Neural Bases of Language Recovery After Stroke Can Only Be Fully Understood Through Longitudinal Studies of Individuals
Abstract
1. Introduction
2. Functional Imaging Studies of Language in People Without Brain Lesions
3. Functional Imaging Studies of Recovery in Groups of People with PSA
4. Functional Imaging Studies of Recovery in Individuals with PSA
4.1. Task-Related fMRI Studies of Recovery in Individuals with PSA
4.2. Functional Connectivity MRI Studies of Individuals with PSA
4.3. Functional Near-Infrared Spectroscopy (fNIRS) Studies of Recovery in Individuals with PSA
4.4. Transcranial Magnetic Stimulation (TMS) with Functional Imaging of Recovery in Individuals with PSA
5. Discussion
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parker Jones, O.; Green, D.W.; Grogan, A.; Pliatsikas, C.; Filippopolitis, K.; Ali, N.; Lee, H.L.; Ramsden, S.; Gazarian, K.; Prejawa, S.; et al. Where, When and Why Brain Activation Differs for Bilinguals and Monolinguals during Picture Naming and Reading Aloud. Cereb. Cortex 2012, 22, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Bartels, A.; Zeki, S. Brain Dynamics during Natural Viewing Conditions—A New Guide for Mapping Connectivity In Vivo. Neuroimage 2005, 24, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Saur, D.; Lange, R.; Baumgaertner, A.; Schraknepper, V.; Willmes, K.; Rijntjes, M.; Weiller, C. Dynamics of Language Reorganization after Stroke. Brain 2006, 129, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Turkeltaub, P.E.; Messing, S.; Norise, C.; Hamilton, R.H. Are Networks for Residual Language Function and Recovery Consistent across Aphasic Patients? Neurology 2011, 76, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Démonet, J.F.; Wise, R.; Frackowiak, R.S.J. Language Functions Explored in Normal Subjects by Positron Emission Tomography: A Critical Review. Hum. Brain Mapp. 1993, 1, 39–47. [Google Scholar] [CrossRef]
- Branco, P.; Seixas, D.; Castro, S.L. Mapping Language with Resting-state Functional Magnetic Resonance Imaging: A Study on the Functional Profile of the Language Network. Hum. Brain Mapp. 2020, 41, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Frith, C.D.; Friston, K.J.; Liddle, P.F.; Frackowiak, R.S.J. A PET Study of Word Finding. Neuropsychologia 1991, 29, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.; Morales, M.; Pickering, M.J.; Hoffman, P. A Common Neural Code for Meaning in Discourse Production and Comprehension. Neuroimage 2023, 279, 120295. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.E.; Small, S.L. Test-Retest Reliability in FMRI of Language: Group and Task Effects. Brain Lang. 2007, 102, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Vikingstad, E.M.; George, K.P.; Johnson, A.F.; Welch, K.M. Cortical Language Activation in Stroke Patients Recovering from Aphasia with Functional MRI. Stroke 1999, 30, 2331–2340. [Google Scholar] [CrossRef] [PubMed]
- Szaflarski, J.P.; Eaton, K.; Ball, A.L.; Banks, C.; Vannest, J.; Allendorfer, J.B.; Page, S.; Holland, S.K. Poststroke Aphasia Recovery Assessed with Functional Magnetic Resonance Imaging and a Picture Identification Task. J. Stroke Cerebrovasc. Dis. 2011, 20, 336–345. [Google Scholar] [CrossRef] [PubMed]
- DeMarco, A.T.; van der Stelt, C.; Paul, S.; Dvorak, E.; Lacey, E.; Snider, S.; Turkeltaub, P.E. Absence of Perilesional Neuroplastic Recruitment in Chronic Poststroke Aphasia. Neurology 2022, 99, e119–e128. [Google Scholar] [CrossRef] [PubMed]
- Turkeltaub, P.E.; Martin, K.C.; Laks, A.B.; DeMarco, A.T. Right Hemisphere Language Network Plasticity in Aphasia. medRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Nenert, R.; Allendorfer, J.B.; Martin, A.M.; Banks, C.; Vannest, J.; Holland, S.K.; Hart, K.W.; Lindsell, C.J.; Szaflarski, J.P. Longitudinal FMRI Study of Language Recovery after a Left Hemispheric Ischemic Stroke. Restor. Neurol. Neurosci. 2018, 36, 359–385. [Google Scholar] [CrossRef] [PubMed]
- Connor, L.T.; DeShazo Braby, T.; Snyder, A.Z.; Lewis, C.; Blasi, V.; Corbetta, M. Cerebellar Activity Switches Hemispheres with Cerebral Recovery in Aphasia. Neuropsychologia 2006, 44, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Szaflarski, J.P.; Allendorfer, J.B.; Banks, C.; Vannest, J.; Holland, S.K. Recovered vs. Not-Recovered from Post-Stroke Aphasia: The Contributions from the Dominant and Non-Dominant Hemispheres. Restor. Neurol. Neurosci. 2013, 31, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Stockert, A.; Wawrzyniak, M.; Klingbeil, J.; Wrede, K.; Kümmerer, D.; Hartwigsen, G.; Kaller, C.P.; Weiller, C.; Saur, D. Dynamics of Language Reorganization after Left Temporo-Parietal and Frontal Stroke. Brain 2020, 143, 844–861. [Google Scholar] [CrossRef] [PubMed]
- Stockbridge, M.D.; Faria, A.V.; Fridriksson, J.; Rorden, C.; Bonilha, L.; Hillis, A.E. Subacute Aphasia Recovery Is Associated with Resting-State Connectivity within and beyond the Language Network. Ann. Clin. Transl. Neurol. 2023, 10, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Abo, M.; Kakita, K.; Mori, Y.; Yoshida, M.; Sasaki, N. The Effect of Selective Transcranial Magnetic Stimulation with Functional Near-Infrared Spectroscopy and Intensive Speech Therapy on Individuals with Post-Stroke Aphasia. Eur. Neurol. 2017, 77, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Hartwigsen, G.; Saur, D. Neuroimaging of Stroke Recovery from Aphasia—Insights into Plasticity of the Human Language Network. Neuroimage 2019, 190, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Saur, D.; Hartwigsen, G. Neurobiology of Language Recovery after Stroke: Lessons from Neuroimaging Studies. Arch. Phys. Med. Rehabil. 2012, 93, S15–S25. [Google Scholar] [CrossRef] [PubMed]
- Bunker, L.B.; Meier, E.; Kim, H.; Durfee, A.; Hillis, A.E. Changes in Activation and Functional Connectivity in Subacute Aphasia Following Treatment: An FNIRS Cares Series Investigation. In Proceedings of the Annual Meeting of the Academy of Aphasia, Reading, UK, 21–23 October 2023; pp. 10–11. [Google Scholar]
- Turkeltaub, P.E.; Coslett, H.B.; Thomas, A.L.; Faseyitan, O.; Benson, J.; Norise, C.; Hamilton, R.H. The Right Hemisphere Is Not Unitary in Its Role in Aphasia Recovery. Cortex 2012, 48, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Crosson, B.; Moore, A.B.; Gopinath, K.; White, K.D.; Wierenga, C.E.; Gaiefsky, M.E.; Fabrizio, K.S.; Peck, K.K.; Soltysik, D.; Milsted, C.; et al. Role of the Right and Left Hemispheres in Recovery of Function during Treatment of Intention in Aphasia. J. Cogn. Neurosci. 2005, 17, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Zahn, R.; Drews, E.; Specht, K.; Kemeny, S.; Reith, W.; Willmes, K.; Schwarz, M.; Huber, W. Recovery of Semantic Word Processing in Global Aphasia: A Functional MRI Study. Brain Res. Cogn. Brain Res. 2004, 18, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Zahn, R.; Huber, W.; Drews, E.; Specht, K.; Kemeny, S.; Reith, W.; Willmes, K.; Schwarz, M. Recovery of Semantic Word Processing in Transcortical Sensory Aphasia: A Functional Magnetic Resonance Imaging Study. Neurocase 2002, 8, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, M.; Shang, D.; Wang, Q.; Luo, B.; Weng, X. Cortical Language Activation in Aphasia: A Functional MRI Study. Chin. Med. J. 2004, 117, 1011–1016. [Google Scholar] [PubMed]
- Kleiser, R.; Wittsack, H.-J.; Bütefisch, C.M.; Jörgens, S.; Seitz, R.J. Functional Activation within the PI-DWI Mismatch Region in Recovery from Ischemic Stroke: Preliminary Observations. Neuroimage 2005, 24, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Jarso, S.; Li, M.; Faria, A.; Davis, C.; Leigh, R.; Sebastian, R.; Tsapkini, K.; Mori, S.; Hillis, A.E. Distinct Mechanisms and Timing of Language Recovery after Stroke. Cogn. Neuropsychol. 2013, 30, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Tilton-Bolowsky, V.; Stockbridge, M.D.; Hillis, A.E. Remapping and Reconnecting the Language Network after Stroke. Brain Sci. 2024, 14, 419. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, A. Western Aphasia Battery–Revised; APA: Washington, DC, USA, 2006. [Google Scholar]
- Hillis, A.E. The Soriano Lecture: Language Recovery after Stroke: Evidence of Reorganization. In Proceedings of the 147th Meeting of the American Neurological Association, Annals of Neurology 92, Chicago, IL, USA, 22–25 October 2022; p. S131. [Google Scholar]
- Purcell, J.; Sebastian, R.; Leigh, R.; Jarso, S.; Davis, C.; Posner, J.; Wright, A.; Hillis, A.E. Recovery of Orthographic Processing after Stroke: A Longitudinal FMRI Study. Cortex 2017, 92, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, R.; Long, C.; Purcell, J.J.; Faria, A.V.; Lindquist, M.; Jarso, S.; Race, D.; Davis, C.; Posner, J.; Wright, A.; et al. Imaging Network Level Language Recovery after Left PCA Stroke. Restor. Neurol. Neurosci. 2016, 34, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.S.; Seitzman, B.A.; Ramsey, L.E.; Ortega, M.; Gordon, E.M.; Dosenbach, N.U.F.; Petersen, S.E.; Shulman, G.L.; Corbetta, M. Re-Emergence of Modular Brain Networks in Stroke Recovery. Cortex 2018, 101, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Winhuisen, L.; Thiel, A.; Schumacher, B.; Kessler, J.; Rudolf, J.; Haupt, W.F.; Heiss, W.D. Role of the Contralateral Inferior Frontal Gyrus in Recovery of Language Function in Poststroke Aphasia: A Combined Repetitive Transcranial Magnetic Stimulation and Positron Emission Tomography Study. Stroke 2005, 36, 1759–1763. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Mukadam, N.; Kiran, S. Functional MRI Evidence for Reorganization of Language Networks after Stroke. Handb. Clin. Neurol. 2022, 185, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Crosson, B.; McGregor, K.; Gopinath, K.S.; Conway, T.W.; Benjamin, M.; Chang, Y.-L.; Moore, A.B.; Raymer, A.M.; Briggs, R.W.; Sherod, M.G.; et al. Functional MRI of Language in Aphasia: A Review of the Literature and the Methodological Challenges. Neuropsychol. Rev. 2007, 17, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Swihart, B.J.; Caffo, B.; James, B.D.; Strand, M.; Schwartz, B.S.; Punjabi, N.M. Lasagna Plots: A Saucy Alternative to Spaghetti Plots. Epidemiology 2010, 21, 621–625. [Google Scholar] [CrossRef] [PubMed]


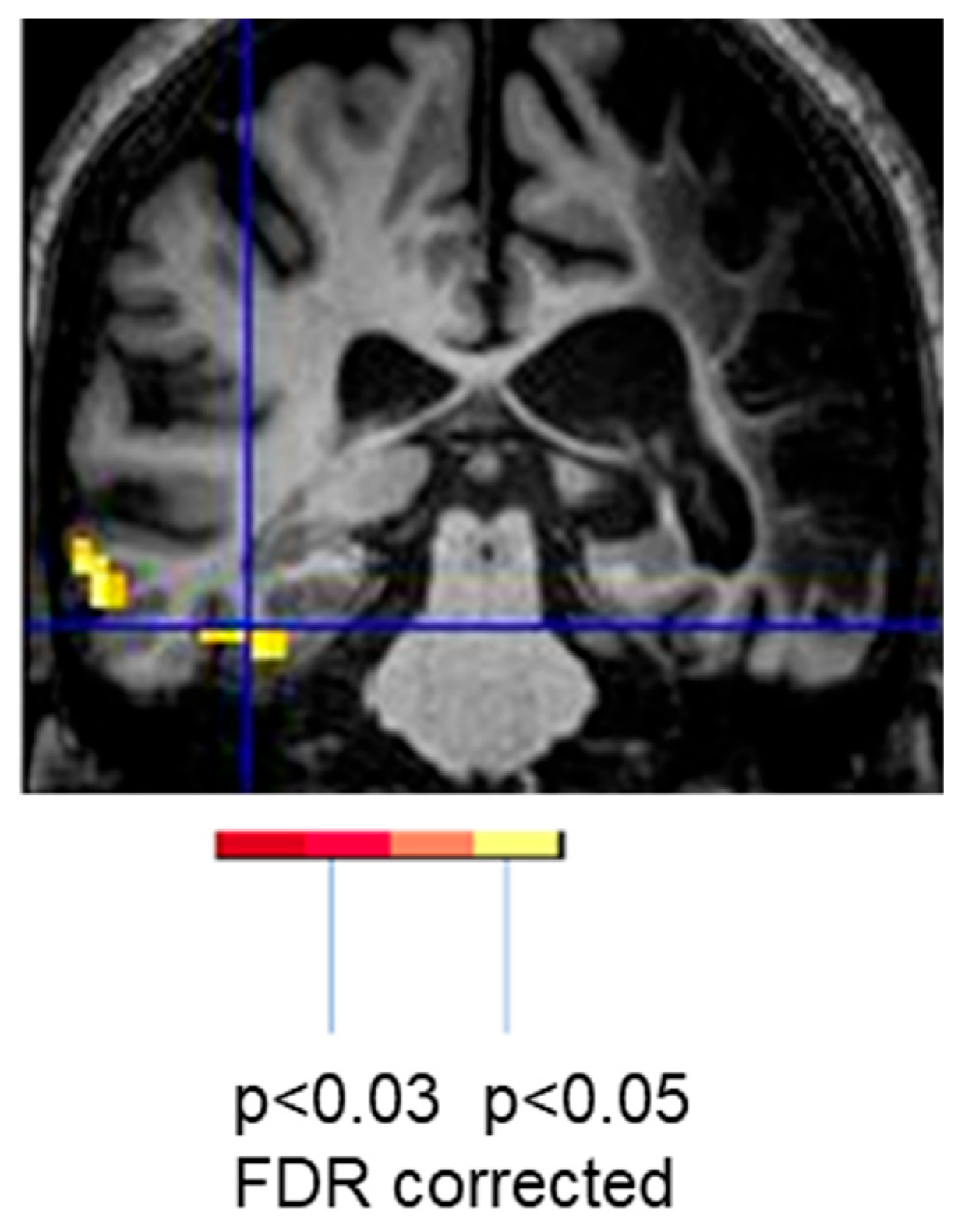
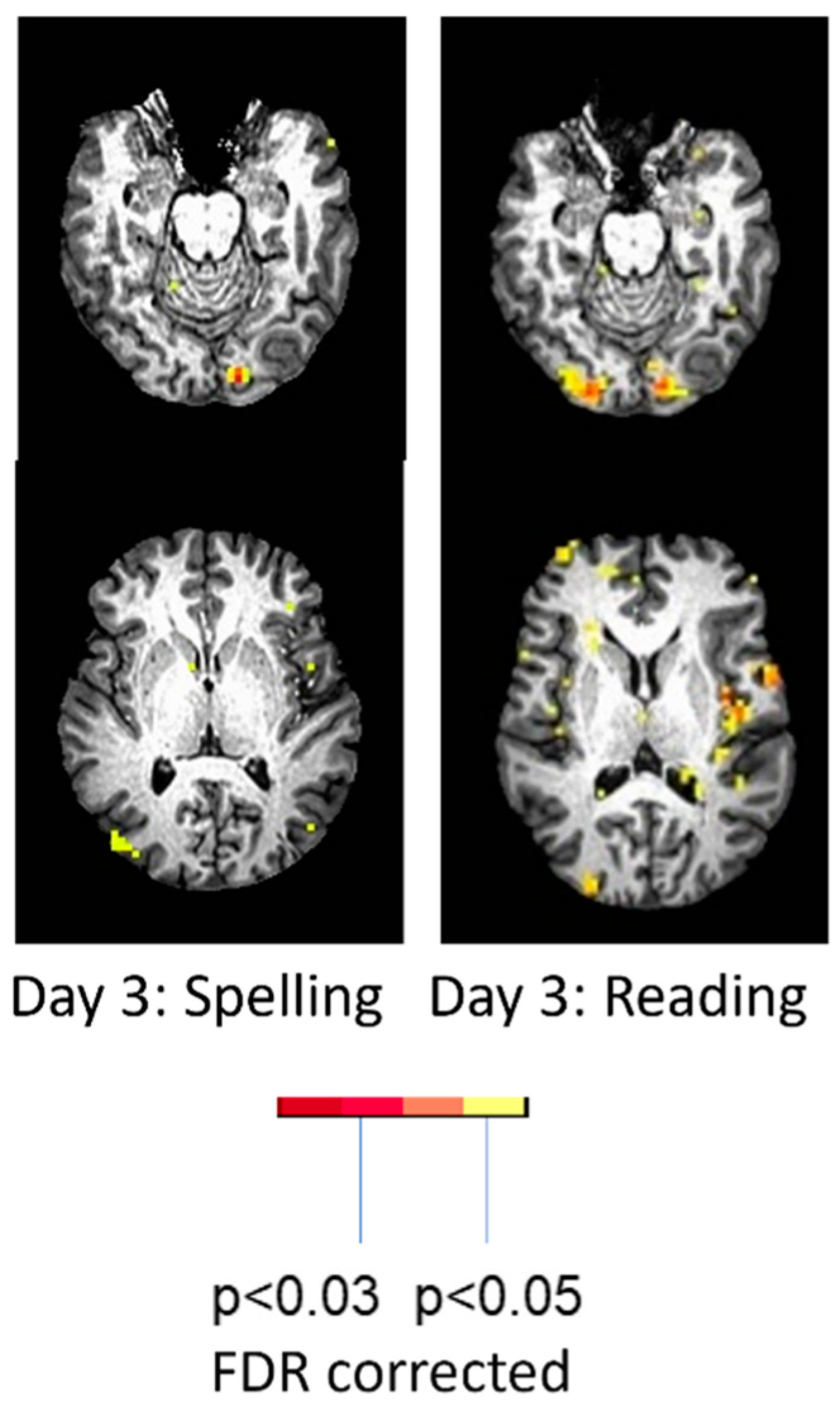
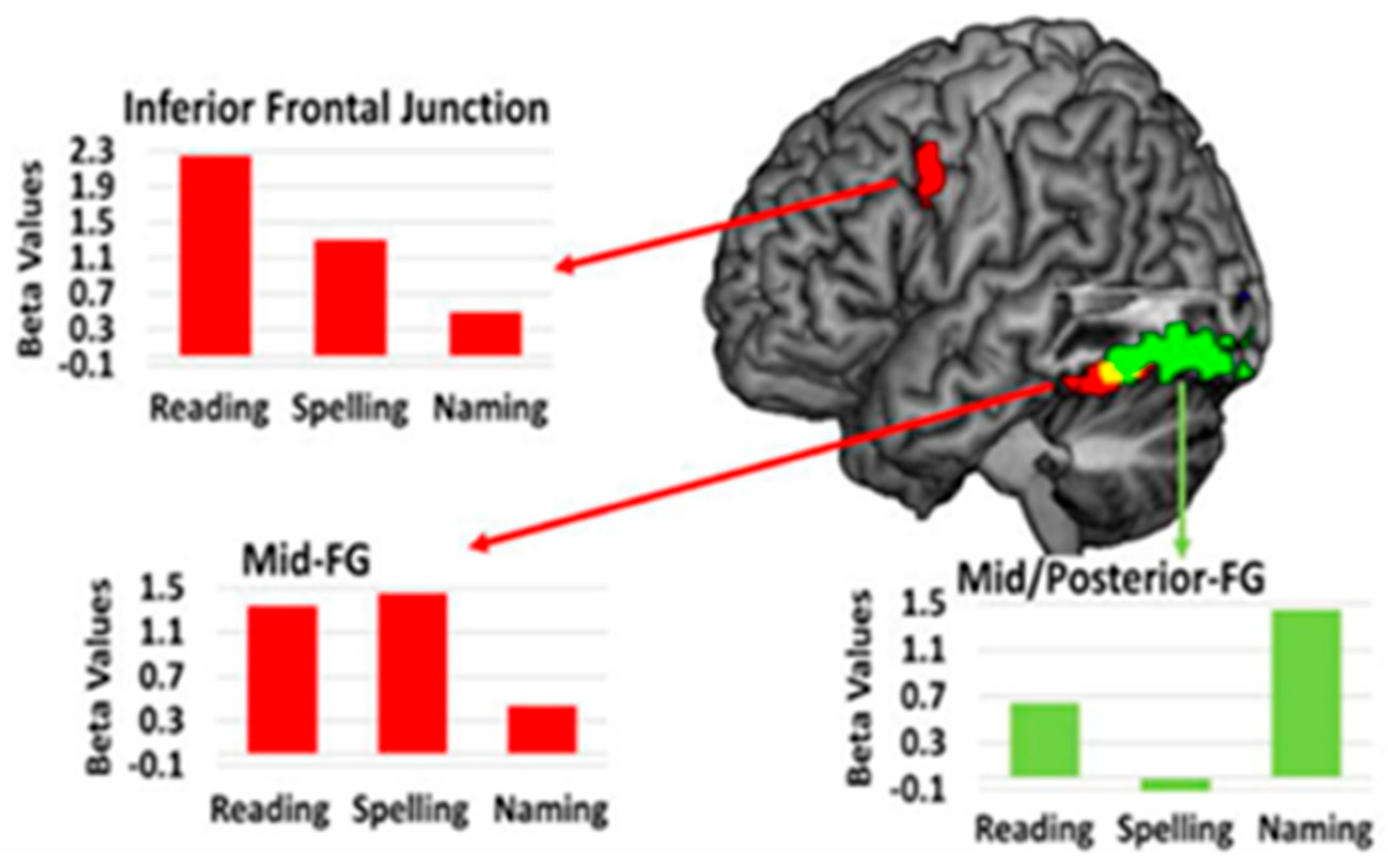
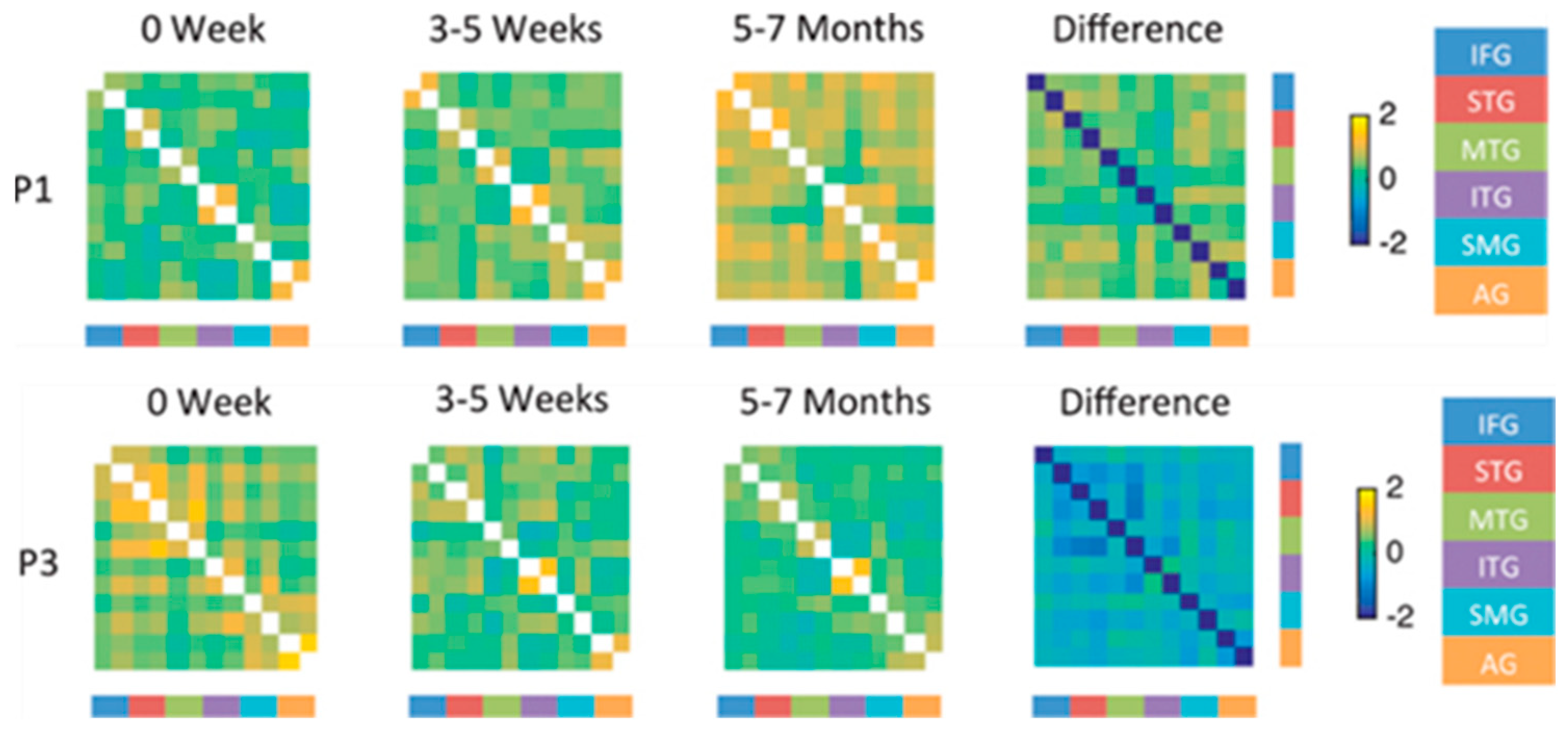
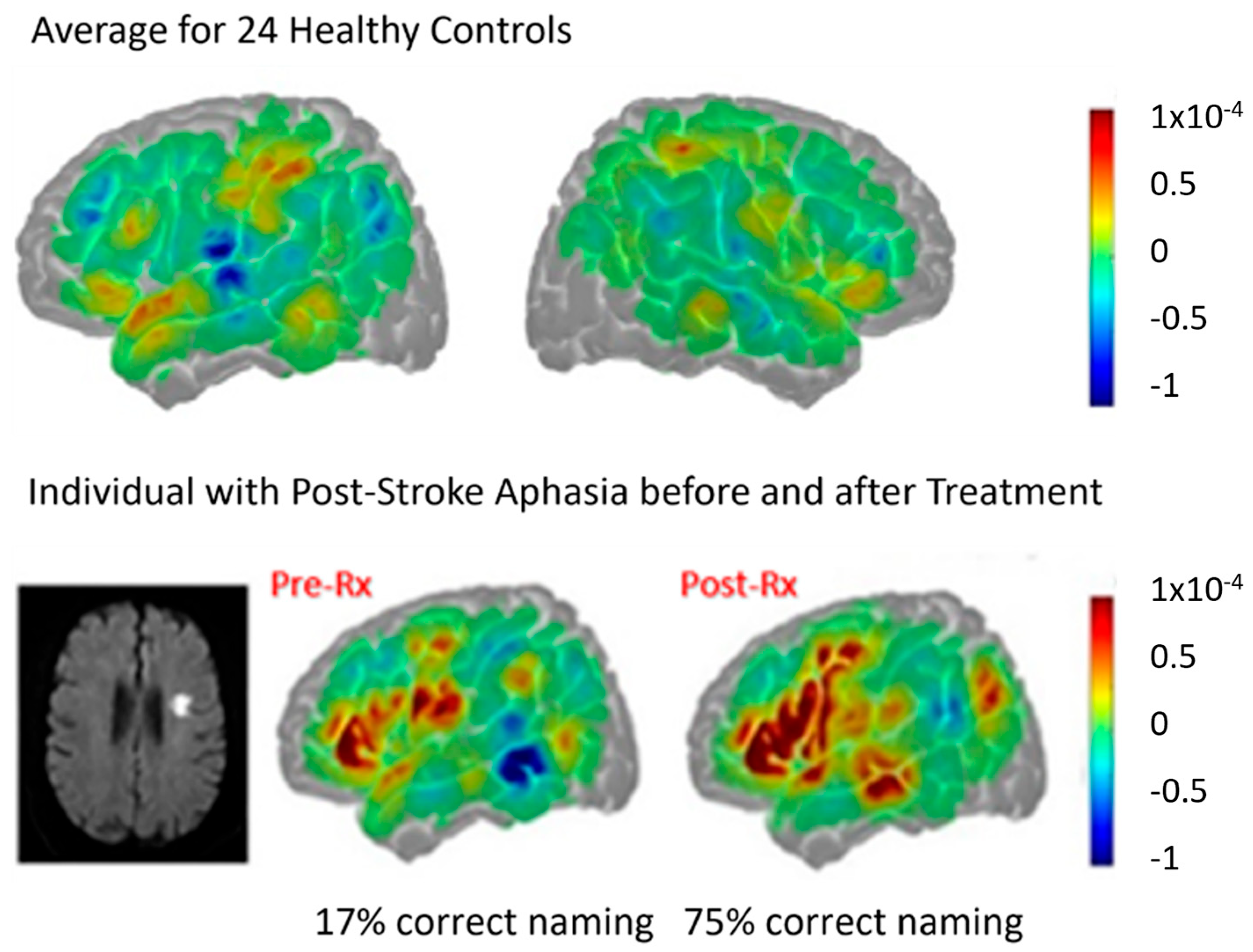
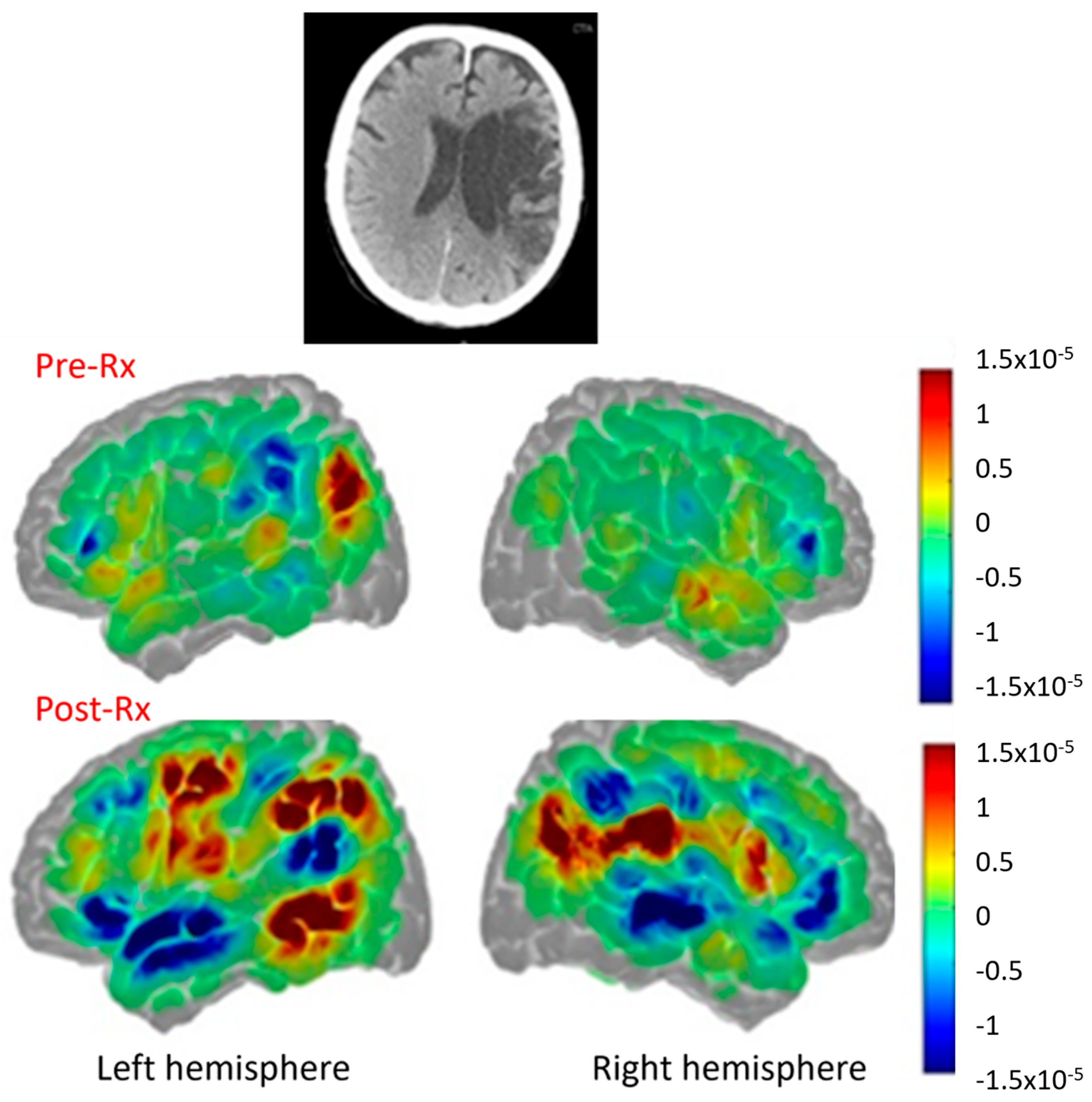
| Reference | Number (N) | Time Since Stroke | Task | Main Results |
|---|---|---|---|---|
| [10] | 7 PSA | 5 months | Lexical–semantic | Increased right hemisphere activation, but bilateral language network activation associated with good recovery |
| [11] | 4 PSA 4 controls | >12 months | Picture–word matching | Perilesional activation in PSA, more widespread frontal and temporal activation than controls; reduced right hemisphere activation in PSA |
| [12] | 82 PSA 82 controls | >6 months | Naming and semantic decision | No evidence of perilesional activation; normalization of the language network +/− activation of alternative cortical areas independent of distance from lesion |
| [13] | 76 PSA 69 controls | >6 months | Semantic decision | Greater right hemisphere activation in PSA than controls, associated with higher education, younger age, left-handedness |
| [3] | 14 PSA 14 controls | 0–4 d *, 2 w, 4–12 months | Sentence judgment | Globally reduced activation acutely, then greater right hemisphere activation at 2–3 w, then normalization of language network activation in PSA |
| [14] | 17 PSA | 2, 6, 17, 26, 52 w | Semantic fluency | Increased left temporal, frontal, and cerebellar activation over time |
| [15] | 8 PSA 14 controls | >6 months | Word stem completion | Less right cerebellar activation in PSA than controls. Increased right frontal and left cerebellar activation associated with learning in PSA |
| Reference | Number (N) | Time Since Stroke | Groups | Results |
|---|---|---|---|---|
| [16] | 27 | >1 year | Good (n = 9) vs. limited (n = 18) recovery | Greater left language network activation in good recovery; greater right homologous activation in PSA with limited recovery |
| [17] | 34 | <1 w *, 2–3 w, >6 m | Frontal (n = 17); temporoparietal (n = 17) lesions | Increased perilesional activation over time; right homologous activation only with frontal lesions |
| [18] | 20 | <3 m, 2 m later | Good (n = 9) vs. limited (n = 11) recovery | Connectivity between left fusiform and other left and right hemisphere language network increased in those with good recovery and decreased with limited recovery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hillis, A.E. Neural Bases of Language Recovery After Stroke Can Only Be Fully Understood Through Longitudinal Studies of Individuals. Brain Sci. 2025, 15, 790. https://doi.org/10.3390/brainsci15080790
Hillis AE. Neural Bases of Language Recovery After Stroke Can Only Be Fully Understood Through Longitudinal Studies of Individuals. Brain Sciences. 2025; 15(8):790. https://doi.org/10.3390/brainsci15080790
Chicago/Turabian StyleHillis, Argye E. 2025. "Neural Bases of Language Recovery After Stroke Can Only Be Fully Understood Through Longitudinal Studies of Individuals" Brain Sciences 15, no. 8: 790. https://doi.org/10.3390/brainsci15080790
APA StyleHillis, A. E. (2025). Neural Bases of Language Recovery After Stroke Can Only Be Fully Understood Through Longitudinal Studies of Individuals. Brain Sciences, 15(8), 790. https://doi.org/10.3390/brainsci15080790





