Canary in the Coal Mine? Mov. Disord. 2020, 35, 1618–1625. [Google Scholar] [CrossRef]
- Barbosa, R.P.; Mendonça, M.D.; Caetano, A.P.; Lampreia, T.M.; Miguel, R.; Bugalho, P.M. Cognitive complaints in Parkinson’s disease patients: From subjective cognitive complaints to dementia and affective disorders. J. Neural Transm. 2019, 126, 1329–1335. [Google Scholar] [CrossRef]
- Hong, J.Y.; Lee, P.H. Subjective Cognitive Complaints in Cognitively Normal Patients With Parkinson’s Disease: A Systematic Review. J. Mov. Disord. 2023, 16, 1–12. [Google Scholar] [CrossRef]
- Stern, Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 2002, 8, 448–460. [Google Scholar] [PubMed]
- Jones, R.N.; Manly, J.; Glymour, M.M.; Rentz, D.M.; Jefferson, A.L.; Stern, Y. Conceptual and measurement challenges in research on cognitive reserve. J. Int. Neuropsychol. Soc. 2011, 17, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, N.; Colombo, B.; Pepe, F.; Magni, E.; Antonietti, A.; Silveri, M.C. Cognitive reserve: A multidimensional protective factor in Parkinson’s disease related cognitive impairment. Aging Neuropsychol. Cogn. 2022, 29, 687–702. [Google Scholar] [CrossRef]
- Muslimovic, D.; Schmand, B.; Speelman, J.D.; de Haan, R.J. Course of cognitive decline in Parkinson’s disease: A meta-analysis. J. Int. Neuropsychol Soc. 2007, 13, 920–932. [Google Scholar] [CrossRef]
- Hindle, J.V.; Martyr, A.; Clare, L. Cognitive reserve in Parkinson’s disease: A systematic review and meta-analysis. Park. Relat Disord. 2014, 20, 1–7. [Google Scholar] [CrossRef]
- Poletti, M.; Emre, M.; Bonuccelli, U. Mild cognitive impairment and cognitive reserve in Parkinson’s disease. Park. Relat. Disord. 2011, 17, 579–586. [Google Scholar] [CrossRef]
- Hindle, J.V.; Martin-Forbes, P.A.; Bastable, A.J.; Pye, K.L.; Martyr, A.; Whitaker, C.J.; Craik, F.I.; Bialystok, E.; Thomas, E.M.; Mueller Gathercole, V.C.; et al. Cognitive reserve in Parkinson’s disease: The effects of welsh-english bilingualism on executive function. Park. Dis. 2015, 2015, 943572. [Google Scholar] [CrossRef]
- Hindle, J.V.; Hurt, C.S.; Burn, D.J.; Brown, R.G.; Samuel, M.; Wilson, K.C.; Clare, L. The effects of cognitive reserve and lifestyle on cognition and dementia in Parkinson’s disease-a longitudinal cohort study. Int. J. Geriatr. Psychiatry 2016, 31, 13–23. [Google Scholar] [CrossRef]
- Ophey, A.; Wirtz, K.; Wolfsgruber, S.; Balzer-Geldsetzer, M.; Berg, D.; Hilker-Roggendorf, R.; Kassubek, J.; Liepelt-Scarfone, I.; Becker, S.; Mollenhauer, B.; et al. Mid- and late-life lifestyle activities as main drivers of general and domain-specific cognitive reserve in individuals with Parkinson’s disease: Cross-sectional and longitudinal evidence from the LANDSCAPE study. J. Neurol. 2024, 271, 5411–5424. [Google Scholar] [CrossRef]
- Leach, L.S.; Christensen, H.; Mackinnon, A.J.; Windsor, T.D.; Butterworth, P. Gender differences in depression and anxiety across the adult lifespan: The role of psychosocial mediators. Soc. Psychiatry Psychiatr. Epidemiol. 2008, 43, 983–998. [Google Scholar] [CrossRef]
- McLean, C.P.; Anderson, E.R. Brave men and timid women? A review of the gender differences in fear and anxiety. Clin. Psychol. Rev. 2009, 29, 496–505. [Google Scholar] [CrossRef]
- Farhane-Medina, N.Z.; Luque, B.; Tabernero, C.; Castillo-Mayén, R. Factors associated with gender and sex differences in anxiety prevalence and comorbidity: A systematic review. Sci. Prog. 2022, 105, 00368504221135469. [Google Scholar] [CrossRef]
- Höglund, P.; Hakelind, C.; Nordin, S. Severity and prevalence of various types of mental ill-health in a general adult population: Age and sex differences. BMC Psychiatry 2020, 20, 209. [Google Scholar] [CrossRef]
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Litvan, I.; Goldman, J.G.; Tröster, A.I.; Schmand, B.A.; Weintraub, D.; Petersen, R.C.; Mollenhauer, B.; Adler, C.H.; Marder, K.; Williams-Gray, C.H.; et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement disorder society task force guidelines. Mov. Disord. 2012, 27, 349–356. [Google Scholar] [CrossRef]
- Garon, M.; Weis, L.; Siquier, A.; Fiorenzato, E.; Pistonesi, F.; Cianci, V.; Canesi, M.; Pesce, F.; Reali, E.; Pozzi, B.; et al. Validation of the Italian version of the Parkinson’s Disease-Cognitive Functional Rating Scale. J. Neural Transm. 2024, 131, 305–314. [Google Scholar] [CrossRef]
- Peto, V.; Jenkinson, C.; Fitzpatrick, R. PDQ-39: A review of the development, validation and application of a Parkinson’s disease quality of life questionnaire and its associated measures. J. Neurol. 1998, 245 (Suppl. S1), S10–S14. [Google Scholar] [CrossRef] [PubMed]
- Carpinelli Mazzi, M.; Iavarone, A.; Russo, G.; Musella, C.; Milan, G.; D’Anna, F.; Garofalo, E.; Chieffi, S.; Sannino, M.; Illario, M.; et al. Mini-Mental State Examination: New normative values on subjects in Southern Italy. Aging Clin. Exp. Res. 2020, 32, 699–702. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Santangelo, G.; Falco, F.; D’Iorio, A.; Cuoco, S.; Raimo, S.; Amboni, M.; Pellecchia, M.T.; Longo, K.; Vitale, C.; Barone, P. Anxiety in early Parkinson’s disease: Validation of the Italian observer-rated version of the Parkinson Anxiety Scale (OR-PAS). J. Neurol. Sci. 2016, 367, 158–161. [Google Scholar] [CrossRef]
- Spielberger, C.; Gorsuch, D.L.; Lushene, R.E. State-Trait Anxiety Inventory; Consulting Psychologists’ Press: San Francisco, CA, USA, 1983. [Google Scholar]
- Starkstein, S.E.; Mayberg, H.S.; Preziosi, T.J.; Andrezejewski, P.; Leiguarda, R.; Robinson, R.G. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 1992, 4, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Brown, G.K. BDI-II: Beck Depression Inventory; Pearson: London, UK, 1996. [Google Scholar]
- Fossati, A.; Di Ceglie, A.; Acquarini, E.; Barratt, E.S. Psychometric properties of an Italian version of the Barratt Impulsiveness Scale-11 (BIS-11) in nonclinical subjects. J. Clin. Psychol. 2001, 57, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Maggi, G.; Vitale, C.; Giacobbe, C.; Barone, A.; Mastromarino, C.; Iannotta, F.; Amboni, M.; Weintraub, D.; Santangelo, G. Validation of the Italian version of the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale (QUIP-RS) in an Italian Parkinson’s disease cohort. Neurol. Sci. 2024, 45, 3153–3161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siciliano, M.; Tessitore, A.; Morgante, F.; Goldman, J.G.; Ricciardi, L. Subjective Cognitive Complaints in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Mov. Disord. 2024, 39, 17–28. [Google Scholar] [CrossRef]
- van Balkom, T.D.; Berendse, H.W.; van der Werf, Y.D.; Twisk, J.W.R.; Peeters, C.F.W.; Hoogendoorn, A.W.; Hagen, R.H.; Berk, T.; van den Heuvel, O.A.; Vriend, C. Effect of eight-week online cognitive training in Parkinson’s disease: A double-blind, randomized, controlled trial. Park. Relat. Disord. 2022, 96, 80–87. [Google Scholar] [CrossRef]
- Han, L.L.; Wang, L.; Xu, Z.H.; Liang, X.N.; Zhang, M.W.; Fan, Y.; Sun, Y.M.; Liu, F.T.; Yu, W.B.; Tang, Y.L. Disease progression in Parkinson’s disease patients with subjective cognitive complaint. Ann. Clin. Transl. Neurol. 2021, 8, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Erro, R.; Santangelo, G.; Barone, P.; Picillo, M.; Amboni, M.; Longo, K.; Giordano, F.; Moccia, M.; Allocca, R.; Pellecchia, M.T.; et al. Do subjective memory complaints herald the onset of mild cognitive impairment in Parkinson disease? J. Geriatr. Psychiatry Neurol. 2014, 27, 276–281. [Google Scholar] [CrossRef]
- Maas, B.R.; Göttgens, I.; Tijsse Klasen, H.P.; Kapelle, W.M.; Radder, D.L.; Bloem, B.R.; Darweesh, S.K. Age and gender differences in non-motor symptoms in people with Parkinson’s disease. Front. Neurol. 2024, 15, 1339716. [Google Scholar] [CrossRef]
- Dalrymple, W.A.; Trach, S.K.; Flanigan, J.L.; Patrie, J.T.; Henry, K.; Harrison, M.B.; Barrett, M.J.; Figari-Jordan, R.; Shah, B.B.; Rossetti, M.A. Psychiatric predictors of quality of life in Parkinson’s disease: A three-year longitudinal study. J. Neurol. Sci. 2024, 466, 123248. [Google Scholar] [CrossRef]
- Cohen, S.; Janicki-Deverts, D.; Turner, R.B.; Doyle, W.J. Does hugging provide stress-buffering social support? A study of susceptibility to upper respiratory infection and illness. Psychol. Sci. 2015, 26, 135–147. [Google Scholar] [CrossRef]
- Geerlings, A.D.; Kapelle, W.M.; Sederel, C.J.; Tenison, E.; Wijngaards-Berenbroek, H.; Meinders, M.J.; Munneke, M.; Ben-Shlomo, Y.; Bloem, B.R.; Darweesh, S.K.L. Caregiver burden in Parkinson’s disease: A mixed-methods study. BMC Med. 2023, 21, 247. [Google Scholar] [CrossRef]
- Bernatzky, G.; Presch, M.; Anderson, M.; Panksepp, J. Emotional foundations of music as a non-pharmacological pain management tool in modern medicine. Neurosci. Biobehav. Rev. 2011, 35, 1989–1999. [Google Scholar] [CrossRef]
- Kwok, J.Y.Y.; Kwan, J.C.Y.; Auyeung, M.; Mok, V.C.T.; Lau, C.K.Y.; Choi, K.C.; Chan, H.Y.L. Effects of Mindfulness Yoga vs Stretching and Resistance Training Exercises on Anxiety and Depression for People With Parkinson’s Disease: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 755–763. [Google Scholar] [CrossRef]
- Sharp, K.; Hewitt, J.; Sharp, K.; Hewitt, J. Dance as an intervention for people with Parkinson’s disease: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2014, 47, 445–456. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).