1. Introduction
At least since the mid-1990s, it has been recognized that hemispatial neglect can occur in distinct reference frames [
1,
2]. In viewer-centered neglect (VCN; also called egocentric neglect), the contralesional side of the viewer or environment (what the viewer sees) is neglected. In stimulus-centered neglect (SCN; also called allocentric neglect), the contralesional side of each stimulus is neglected, irrespective of the location with respect to the viewer. Both left hemisphere (LH) and right hemisphere (RH) strokes can cause contralesional VCN or SCN [
2,
3,
4,
5,
6]. However, most studies indicate that VCN is more common than SCN. Most of these studies have included only right hemisphere stroke [
7,
8,
9]. However, two studies that have also included left hemisphere stroke have found that SCN is more common than VCN in left hemisphere stroke, while VCN is more common in right hemisphere stroke [
3,
10].
A few investigators have questioned the dissociation between VCN and SCN because of the common co-occurrence [
11] or reported that each type of neglect can be detected in the same participants, but with different tasks or task demands [
12]. However, several studies using the same task and task requirements to detect each have shown a clear dissociation or even double dissociation between VCN and SCN [
4,
9].
Several studies have attempted to identify the lesions associated with VCN versus SCN. Some have studied participants with chronic or subacute stroke. This approach can miss some critical lesion sites because participants with those lesions may have recovered. The studies of later strokes show areas where recovery of neglect is unlikely, whereas studies of acute strokes show areas that, when damaged, cause neglect (before the opportunity for reorganization or recovery). Nevertheless, there are some commonalities across studies, such as more frontal or dorsal lesions associated with VCN and more temporal or ventral lesions associated with SCN [
5,
7,
13,
14,
15]. Studies that have evaluated the influence of age have also shown that older age is associated with greater frequency and severity of any neglect [
16].
One limitation in studying individuals with acute stroke is that areas of hypoperfusion beyond the infarct may be responsible for acute deficits [
17,
18]. Studies using perfusion imaging have shown that in acute stroke, hypoperfusion beyond the infarct contributes to deficits, including neglect [
17,
19,
20]. Studies have also shown that reperfusion of dorsal regions results in improvement of VCN, and reperfusion of ventral regions results in recovery of SCN [
21]. However, perfusion imaging is not always available. In its absence, previous work has shown that FLAIR hyperintense vessel (FHV) number and site can be used to estimate the volume [
22,
23] and site [
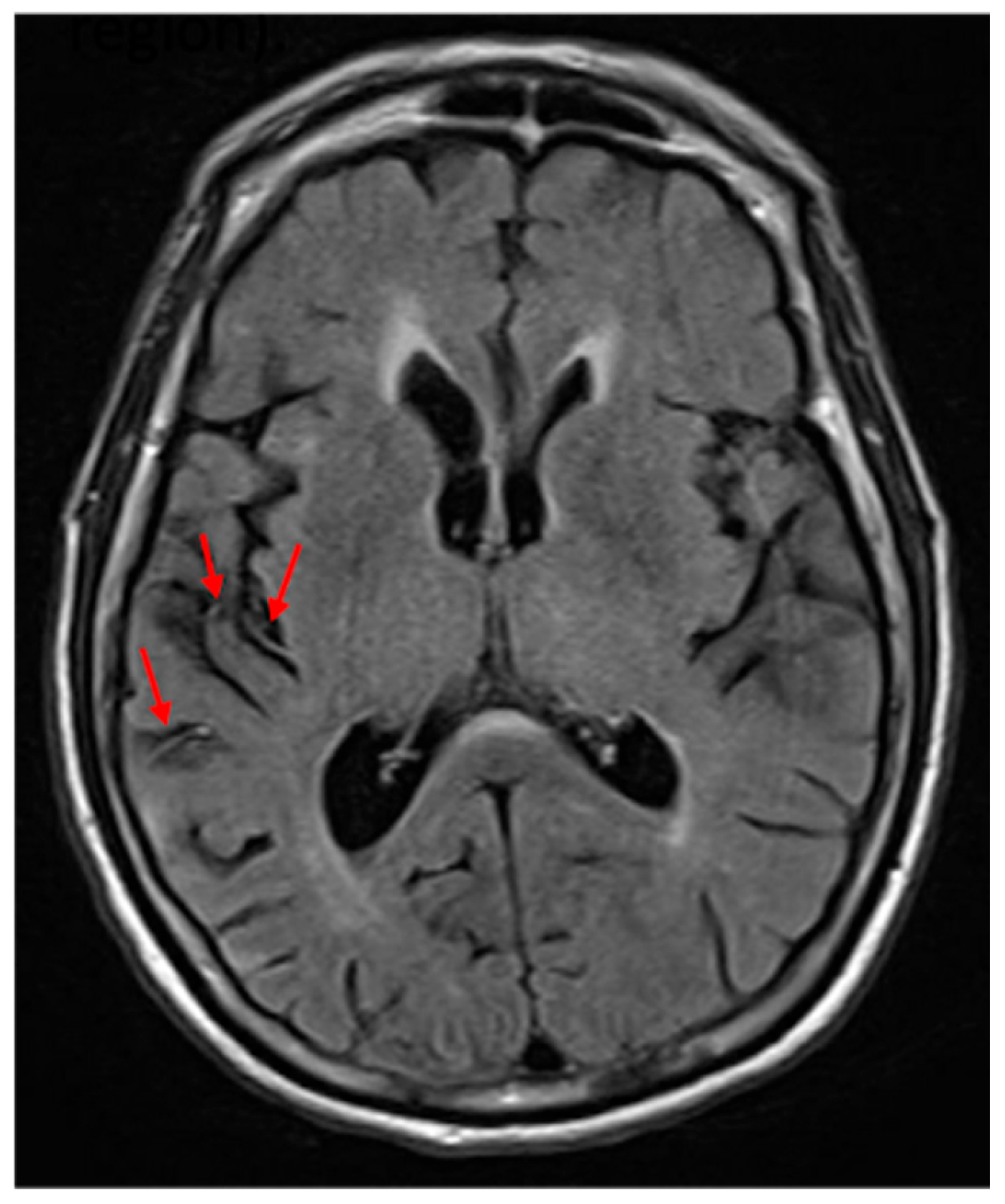
24] of hypoperfusion. An FHV (see
Figure 1) indicates slow blood flow in that area, and the total number can be used to estimate the volume of hypoperfusion [
22]. Furthermore, the site of the FVH is strongly associated with the site of significant hypoperfusion [
24].
In this study, we tested three hypotheses in a relatively large number of individuals with acute ischemic stroke with a single task and MRI of the brain within 5 days of stroke: 1. VCN and SCN dissociate, even on the same task/with the same task demands, and VCN is more common after right hemisphere stroke, while SCN is more common after left hemisphere stroke. 2. Partially distinct areas of infarct contribute to VCN and SCN. 3. Information about the areas of hypoperfusion (estimated with FLAIR hyperintense vessels) contributes additional information to areas of dysfunction (infarct and/or hypoperfusion) that account for the variance in each type of neglect.
2. Materials and Methods
This study was approved by the Johns Hopkins Institutional Review Board (protocol: NA_00042097). A consecutive series of 233 individuals with acute ischemic stroke (73 LH and 160 RH) who provided informed consent were enrolled. Inclusion criteria included the following: the ability to complete testing and MRI within 5 days of ischemic stroke; the ability to provide informed consent or indicate a surrogate to provide informed consent; the ability to understand directions for the task; and premorbid competency in English by self-report. Exclusion criteria included the following: neurological disease involving the brain other than stroke; prior symptomatic stroke (asymptomatic lacunar infarcts were not excluded); uncorrected hearing or visual acuity loss; and reduced level of consciousness or ongoing sedation. Every attempt was made to enroll patients within 24 h of admission to the hospital. Patients were tested seven days per week. Fewer left hemisphere stroke survivors were included because of difficulty understanding the task in the presence of aphasia.
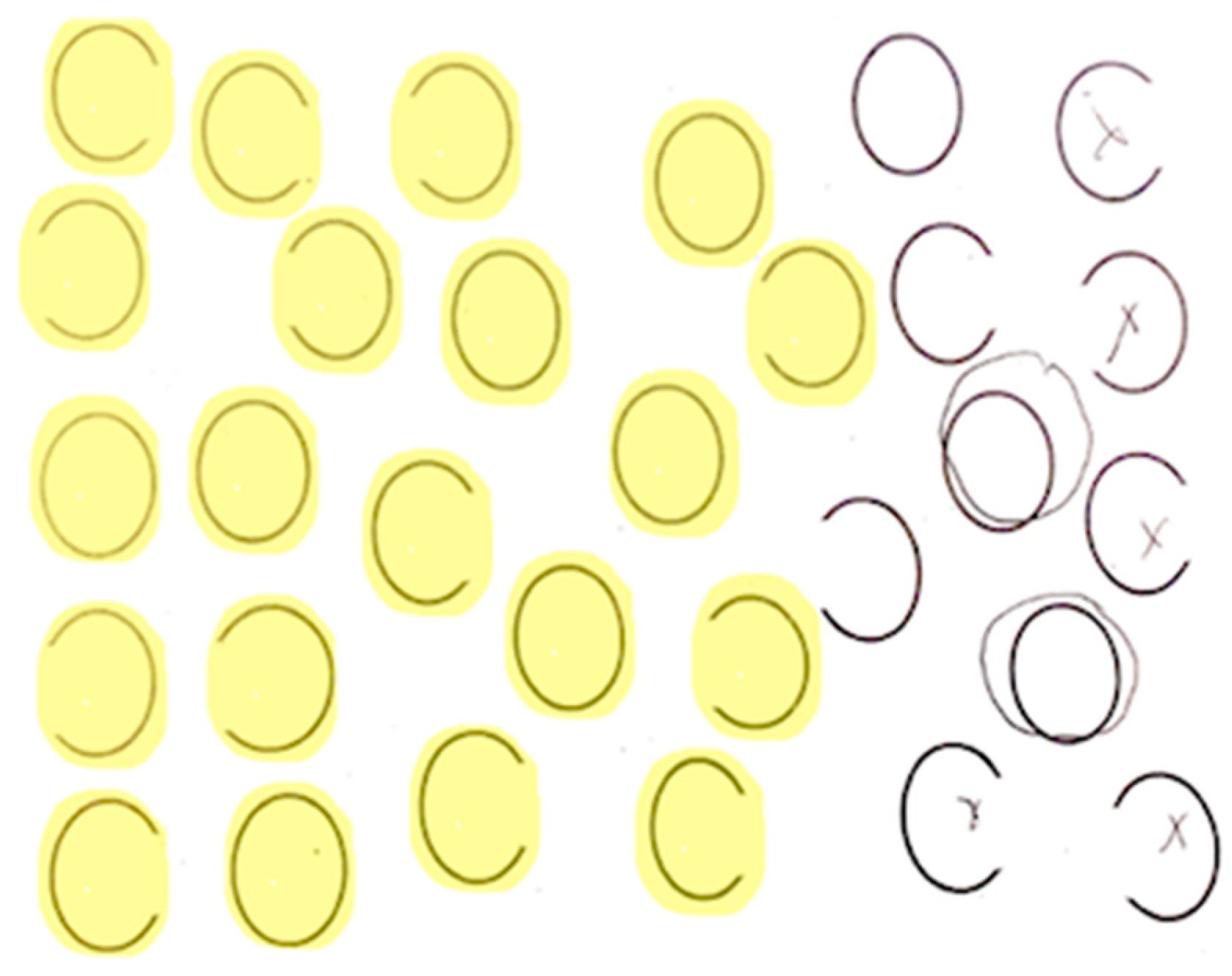
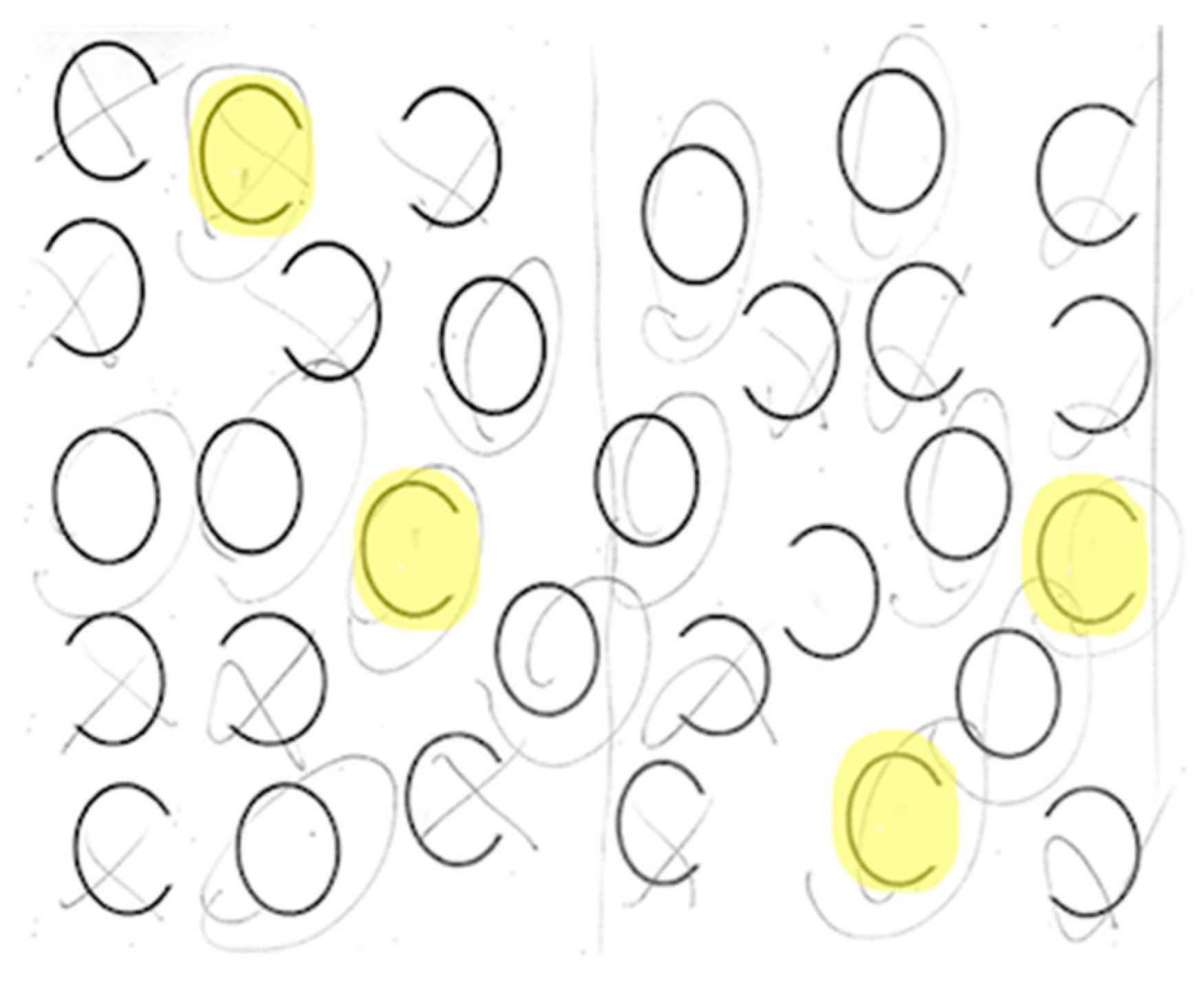
Each participant completed a neglect task with 30 ovals: 10 full, 10 with a gap on the left, and 10 with a gap on the right (adapted from Ota). Participants were asked to circle full ovals and cross out ovals with a gap on either side. We defined VCN as >10% of the total number of ovals left unmarked after the most extreme mark on the contralesional side (
Figure 2) and SCN as incorrectly marking >10% of contralesional gaps (
Figure 3). All participants completed neglect testing and brain MRI within 5 days of stroke onset.
On FLAIR sequences, we identified FHVs in 4 MCA regions, according to a published method [
22]: frontal, temporal, parietal, and insular, as well as ACA and PCA territories (
Figure 1). Each region was scored from 0 to 2: 0 = no FHVs; 1 = 1–2 FHVs on 1–2 slices; 2 = 3 or more vessels on 1 slice; or 3 or more slices with at least 1 FHV (total = 0–12). The stroke core was automatically delineated with ADS (
https://www.nitrc.org/projects/ads, accessed on,
https://www.nature.com/articles/s43856-021-00062-8, accessed on 1 December 2024) and revised by an experienced neuroradiologist (AVF). The vascular territories affected were defined according to the digital atlas of arterial territories (
https://www.nitrc.org/projects/arterialatlas, accessed on 1 December 2024,
https://www.nature.com/articles/s41597-022-01923-0, accessed on 1 December 2024). Total infarct volume and lesion load in the right and left anterior cerebral artery, middle cerebral artery—frontal, middle cerebral artery—parietal, middle cerebral artery—temporal, middle cerebral artery—occipital, posterior cerebral artery—temporal, and posterior cerebral artery—occipital were calculated using the pipeline that utilized b0, Diffusion Weighted Imaging (DWI), Apparent Diffusion Coefficient (ADC), and Fluid Attenuated Inversion Recovery (FLAIR) sequences.
We used multivariable logistic regression, with the presence of VCN or SCN in each hemisphere as the dependent variables and FHV ratings in each area, lesion load in each territory, and age as independent variables.
3. Results
There were no significant differences between left hemisphere and right hemisphere stroke patients with respect to age, sex, racial distribution, or infarct volume. There was a marginally significant difference in education, with more years of education in the left hemisphere stroke patients (mean 14.4 versus 13.5). Right hemisphere stroke patients showed a numerically higher average volume of infarct and a significantly larger volume of hypoperfused tissue estimated by FVH. Demographics are shown in
Table 1.
3.1. Dissociation Between VCN and SCN and the Incidence of Each After Left and Right Hemisphere Stroke
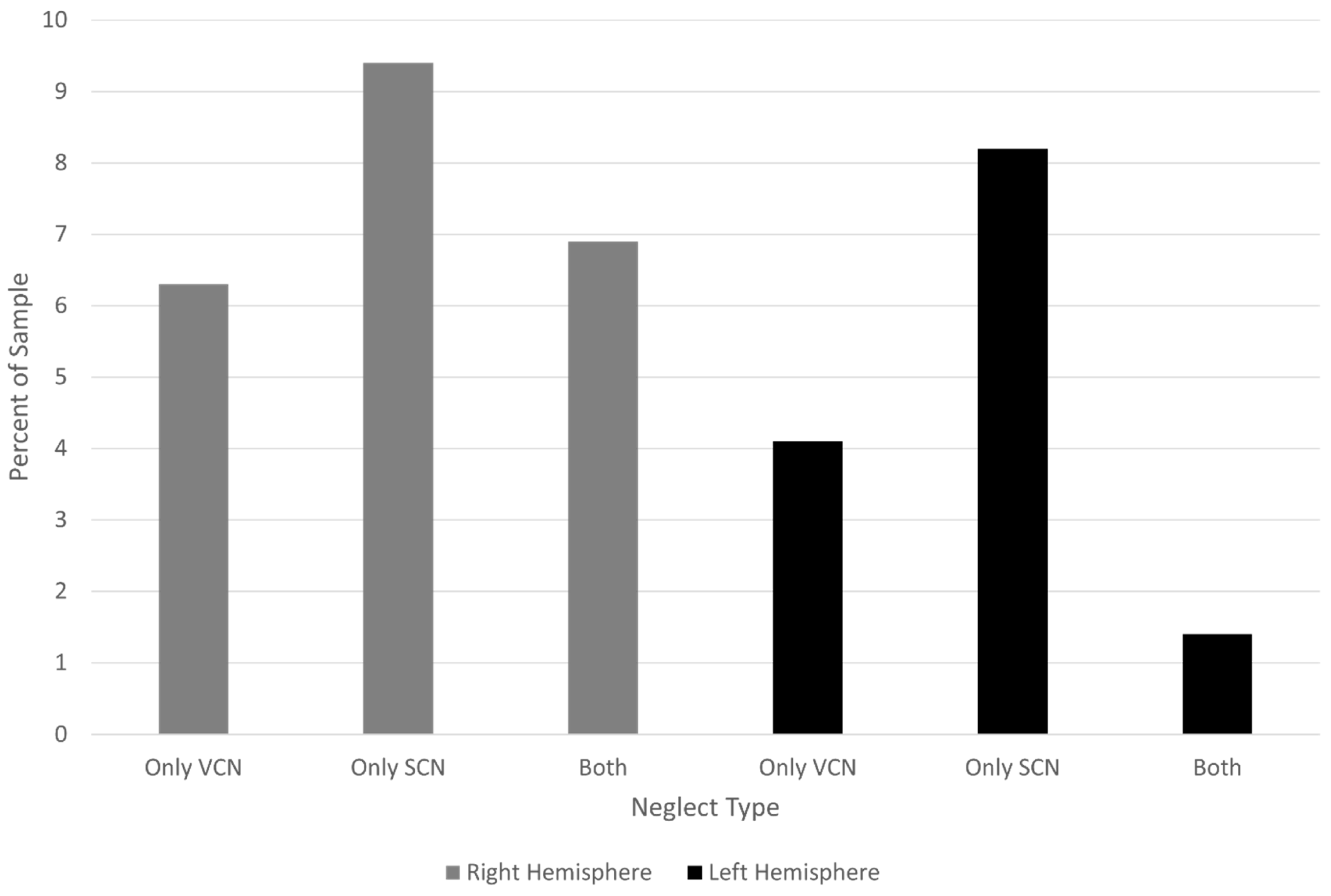
Among participants with right hemisphere stroke, 21 (13.1%) had VCN (with or without SCN), 26 (16.3%) had SCN (with or without VCN), and 11 (6.9%) had both VCN and SCN. Among participants with left hemisphere stroke, four (5.5%) had VCN (with or without SCN), seven (9.6%) had SCN (with or without VCN), and one (1.4%) had both VCN and SCN.
Figure 4 shows the percentage with VCN and SCN alone in each hemisphere group.
Put another way, VCN occurred in 13.1% of participants with right hemisphere stroke and 5.5% of those with left hemisphere stroke. SCN occurred in 16.3% of participants with right hemisphere stroke and 9.6% of those with left hemisphere stroke. The co-occurrence was less common than either type of neglect in isolation after left hemisphere stroke and less common than SCN alone in right hemisphere stroke. (
Figure 4).
3.2. Partially Distinct Areas of Infarct Contribute to VCN and SCN
For right hemisphere stroke participants, the lesion load across the right hemisphere vascular territories, along with age, predicted 45.0% of the variance in incidence of left VCN (
p < 0.00001). The presence of VCN was independently predicted by age (
p = 0.002) and lesion load in the right ACA territory (
p = 0.001) and right MCA occipital portion (
p = 0.03). The lesion load across the right hemisphere vascular territories, along with age, predicted 15.5% of the variance in incidence of left SCN (
p = 0.0048). None of the lesioned areas alone or age independently predicted left SCN. Refer to
Table 2 and
Table 3 for odds ratios and 95% confidence intervals.
In the left hemisphere stroke, none of the lesioned areas alone or age independently or together predicted the right VCN. However, the lesion load across the left hemisphere vascular territories, along with age, predicted 49.0% of the variance in the incidence of right SCN (
p = 0.0063). The absence of any damage to the left PCA temporal region predicted the absence of the right SCN perfectly and was omitted from the model. Right SCN was independently predicted lesion load in the left ACA (
p = 0.024), left PCA occipital region (
p = 0.015), and age (
p = 0.021). Refer to
Table 4 and
Table 5 for odds ratios and 95% confidence intervals.
3.3. The Contribution of Areas of Hypoperfusion (Estimated with FLAIR Vessel Hyperintensities) in Accounting for the Variance in Each Type of Neglect
In the right hemisphere stroke participants, adding the FVH values to the model for left VCN improved the amount of variance explained from 44.5% to 55.0% (
p < 0.00001). In this model (like the model without FVH), age (
p = 0.030), lesion load in the right ACA territory (
p = 0.003), and MCA occipital region (
p = 0.03) independently predicted left VCN. Likewise, adding the FVH values to the model for the left SCN improved the amount of variance explained from 15.6% to 20.1% (
p = 0.012). In this model, FVH (indicating hypoperfusion) in the parietal cortex (
p = 0.043) and age (
p = 0.009) independently predicted left SCN. Refer to
Table 6 and
Table 7 for odds ratios and 95% confidence intervals.
In the left hemisphere stroke participants, adding the FVH values to the model for right VCN improved the amount of variance explained from 8.4% (ns) to 20.7% (
p = 0.03). None of the lesioned areas alone or age independently predicted right VCN (see
Table 8). However, adding FVH to the model for right SCN improved the amount of variance explained from 49.1% (
p = 0.0063) to 100% (incalculable odds ratios for each independent variable, likely due to the relatively small number of participants with neglect due to left hemisphere lesions).
4. Discussion
Here, we evaluated three hypotheses. We partially confirmed the first hypothesis, that VCN and SCN dissociate, even on the same tasks/with the same task demands; and VCN is more common after right hemisphere stroke. However, SCN was more common after right than left hemisphere stroke. The co-occurrence was relatively uncommon after stroke in either hemisphere. Results are consistent with most previous studies that have tested both types of neglect in acute and chronic strokes [
4,
5,
6,
9]. However, they contrast with the findings of Rorden et al. [
11], in which the two types of neglect were highly correlated. One plausible explanation for the contrasting findings is that patients in the Rorden study [
11] had larger strokes, including areas important for both VCN and SCN. The slightly greater frequency of SCN alone (without VCN) after right than left hemisphere stroke (9.3% vs. 8.2%) was unexpected but is likely explained by the larger infarct volume (30 vs. 19 cc) and larger volume of hypoperfusion estimated by the number of FVH (1.7 vs. 1;
p = 0.008) in right versus left hemisphere strokes. Alternatively, the results may simply reflect the distribution of lesion sites in right and left hemisphere stroke participants in this study or the relatively small number of individuals with left hemisphere stroke. Most studies of the incidence of neglect have tested only VCN, which is more common after the right hemisphere stroke in this and other studies. This bias toward evaluating VCN may account for the common assertion that neglect is more common and severe in right than left hemisphere stroke (although we found that both types of neglect are more common after right than left hemisphere stroke).
We also confirmed the second hypothesis, that partially distinct areas of infarct contribute to VCN and SCN. While lesion load in right MCA—occipital and right ACA regions (and age) independently predicted the presence of left VCN, right parietal hypoperfusion (indicated by FVH), and other areas together were necessary for predicting the presence of SCN in the same individuals. Unlike previous studies, we did not carry out voxel-based analyses to identify smaller areas most strongly associated with either type of neglect. Similarly, in left hemisphere stroke, right VCN was only predicted by all of the left hemisphere regions together, along with age and FVH. In contrast, right SCN was predicted by the lesion load in left PCA occipital and left ACA regions (and age). We did confirm that more frontal (ACA) infarcts were more associated with VCN, and more ventral (PCA) infarcts were associated with SCN. We did not evaluate the proposal that the dorsal visual processing stream, when damaged, is more responsible for VCN versus the ventral stream being more responsible for SCN [
15].
Finally, we confirmed the last hypothesis, that information about the areas of hypoperfusion (estimated with FLAIR hyperintense vessel) contributes additional information to areas of dysfunction (infarct and/or hypoperfusion) that account for the variance in each type of neglect. The estimated percentage of variance explained by the anatomical model (based on pseudo r2) improved from 44.5% to 55.0% for left VCN, from 15.6% to 20.1% for left SCN, from 8.0% to 20.7% for right VCN, and from 49.1% to 100% for right SCN when we added data on the location and number of FHVs in each area. These results demonstrate that brain mapping studies in acute stroke can include approximate areas of hypoperfusion beyond the infarct that may account for acute deficits, even when perfusion MRI is not available.
Limitations of our study include the relatively small number of participants with left hemisphere stroke and the smaller infarct volume and volume of hypoperfusion (estimated with FVH) in left compared to right hemisphere stroke. These two limitations both reflect the fact that individuals with larger left hemisphere strokes often had comprehension deficits that precluded them from understanding the task. But these limitations may have precluded us from identifying the reliable location of infarct and/or hypoperfusion responsible for VCN and SCN after left hemisphere stroke. The pseudo r
2 of 1 identified for the model of right SCN is clearly not reliable but reflects the small number of people with the right SCN in this study. Another limitation is that we did not have perfusion-weighted images to identify the more exact areas of hypoperfusion that could cause neglect in our participants. But early studies have shown that FVH accurately reflects both the volume [
22,
24] and location of hypoperfusion, as measured in participants with both FLAIR and perfusion-weighted imaging at the same time, although clearly arterial spin labeling (ASL) MRI or dynamic contrast tracking perfusion-weighted imaging would have been more sensitive for the precise location and quantity of hypoperfusion. Finally, we did not evaluate the contribution of white matter tracts in accounting for each type of neglect. Previous studies have shown that disruption of white matter tracts can account for hemispatial neglect [
25,
26]. Future studies will further assess the contributions of disrupted white matter tracts and atrophy in each hemisphere.
5. Conclusions
Despite its limitations, this study provided confirmatory evidence on the dissociation and relative frequency of VCN and SCN in acute stroke, which should lead to better detection of hemispatial neglect in the left as well as the right hemisphere stroke. We also provided new evidence on the potential contribution of FVH ratings in accounting for both VCN and SCN. Thus, clinical MRI with FLAIR sequences can provide valuable additional information about hypoperfusion that may be causing neglect and should be useful in guiding reperfusion therapies to improve neglect and other cognitive deficits after stroke.
Author Contributions
Conceptualization, A.R. and A.E.H.; methodology, A.E.H. and A.V.F.; formal analysis, A.E.H. and A.V.F.; investigation, A.R. and A.E.H.; data curation, M.C.; writing—original draft preparation, A.R. and A.E.H.; writing—review and editing, A.R. and A.E.H.; visualization, M.C. and A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by National Institutes of Health, through NIDCD (R01DC05375 and R01DC025466) and NIBIB (P41EB031771).
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Johns Hopkins Institutional Review Board (protocol: NA_00042097; date of last approval: 31 October 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
We gratefully acknowledge the support of NIDCD and the involvement of the participants. Members of the Stroke Cognitive Outcomes and REcovery (SCORE) Lab administered the assessments, scored, and entered data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chatterjee, A. Picturing unilateral spatial neglect: Viewer versus object centred reference frames. J. Neurol. Neurosurg. Psychiatry 1994, 57, 1236–1240. Available online: http://www.ncbi.nlm.nih.gov/pubmed/7931386 (accessed on 30 November 2024). [CrossRef] [PubMed]
- Hillis, A.E.; Caramazza, A. A framework for interpreting distinct patterns of hemispatial neglect. Neurocase 1995, 1, 189–207. [Google Scholar] [CrossRef]
- Kleinman, J.T.; Newhart, M.; Davis, C.; Heidler-Gary, J.; Gottesman, R.F.; Hillis, A.E. Right hemispatial neglect: Frequency and characterization following acute left hemisphere stroke. Brain Cogn. 2007, 64, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Demeyere, N.; Gillebert, C.R. Ego- and allocentric visuospatial neglect: Dissociations, prevalence, and laterality in acute stroke. Neuropsychology 2019, 33, 490–498. [Google Scholar] [CrossRef]
- Kenzie, J.M.; Girgulis, K.A.; Semrau, J.A.; Findlater, S.E.; Desai, J.A.; Dukelow, S.P. Lesion Sites Associated with Allocentric and Egocentric Visuospatial Neglect in Acute Stroke. Brain Connect. 2015, 5, 413–422. [Google Scholar] [CrossRef]
- Chechlacz, M.; Rotshtein, P.; Roberts, K.L.; Bickerton, W.L.; Lau, J.K.L.; Humphreys, G.W. The prognosis of allocentric and egocentric neglect: Evidence from clinical scans. PLoS ONE 2012, 7, e47821. [Google Scholar] [CrossRef] [PubMed]
- Suarez, A.; Saxena, S.; Oishi, K.; Oishi, K.; Walker, A.; Rorden, C.; Hillis, A.E. Influence of age, lesion volume, and damage to dorsal versus ventral streams to viewer- and stimulus-centered hemispatial neglect in acute right hemisphere stroke. Cortex 2020, 126, 73–82. [Google Scholar] [CrossRef]
- Rode, G.; Pagliari, C.; Huchon, L.; Rossetti, Y.; Pisella, L. Semiology of neglect: An update. Ann. Phys. Rehabil. Med. 2017, 60, 177–185. [Google Scholar] [CrossRef]
- Turgut, N.; Mödden, C.; Brumund, T.; Eling, P.; Hildebrandt, H. A study on the independence of egocentric and allocentric neglect. Cortex 2017, 96, 95–104. [Google Scholar] [CrossRef]
- Edmonds, J.; Lincoln, N.B. The frequency of perceptual deficits after stroke. Clin. Rehabil. 1987, 1, 273–281. [Google Scholar]
- Rorden, C.; Hjaltason, H.; Fillmore, P.; Fridriksson, J.; Kjartansson, O.; Magnusdottir, S.; Karnath, H.-O. Allocentric neglect strongly associated with egocentric neglect. Neuropsychologia 2012, 50, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Leyland, L.A.; Godwin, H.J.; Benson, V.; Liversedge, S.P. Neglect Patients Exhibit Egocentric or Allocentric Neglect for the Same Stimulus Contingent upon Task Demands. Sci. Rep. 2017, 7, 1941. [Google Scholar] [CrossRef]
- Grimsen, C.; Hildebrandt, H.; Fahle, M. Dissociation of egocentric and allocentric coding of space in visual search after right middle cerebral artery stroke. Neuropsychologia 2008, 46, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Song, W.; Huo, S.; Wang, M. Study on the occurrence and neural bases of hemispatial neglect with different reference frames. Arch. Phys. Med. Rehabil. 2012, 93, 156–162. [Google Scholar] [CrossRef]
- Medina, J.; Kannan, V.; Pawlak, M.A.; Kleinman, J.T.; Newhart, M.; Davis, C.; Heidler-Gary, J.E.; Herskovits, E.H.; Hillis, A.E. Neural substrates of visuospatial processing in distinct reference frames: Evidence from unilateral spatial neglect. J. Cogn. Neurosci. 2009, 21, 2073–2084. [Google Scholar] [CrossRef]
- Gottesman, R.F.; Kleinman, J.T.; Davis, C.; Heidler-Gary, J.; Newhart, M.; Kannan, V.; Hillis, A.E. Unilateral neglect is more severe and common in older patients with right hemispheric stroke. Neurology 2008, 71, 1439–1444. [Google Scholar] [CrossRef]
- Hillis, A.E.; Barker, P.B.; Beauchamp, N.J.; Gordon, B.; Wityk, R.J. MR perfusion imaging reveals regions of hypoperfusion associated with aphasia and neglect. Neurology 2000, 55, 782–788. [Google Scholar] [CrossRef]
- Croquelois, A.; Wintermark, M.; Reichhart, M.; Meuli, R.; Bogousslavsky, J. Aphasia in hyperacute stroke: Language follows brain penumbra dynamics. Ann. Neurol. 2003, 54, 321–329. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12953264 (accessed on 30 November 2024). [CrossRef]
- Hillis, A.E.; Wityk, R.J.; Barker, P.B.; Beauchamp, N.J.; Gailloud, P.; Murphy, K.; Cooper, O.; Metter, E.J. Subcortical aphasia and neglect in acute stroke: The role of cortical hypoperfusion. Brain 2002, 125, 1094–1104. [Google Scholar] [CrossRef]
- Shirani, P.; Thorn, J.; Davis, C.; Heidler-Gary, J.; Newhart, M.; Gottesman, R.F.; Hillis, A.E. Severity of hypoperfusion in distinct brain regions predicts severity of hemispatial neglect in different reference frames. Stroke 2009, 40, 3563–3566. [Google Scholar] [CrossRef]
- Khurshid, S.; Trupe, L.A.; Newhart, M.; Davis, C.; Molitoris, J.J.; Medina, J.; Leigh, R.; Hillis, A.E. Reperfusion of specific cortical areas is associated with improvement in distinct forms of hemispatial neglect. Cortex 2012, 48, 530–539. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reyes, D.; Simpkins, A.N.; Hitomi, E.; Lynch, J.K.; Hsia, A.W.; Nadareishvili, Z.; Luby, M.; Latour, L.L.; Leigh, R. Estimating Perfusion Deficits in Acute Stroke Patients Without Perfusion Imaging. Stroke 2022, 53, 3439–3445. [Google Scholar] [CrossRef] [PubMed]
- Bunker, L.; Walker, A.; Meier, E.; Goldberg, E.; Leigh, R.; Hillis, A.E. Hyperintense vessels on imaging account for neurological function independent of lesion volume in acute ischemic stroke. Neuroimage Clin. 2022, 34, 102991. [Google Scholar] [CrossRef] [PubMed]
- Bunker, L.D.; Hillis, A.E. Location of Hyperintense Vessels on FLAIR Associated with the Location of Perfusion Deficits in PWI. J. Clin. Med. 2023, 12, 1554. [Google Scholar] [CrossRef]
- Bartolomeo, P.; de Schotten, M.T.; Doricchi, F. Left unilateral neglect as a disconnection syndrome. Cereb. Cortex 2007, 17, 2479–2490. [Google Scholar] [CrossRef]
- Saxena, S.; Keser, Z.; Rorden, C.; Bonilha, L.; Fridriksson, J.; Walker, A.; Hillis, A.E. Disruptions of the Human Connectome Associated with Hemispatial Neglect. Neurology 2022, 98, e107–e114. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).