The Effect of Skating Exercises as High-Intensity Interval Training on Elderly Stroke Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. HIIT Protocol
2.4. MICT Protocol
2.5. Outcome Measures
2.6. Statistics
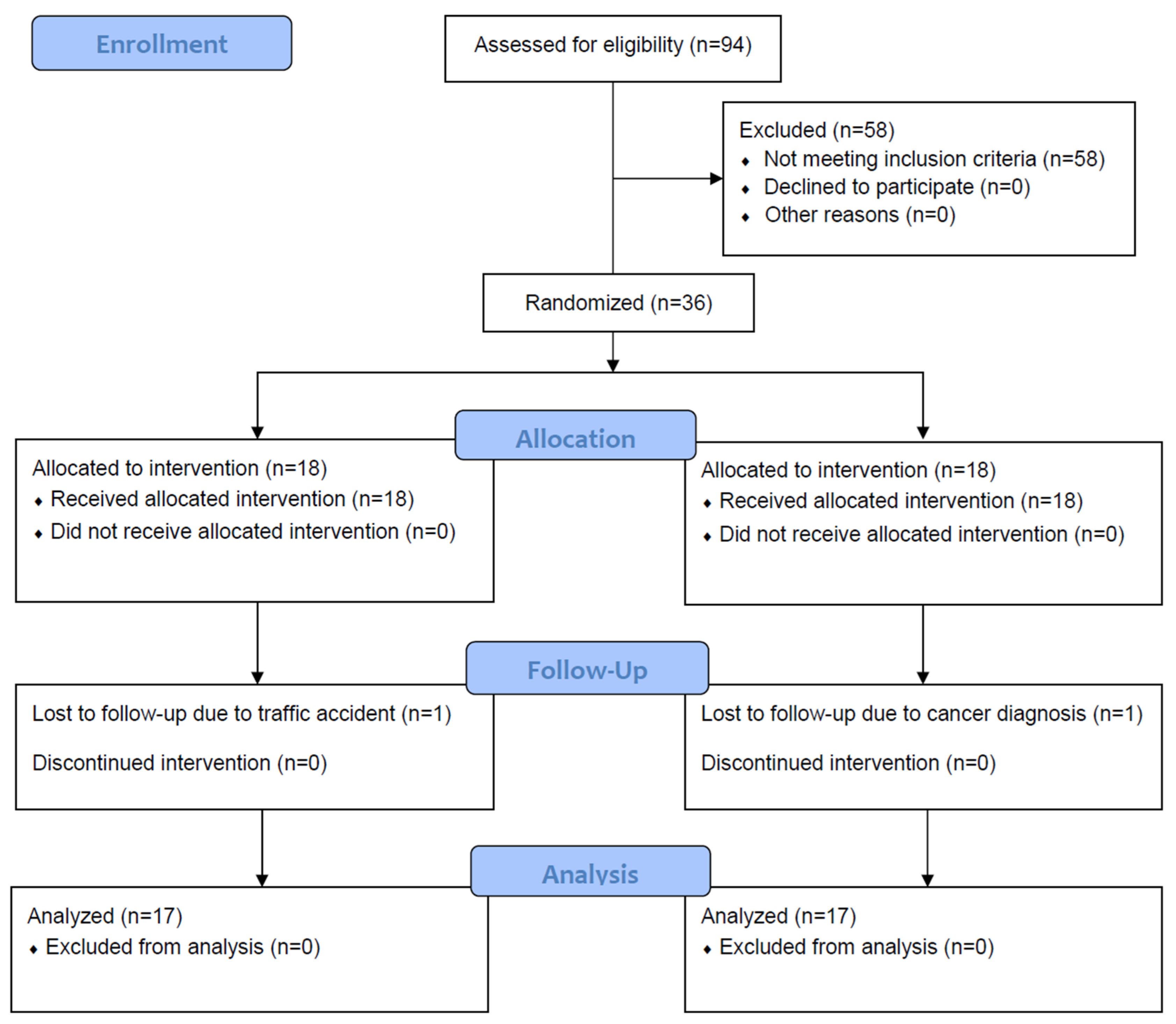
3. Results
3.1. Demographic and Clinical Characteristics of the Participants
3.2. Effects of HIIT and MICT on Cardiorespiratory Fitness
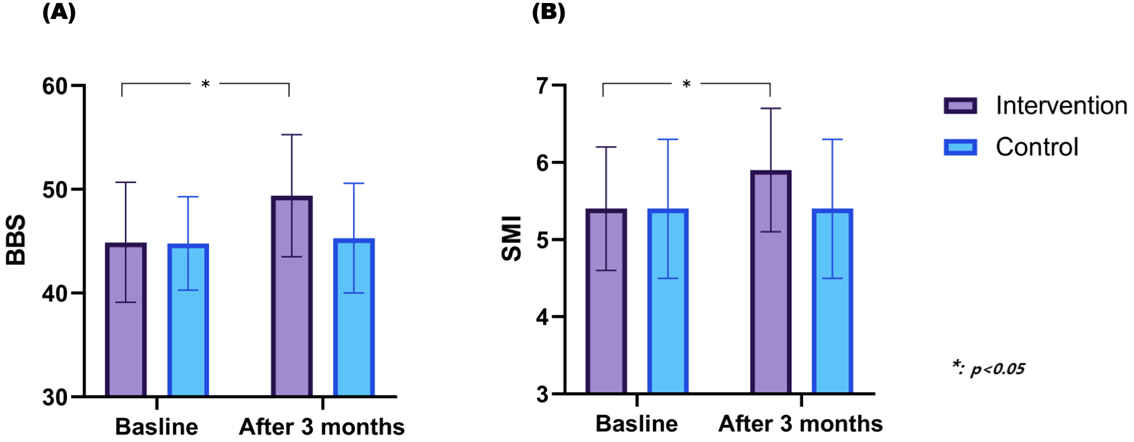
3.3. Changes in Balance and Muscle Mass After HIIT and MICT
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- von Weitzel-Mudersbach, P.; Andersen, G.; Hundborg, H.H.; Johnsen, S.P. Transient ischemic attack and minor stroke are the most common manifestations of acute cerebrovascular disease: A prospective, population-based study-the Aarhus TIA study. Neuroepidemiology 2013, 40, 50–55. [Google Scholar] [CrossRef]
- Fischer, U.; Baumgartner, A.; Arnold, M.; Nedeltchev, K.; Gralla, J.; De Marchis, G.M.; Kappeler, L.; Mono, M.L.; Brekenfeld, C.; Schroth, G.; et al. What is a minor stroke? Stroke 2010, 41, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Cucchiara, B.; Elm, J.; Easton, J.D.; Coutts, S.B.; Willey, J.Z.; Biros, M.H.; Ross, M.A.; Johnston, S.C. Disability After Minor Stroke and Transient Ischemic Attack in the POINT Trial. Stroke 2020, 51, 792–799. [Google Scholar] [CrossRef]
- Cucchiara, B.; George, D.K.; Kasner, S.E.; Knutsson, M.; Denison, H.; Ladenvall, P.; Amarenco, P.; Johnston, S.C. Disability after minor stroke and TIA: A secondary analysis of the SOCRATES trial. Neurology 2019, 93, e708–e716. [Google Scholar] [CrossRef] [PubMed]
- Xinyi, L.; David, W. Editorial: Minor stroke is not minor. Stroke Vasc. Neurol. 2023, 8, 175–177. [Google Scholar] [CrossRef]
- Khatri, P.; Kleindorfer, D.O.; Devlin, T.; Sawyer, R.N., Jr.; Starr, M.; Mejilla, J.; Broderick, J.; Chatterjee, A.; Jauch, E.C.; Levine, S.R.; et al. Effect of Alteplase vs Aspirin on Functional Outcome for Patients with Acute Ischemic Stroke and Minor Nondisabling Neurologic Deficits: The PRISMS Randomized Clinical Trial. JAMA 2018, 320, 156–166. [Google Scholar] [CrossRef]
- Prestgaard, E.; Mariampillai, J.; Engeseth, K.; Erikssen, J.; Bodegård, J.; Liestøl, K.; Gjesdal, K.; Kjeldsen, S.; Grundvold, I.; Berge, E. Change in Cardiorespiratory Fitness and Risk of Stroke and Death: Long-Term Follow-Up of Healthy Middle-Aged Men. Stroke 2019, 50, 155–161. [Google Scholar] [CrossRef]
- Lang, J.J.; Prince, S.A.; Merucci, K.; Cadenas-Sanchez, C.; Chaput, J.-P.; Fraser, B.J.; Manyanga, T.; McGrath, R.; Ortega, F.B.; Singh, B.; et al. Cardiorespiratory fitness is a strong and consistent predictor of morbidity and mortality among adults: An overview of meta-analyses representing over 20.9 million observations from 199 unique cohort studies. Br. J. Sports Med. 2024, 58, 556–566. [Google Scholar] [CrossRef]
- Raghuveer, G.; Hartz, J.; Lubans, D.R.; Takken, T.; Wiltz, J.L.; Mietus-Snyder, M.; Perak, A.M.; Baker-Smith, C.; Pietris, N.; Edwards, N.M. Cardiorespiratory Fitness in Youth: An Important Marker of Health: A Scientific Statement from the American Heart Association. Circulation 2020, 142, e101–e118. [Google Scholar] [CrossRef]
- MacKay-Lyons, M.; Billinger, S.A.; Eng, J.J.; Dromerick, A.; Giacomantonio, N.; Hafer-Macko, C.; Macko, R.; Nguyen, E.; Prior, P.; Suskin, N.; et al. Aerobic Exercise Recommendations to Optimize Best Practices in Care After Stroke: AEROBICS 2019 Update. Phys. Ther. 2020, 100, 149–156. [Google Scholar] [CrossRef]
- Marsden, D.L.; Dunn, A.; Callister, R.; Levi, C.R.; Spratt, N.J. Characteristics of Exercise Training Interventions to Improve Cardiorespiratory Fitness After Stroke:A Systematic Review with Meta-analysis. Neurorehabilit. Neural Repair 2013, 27, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.; Johnson, F.; Uy, J.; Serrada, I.; Benyamin, B.; van den Berg, M.; Hordacre, B. Moderate intensity aerobic exercise may enhance neuroplasticity of the contralesional hemisphere after stroke: A randomised controlled study. Sci. Rep. 2023, 13, 14440. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Meng, H.; Wang, Z.; Zhu, S.; Yuan, S.; Wang, Y.; Wang, Q. Effect of high-intensity exercise on cardiorespiratory fitness in stroke survivors: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2020, 63, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Moncion, K.; Rodrigues, L.; De Las Heras, B.; Noguchi, K.S.; Wiley, E.; Eng, J.J.; MacKay-Lyons, M.; Sweet, S.N.; Thiel, A.; Fung, J.; et al. Cardiorespiratory Fitness Benefits of High-Intensity Interval Training After Stroke: A Randomized Controlled Trial. Stroke 2024, 55, 2202–2211. [Google Scholar] [CrossRef]
- Crozier, J.; Roig, M.; Eng, J.J.; MacKay-Lyons, M.; Fung, J.; Ploughman, M.; Bailey, D.M.; Sweet, S.N.; Giacomantonio, N.; Thiel, A.; et al. High-Intensity Interval Training After Stroke: An Opportunity to Promote Functional Recovery, Cardiovascular Health, and Neuroplasticity. Neurorehabil. Neural Repair 2018, 32, 543–556. [Google Scholar] [CrossRef]
- Ashcroft, S.K.; Johnson, L.; Kuys, S.S.; Thompson-Butel, A.G. High Intensity Interval Training POst-STroke (HIIT-POST): Perspectives of People Living With Stroke and Health Professionals. Neurorehabil. Neural Repair 2025, 39, 343–354. [Google Scholar] [CrossRef]
- Gjellesvik, T.I.; Becker, F.; Tjønna, A.E.; Indredavik, B.; Lundgaard, E.; Solbakken, H.; Brurok, B.; Tørhaug, T.; Lydersen, S.; Askim, T. Effects of High-Intensity Interval Training After Stroke (The HIIT Stroke Study) on Physical and Cognitive Function: A Multicenter Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2021, 102, 1683–1691. [Google Scholar] [CrossRef]
- Boyne, P.; Dunning, K.; Carl, D.; Gerson, M.; Khoury, J.; Rockwell, B.; Keeton, G.; Westover, J.; Williams, A.; McCarthy, M.; et al. High-Intensity Interval Training and Moderate-Intensity Continuous Training in Ambulatory Chronic Stroke: Feasibility Study. Phys. Ther. 2016, 96, 1533–1544. [Google Scholar] [CrossRef]
- Yousufuddin, M.; Young, N. Aging and ischemic stroke. Aging 2019, 11, 2542–2544. [Google Scholar] [CrossRef]
- Da Campo, L.; Hauck, M.; Marcolino, M.A.Z.; Pinheiro, D.; Plentz, R.D.M.; Cechetti, F. Effects of aerobic exercise using cycle ergometry on balance and functional capacity in post-stroke patients: A systematic review and meta-analysis of randomised clinical trials. Disabil. Rehabil. 2021, 43, 1558–1564. [Google Scholar] [CrossRef]
- Romero-Franco, N.; Jiménez-Reyes, P. Unipedal Postural Balance and Countermovement Jumps After a Warm-up and Plyometric Training Session: A Randomized Controlled Trial. J. Strength. Cond. Res. 2015, 29, 3216–3222. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, J.; Ruby, B.C.; Dumke, C.L. Effect of Plyometrics on the Energy Cost of Running and MHC and Titin Isoforms. Med. Sci. Sports Exerc. 2016, 48, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Mau-Moeller, A.; Mueller, K.; Heise, S.; Gube, M.; Beuster, N.; Herlyn, P.K.; Fischer, D.C.; Bruhn, S. Plyometric training improves voluntary activation and strength during isometric, concentric and eccentric contractions. J. Sci. Med. Sport 2016, 19, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Soh, S.H.; Joo, M.C.; Yun, N.R.; Kim, M.S. Randomized Controlled Trial of the Lateral Push-Off Skater Exercise for High-Intensity Interval Training vs Conventional Treadmill Training. Arch. Phys. Med. Rehabil. 2020, 101, 187–195. [Google Scholar] [CrossRef]
- Mueller, S.; Winzer, E.B.; Duvinage, A.; Gevaert, A.B.; Edelmann, F.; Haller, B.; Pieske-Kraigher, E.; Beckers, P.; Bobenko, A.; Hommel, J.; et al. Effect of High-Intensity Interval Training, Moderate Continuous Training, or Guideline-Based Physical Activity Advice on Peak Oxygen Consumption in Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. Jama 2021, 325, 542–551. [Google Scholar] [CrossRef]
- Globas, C.; Becker, C.; Cerny, J.; Lam, J.M.; Lindemann, U.; Forrester, L.W.; Macko, R.F.; Luft, A.R. Chronic stroke survivors benefit from high-intensity aerobic treadmill exercise: A randomized control trial. Neurorehabil. Neural Repair 2012, 26, 85–95. [Google Scholar] [CrossRef]
- Lamberti, N.; Straudi, S.; Malagoni, A.M.; Argirò, M.; Felisatti, M.; Nardini, E.; Zambon, C.; Basaglia, N.; Manfredini, F. Effects of low-intensity endurance and resistance training on mobility in chronic stroke survivors: A pilot randomized controlled study. Eur. J. Phys. Rehabil. Med. 2017, 53, 228–239. [Google Scholar] [CrossRef]
- Macko, R.F.; Katzel, L.I.; Yataco, A.; Tretter, L.D.; DeSouza, C.A.; Dengel, D.R.; Smith, G.V.; Silver, K.H. Low-velocity graded treadmill stress testing in hemiparetic stroke patients. Stroke 1997, 28, 988–992. [Google Scholar] [CrossRef]
- Moore, J.; Raad, J. Measurement Characteristics and Clinical Utility of the Berg Balance Scale Among Individuals With Stroke. Arch. Phys. Med. Rehabil. 2013, 94, 217–218. [Google Scholar] [CrossRef]
- Boo, S.H.; Joo, M.C.; Lee, J.M.; Kim, S.C.; Yu, Y.M.; Kim, M.S. Association between skeletal muscle mass and cardiorespiratory fitness in community-dwelling elderly men. Aging Clin. Exp. Res. 2019, 31, 49–57. [Google Scholar] [CrossRef]
- Weatherwax, R.M.; Nelson, M.C.; Dalleck, L.C. The Impact of Personalized versus Standardized Cardiorespiratory and Muscular Training on Health-Related Outcomes and Rate of Responders. J. Sports Sci. Med. 2024, 23, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Billinger, S.A.; Arena, R.; Bernhardt, J.; Eng, J.J.; Franklin, B.A.; Johnson, C.M.; MacKay-Lyons, M.; Macko, R.F.; Mead, G.E.; Roth, E.J.; et al. Physical activity and exercise recommendations for stroke survivors: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 2532–2553. [Google Scholar] [CrossRef]
- Atakan, M.M.; Li, Y.; Koşar, Ş.N.; Turnagöl, H.H.; Yan, X. Evidence-Based Effects of High-Intensity Interval Training on Exercise Capacity and Health: A Review with Historical Perspective. Int. J. Environ. Res. Public Health 2021, 18, 7201. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Yang, G.Y.; Jin, K. Age-Related Dysfunction in Balance: A Comprehensive Review of Causes, Consequences, and Interventions. Aging Dis. 2024, 16, 714–737. [Google Scholar] [CrossRef]
- Gobezie, M.; Kassa, T.; Suliman, J.; Eriku, G.A.; Takele, M.D.; Bitew, D.A.; Wubante, S.M.; Kibret, A.K. Balance impairment and associated factors among stroke survivors in public hospitals of Amhara regional state: A multicenter cross-sectional study. BMC Neurol. 2024, 24, 387. [Google Scholar] [CrossRef]
- Khan, F.; Chevidikunnan, M.F. Prevalence of Balance Impairment and Factors Associated with Balance among Patients with Stroke. A Cross Sectional Retrospective Case Control Study. Healthcare 2021, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Yoon, S.Y. The Safety and Efficacy of Balance Training on Stroke Patients With Reduced Balance Ability: A Meta-Analysis of Randomized Controlled Trials. Brain Neurorehabil. 2024, 17, e15. [Google Scholar] [CrossRef]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef]
- Ozkan, H.; Ambler, G.; Banerjee, G.; Mitchell, J.J.; Barbato, C.; Browning, S.; Leff, A.P.; Simister, R.J.; Werring, D.J. Prevalence, predictors, and patterns of patient reported non-motor outcomes six months after stroke: A prospective cohort study. Lancet Reg. Health Eur. 2024, 47, 101080. [Google Scholar] [CrossRef]
- Orr, R. Contribution of muscle weakness to postural instability in the elderly. A systematic review. Eur. J. Phys. Rehabil. Med. 2010, 46, 183–220. [Google Scholar] [PubMed]
- Plotkin, D.L.; Roberts, M.D.; Haun, C.T.; Schoenfeld, B.J. Muscle Fiber Type Transitions with Exercise Training: Shifting Perspectives. Sports 2021, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Prieto-González, P.; Sedlacek, J. Effects of Running-Specific Strength Training, Endurance Training, and Concurrent Training on Recreational Endurance Athletes’ Performance and Selected Anthropometric Parameters. Int. J. Environ. Res. Public Health 2022, 19, 10773. [Google Scholar] [CrossRef] [PubMed]

| Factor | Intervention Group (n = 17) | Control Group (n = 17) | p-Value | |
|---|---|---|---|---|
| Age | (years, mean ± SD) | 70.5 ± 5.5 | 70.8 ± 5.7 | 0.562 |
| Sex | Male | 7 | 7 | 0.974 |
| Female | 10 | 10 | ||
| Stroke type | Infarct | 17 | 17 | 0.942 |
| Hemorrhage | 0 | 0 | ||
| Weight | kg | 57 ± 8 | 58 ± 9 | 0.321 |
| Height | cm | 166 ± 9 | 165 ± 9 | 0.488 |
| BMI | kg/m2 | 20.7 ± 2.6 | 21.3 ± 3.1 | 0.105 |
| Duration after stroke onset | (days) | 76.1 ± 8.1 | 74.1 ± 8.2 | 0.237 |
| Comorbidity | Hypertension | 17 | 17 | 0.408 |
| Diabetes | 7 | 6 | ||
| Hyperlipidemia | 15 | 15 | ||
| NIHSS | Onset | 1.9 ± 0.8 | 2.0 ± 0.6 | 0.207 |
| K-MMSE | (at the beginning of the study) | 19.3 ± 4.0 | 18.5 ± 4.5 | 0.188 |
| Cardiorespiratory Fitness | Intervention Group (n = 17) | Control Group (n = 17) | p-Value | ||
|---|---|---|---|---|---|
| Baseline | After 3 Months | Baseline | After 3 Months | (Cohen’s d) | |
| Aerobic capacities | |||||
| VO2peak (mL/kg/min) | 20.4 (15.0–25.3) | 25.8 (16.5–32.8) | 20.6 (14.8–26.1) | 23.7 (15.3–29.9) | 0.005 * (0.20) |
| VT (mL/kg/min) | 10.6 (4.3–16.4) | 15.2 (10.5–19.9) | 10.7 (4.5–16.2) | 13.1 (5.8–17.3) | 0.002 * (0.22) |
| METpeak | 6.3 (2.2–10.1) | 8.5 (4.3–13.1) | 6.5 (2.5–10.5) | 7.3 (2.7–11.4) | 0.024 * (0.18) |
| Cardiovascular responses | |||||
| SBPrest (mmHg) | 125 (101–147) | 120 (96–146) | 123 (102–146) | 119 (100–138) | 0.432 (0.12) |
| DBPrest (mmHg) | 76 (60–91) | 77 (63–88) | 75 (59–90) | 78 (60–93) | 0.651 (0.14) |
| SBPpeak (mmHg) | 171 (122–201) | 173 (121–204) | 169 (114–200) | 174 (117–202) | 0.655 (0.12) |
| DBPpeak (mmHg) | 77 (57–92) | 77 (58–90) | 79 (59–93) | 80 (60–94) | 0.526 (0.18) |
| HRrest (beats/min) | 68 (50–84) | 65 (51–80) | 66 (49–82) | 65 (47–80) | 0.871 (0.25) |
| HRpeak (beats/min) | 137 (118–156) | 140 (122–159) | 136 (115–154) | 141 (116–153) | 0.329 (0.24) |
| O2pulsepeak (mL/beat) | 8.2 (4.1–11.7) | 10.5 (5.3–15.1) | 8.0 (4.0–11.1) | 10.2 (5.2–14.9) | 0.105 (0.24) |
| Ventilatory responses | |||||
| VEpeak (L/min) | 55 (38–68) | 58 (38–66) | 56 (39–69) | 60 (39–67) | 0.210 (0.21) |
| Vtpeak (L) | 1.6 (1.3–1.9) | 1.6 (1.3–1.9) | 1.7 (1.3–2.1) | 1.7 (1.3–2.1) | 0.488 (0.19) |
| VE/VCO2peak | 31.5 (25.4–37.4) | 30.9 (25.3–37.2) | 30.9 (24.3–37.0) | 30.5 (25.0–36.7) | 0.429 (0.18) |
| RRpeak (rates/min) | 31.2 (23.6–38.5) | 32.8 (23.8–38.8) | 31.3 (22.5–38.1) | 32.6 (23.5–38.1) | 0.208 (0.25) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-S. The Effect of Skating Exercises as High-Intensity Interval Training on Elderly Stroke Patients. Brain Sci. 2025, 15, 676. https://doi.org/10.3390/brainsci15070676
Kim M-S. The Effect of Skating Exercises as High-Intensity Interval Training on Elderly Stroke Patients. Brain Sciences. 2025; 15(7):676. https://doi.org/10.3390/brainsci15070676
Chicago/Turabian StyleKim, Min-Su. 2025. "The Effect of Skating Exercises as High-Intensity Interval Training on Elderly Stroke Patients" Brain Sciences 15, no. 7: 676. https://doi.org/10.3390/brainsci15070676
APA StyleKim, M.-S. (2025). The Effect of Skating Exercises as High-Intensity Interval Training on Elderly Stroke Patients. Brain Sciences, 15(7), 676. https://doi.org/10.3390/brainsci15070676





