The Safety and Efficacy of Glibenclamide in Managing Cerebral Edema After Aneurysmal Subarachnoid Hemorrhage: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Data Sources and Search Strategy
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Extraction
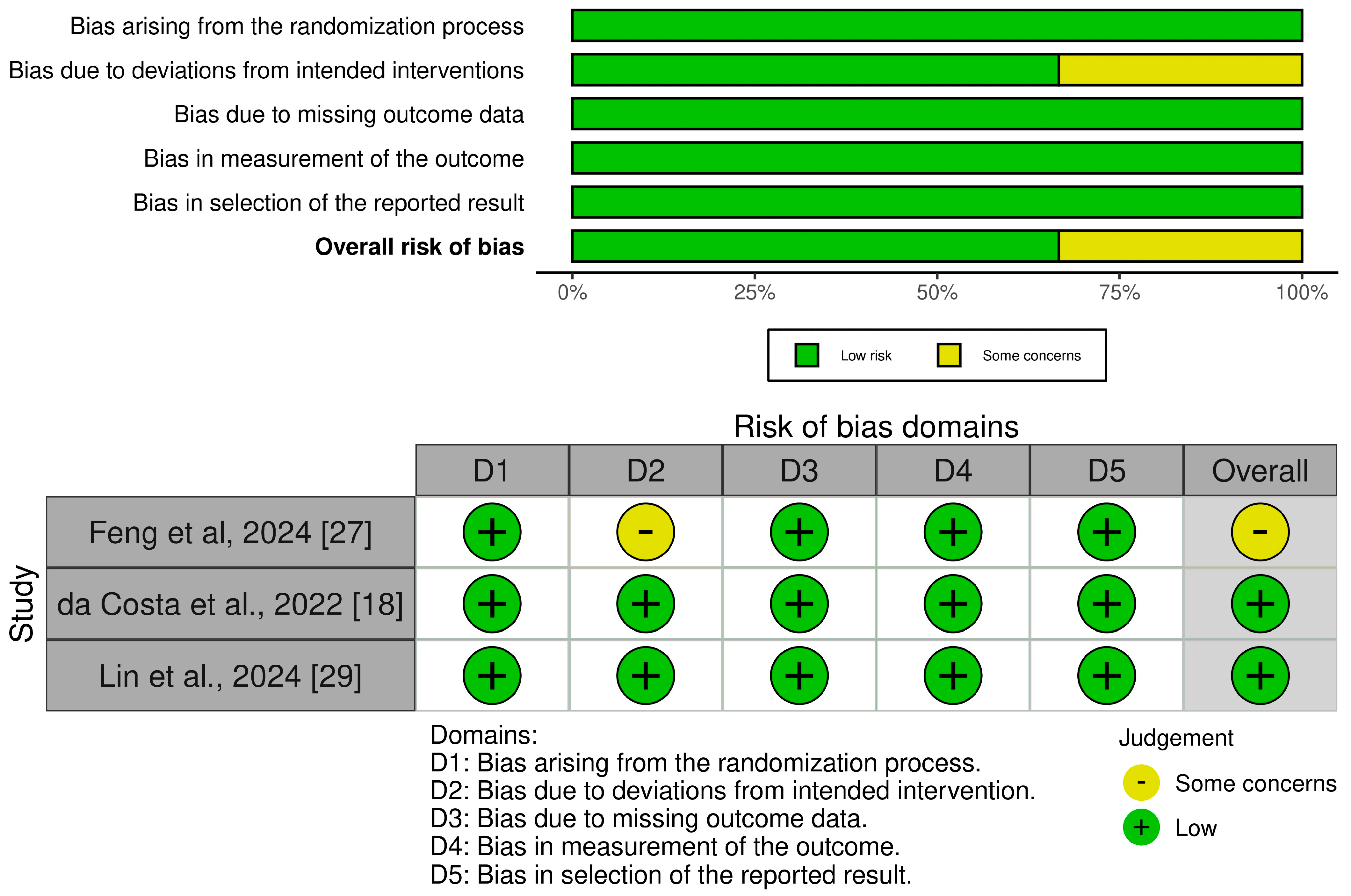
2.6. Risk-of-Bias Assessment and Certainty of Evidence
2.7. Statistical Analysis
3. Results
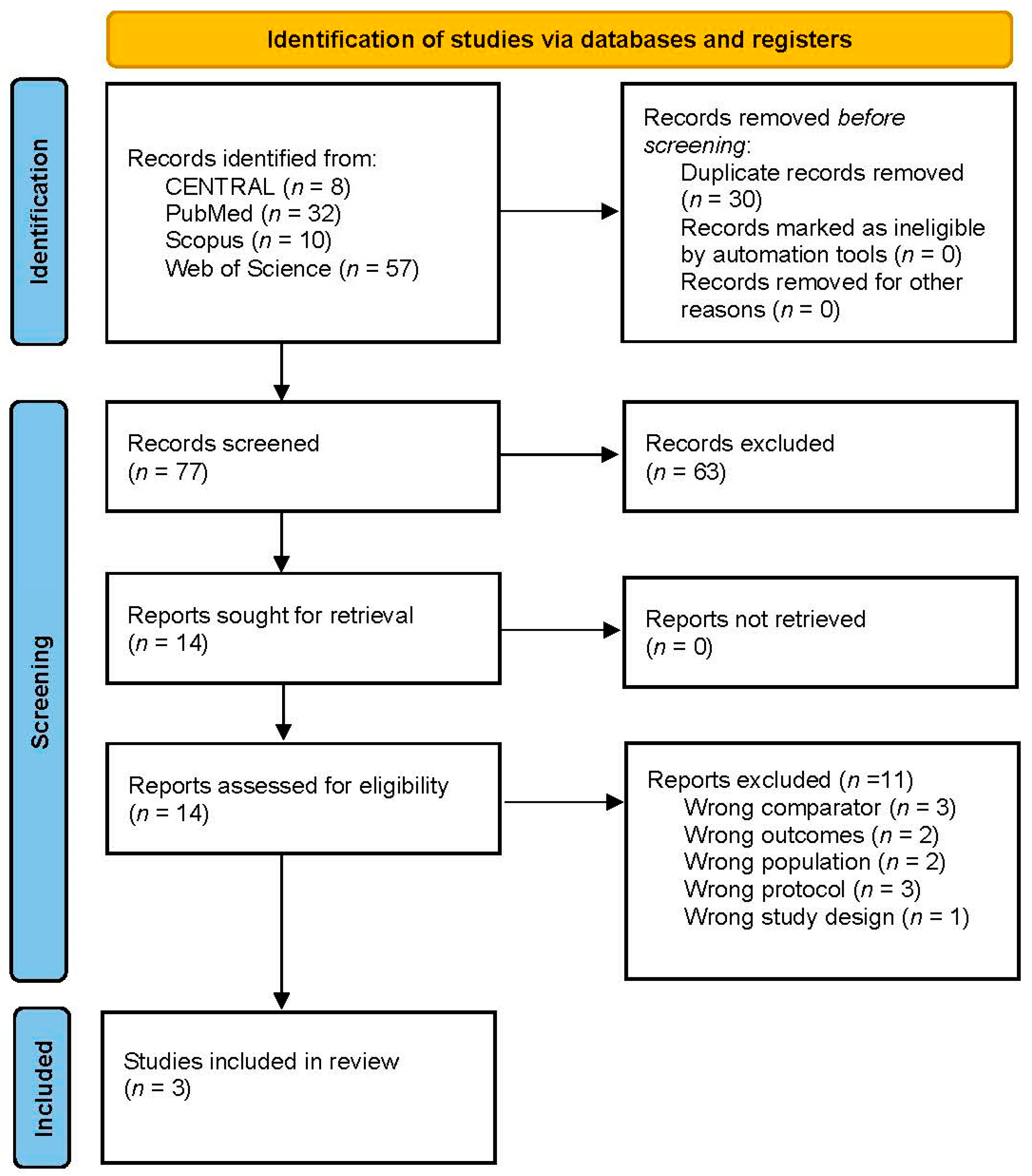
3.1. Search Results and Study Selection
3.2. Characteristics of Included Studies
3.3. Risk of Bias and Quality of Evidence
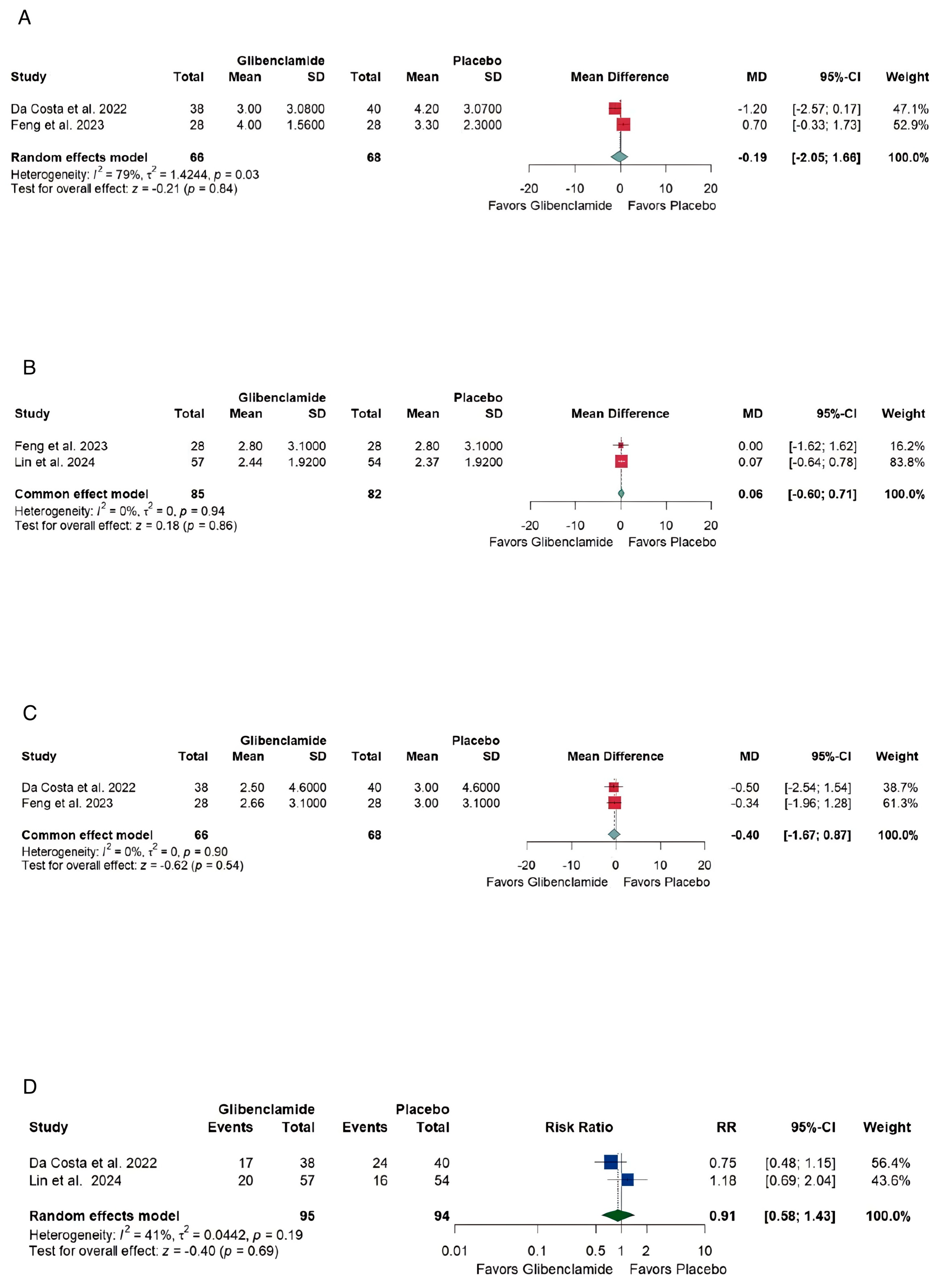
3.4. Efficacy
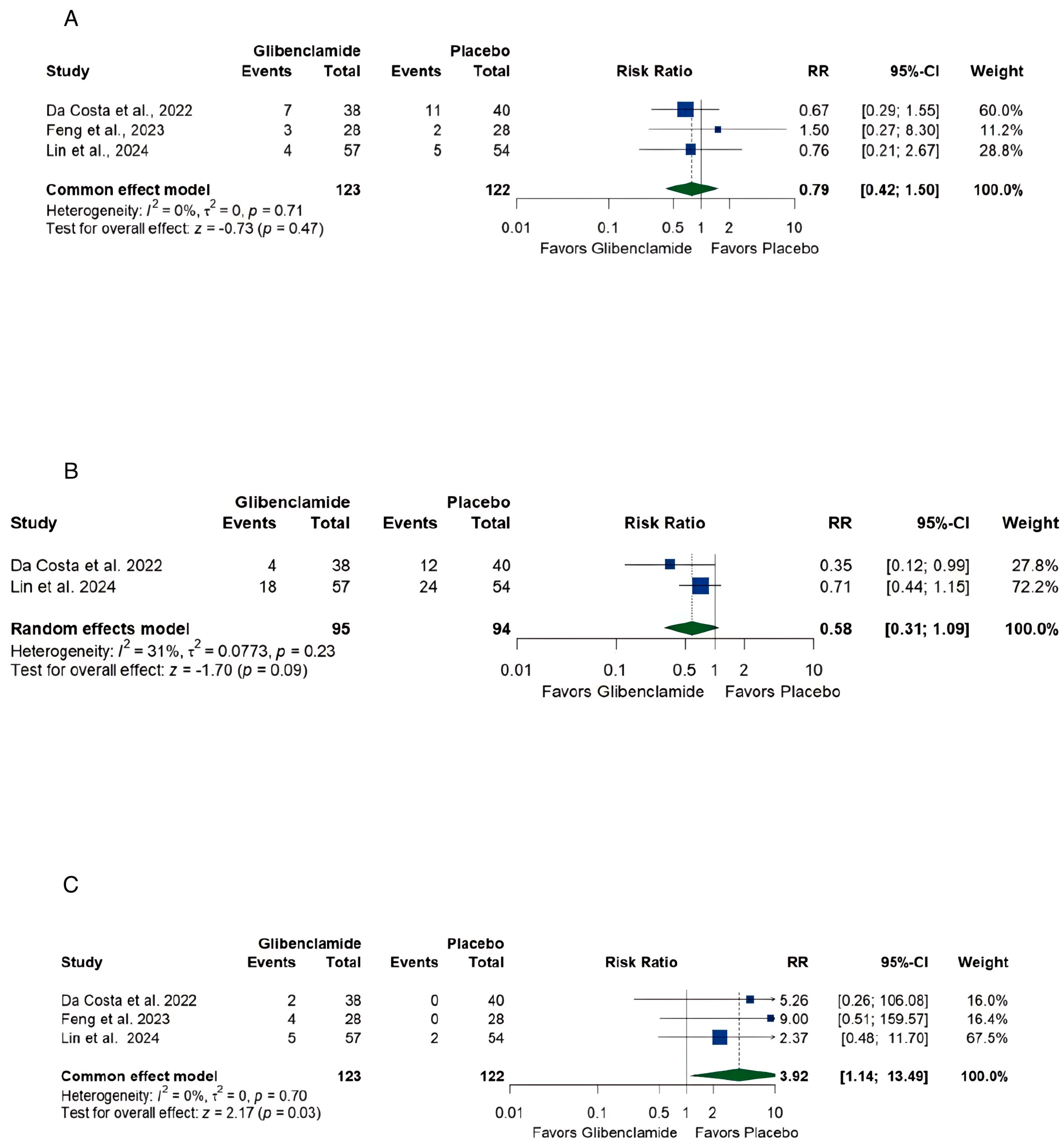
3.5. Safety
4. Discussion
4.1. Unmet Needs in aSAH Management
4.2. Limited Efficacy of Oral Glibenclamide
4.2.1. Functional Outcomes
- At discharge, the pooled mean difference in mRS [21] was [MD −0.19, (−2.05, 1.66)] with high heterogeneity, reflecting variability in study designs and patient populations.
- At 3 months, the mRS [21] pooled mean difference was [MD 0.06, (−0.60,0.71)] with no heterogeneity, indicating consistent findings across studies but no significant benefit.
4.2.2. Neurological Recovery and Independence
4.3. Safety Profile
4.4. Mechanism of Action
4.5. Translation of Preclinical Findings to Clinical Practice
4.6. Timing of Intervention
4.7. Limitations
- Complex Pathophysiology: The multifaceted nature of aSAH-induced brain injury, involving edema, inflammation, vasospasm, and apoptosis, makes it challenging to develop targeted therapies. Therapies like glibenclamide, which focus on a single pathway, may fail to address the broader spectrum of injury mechanism [12,16].
- Heterogeneity of patients: Variability in patient demographics, aneurysm location and size, and comorbidities introduces confounding factors in clinical trials. Studies show that responses to treatments can differ significantly based on these variables, making it difficult to achieve uniform outcomes [7,18,25,27,31,32].
- Translation Challenges: Differences in dosing regimens, injury models, and treatment timelines between preclinical and clinical studies often result in inconsistent outcomes. For example, glibenclamide doses used in animal models are frequently higher and initiated earlier, compared to clinical settings [12,17,18,25,27,31,32].
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aSAH | Aneurysmal subarachnoid hemorrhage |
| BMI | Body mass index |
| CI | Confidence interval |
| DCI | Delayed cerebral ischemia |
| ICP | Increased intracranial pressure |
| MD | Mean difference |
| mFS | Modified Fisher Scale [23] |
| mRS | Modified Rankin Scale [21] |
| N | Number |
| NS | Not significant |
| NSE | Neuron-specific enolase |
| p | Probability |
| PRISMA | Preferred Reporting Items for Systematic reviews and Meta-Analyses [19,20] |
| PROSPERO | Prospective Register of Systematic Reviews |
| RCT | Randomized controlled trial |
| RR | Risk ratio |
| SEBES | Subarachnoid Hemorrhage Early Brain Edema Score [22] |
| SUR1-TRPM4 | Sulfonylurea receptor 1-transient receptor potential melastatin 4 |
| WOS | Web of Science |
References
- Macdonald, R.L.; Schweizer, T.A. Spontaneous subarachnoid haemorrhage. Lancet. 2017, 389, 655–666. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, N.K.; Linn, F.H.H.; Van Der Plas, J.A.; Algra, A.; Rinkel, G.J.E. Incidence of subarachnoid haemorrhage: A systematic review with emphasis on region, age, gender and time trends. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Zacharia, B.E.; Hickman, Z.L.; Grobelny, B.T.; DeRosa, P.; Kotchetkov, I.; Ducruet, A.F.; Connolly, E.S. Epidemiology of aneurysmal subarachnoid hemorrhage. Neurosurg. Clin. N. Am. 2010, 21, 221–233. [Google Scholar] [CrossRef]
- Connolly, E.S.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, B.L.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.C.; et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef] [PubMed]
- Lantigua, H.; Ortega-Gutierrez, S.; Schmidt, J.M.; Lee, K.; Badjatia, N.; Agarwal, S.; Claassen, J.; Connolly, E.S.; Mayer, S.A. Subarachnoid hemorrhage: Who dies, and why? Crit. Care. 2015, 19, 309. [Google Scholar] [CrossRef]
- Kreiter, K.T.; Copeland, D.; Bernardini, G.L.; Bates, J.E.; Peery, S.; Claassen, J.; Du, E.; Stern, Y.; Connolly, E.S.; Mayer, S.A. Predictors of cognitive dysfunction after subarachnoid hemorrhage. Stroke 2002, 33, 200–208. [Google Scholar] [CrossRef]
- Darkwah Oppong, M.; Golubovic, J.; Hauck, E.F.; Wrede, K.H.; Sure, U.; Jabbarli, R. Decompressive craniectomy in aneurysmal subarachnoid hemorrhage: Who and when? —A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2020, 199, 106252. [Google Scholar] [CrossRef]
- Alotaibi, N.M.; Elkarim, G.A.; Samuel, N.; Ayling, O.G.S.; Guha, D.; Fallah, A.; Aldakkan, A.; Jaja, B.N.R.; Manoel, A.L.O.; Ibrahim, G.M.; et al. Effects of decompressive craniectomy on functional outcomes and death in poor-grade aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. J. Neurosurg. 2017, 127, 1315–1325. [Google Scholar] [CrossRef]
- Pickard, J.D.; Murray, G.D.; Illingworth, R.; Shaw, M.D.; Teasdale, G.M.; Foy, P.M.; Humphrey, P.M.; Lang, D.A.; Nelson, R.; Richards, P.; et al. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid haemorrhage: British aneurysm nimodipine trial. BMJ 1989, 298, 636–642. [Google Scholar] [CrossRef]
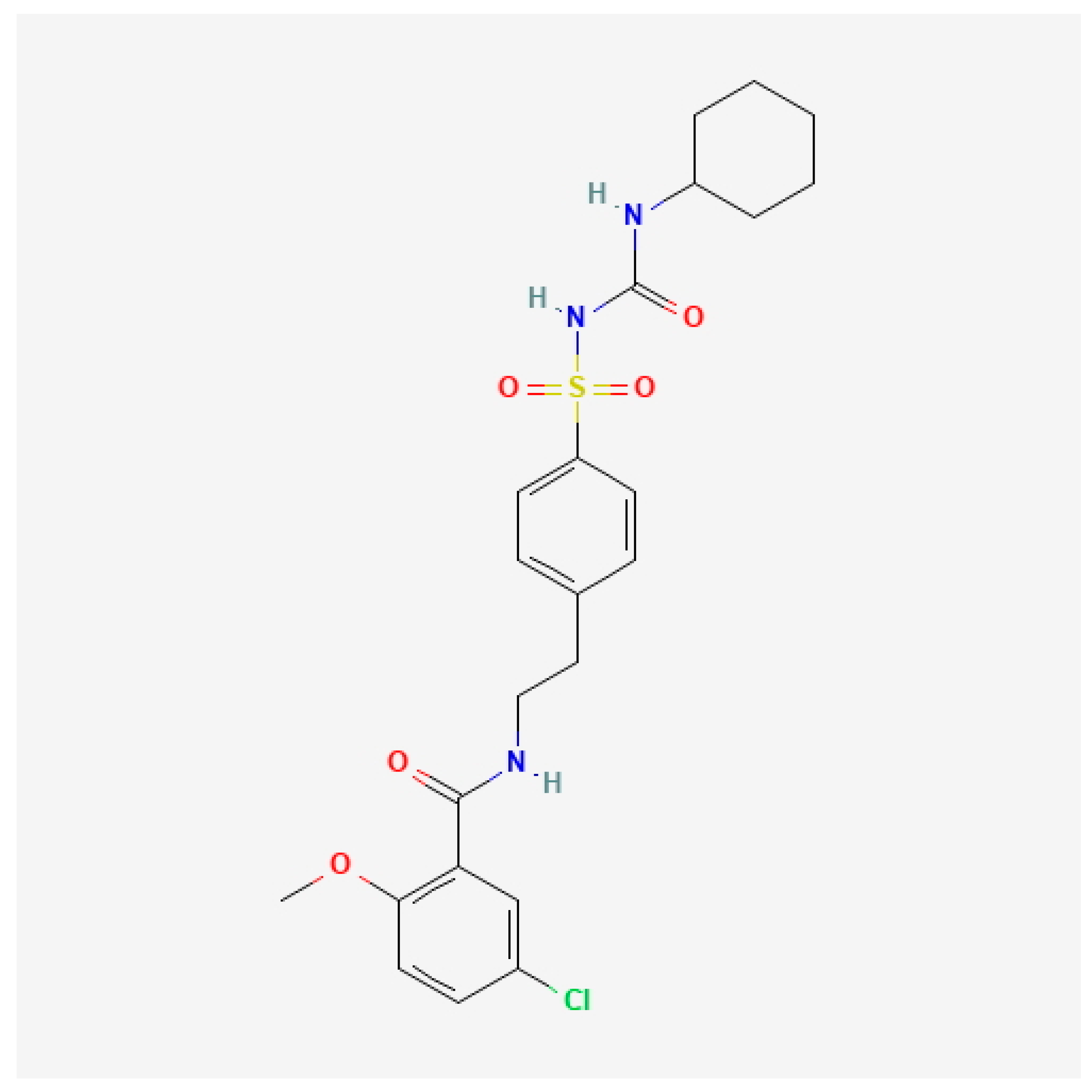
- National Center for Biotechnology Information. PubChem Compound Summary for CID 129848290, Glibenclamide Hydrochloride. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Glibenclamide-hydrochloride (accessed on 19 June 2025).
- Suresh, K.; Khandavilli, U.B.R.; Gunnama, A.; Nangia, A. Polymorphism, isostructurality and physicochemical properties of glibenclamide salts. CrystEngComm 2017, 19, 918–929. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, J.; Chen, J.; Lin, W.; Shen, H.; Fang, Y.; Yu, W. Multifaceted role of thrombin in subarachnoid hemorrhage: Focusing on cerebrospinal fluid circulation disorder. Exp. Neurol. 2025, 383, 115036. [Google Scholar] [CrossRef]
- Caffes, N.; Kurland, D.B.; Gerzanich, V.; Simard, M. Glibenclamide for the treatment of ischemic and hemorrhagic stroke. Int. J. Mol. Sci. 2015, 16, 4973–4984. [Google Scholar] [CrossRef]
- Simard, J.M.; Tsymbalyuk, N.; Tsymbalyuk, O.; Ivanova, S.; Yurovsky, V.; Gerzanich, V. Glibenclamide is superior to decompressive craniectomy in a rat model of malignant stroke. Stroke 2010, 41, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.M.; Kilbourne, M.; Tsymbalyuk, O.; Tosun, C.; Caridi, J.; Ivanova, S.; Keledjian, K.; Bochicchio, G.; Gerzanich, V. Key role of sulfonylurea receptor 1 in progressive secondary hemorrhage after brain contusion. J. Neurotrauma 2009, 26, 2257–2267. [Google Scholar] [CrossRef]
- Simard, J.M.; Geng, Z.; Kyoon Woo, S.; Ivanova, S.; Tosun, C.; Melnichenko, L.; Gerzanich, V. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2009, 29, 317–330. [Google Scholar] [CrossRef]
- Kajimoto, R.; Igarashi, T.; Moro, N.; Oshima, H.; Suma, T.; Otani, N.; Yoshino, A. Glibenclamide reduces secondary brain injury in a SAH rat model by reducing brain swelling and modulating inflammatory response. J. Neurosurg. Sci. 2023, 67, 431–438. [Google Scholar] [CrossRef] [PubMed]
- da Costa, B.B.S.; Windlin, I.C.; Koterba, E.; Yamaki, V.N.; Rabelo, N.N.; Solla, D.J.F.; Samaia da Silva Coelho, A.C.S.; Telles, J.P.M.; Teixeira, M.J.; Figueiredo, E.G. Glibenclamide in aneurysmal subarachnoid hemorrhage: A randomized controlled clinical trial. J. Neurosurg. 2022, 137, 121–128. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019; pp. 1–694. [Google Scholar] [CrossRef]
- Saver, J.L.; Chaisinanunkul, N.; Campbell, B.C.V.; Grotta, J.C.; Hill, M.D.; Khatri, P.; Landen, J.; Lansberg, M.G.; Venkatasubramanian, C.; Albers, G.W.; et al. Standardized Nomenclature for Modified Rankin Scale Global Disability Outcomes: Consensus Recommendations From Stroke Therapy Academic Industry Roundtable XI. Stroke 2021, 52, 3054–3062. [Google Scholar] [CrossRef]
- Ahn, S.H.; Savarraj, J.P.; Pervez, M.; Jones, W.; Park, J.; Jeon, S.B.; Kwon, S.U.; Chang, T.R.; Lee, K.; Kim, D.H.; et al. The Subarachnoid Hemorrhage Early Brain Edema Score predicts delayed cerebral ischemia and clinical outcomes. Neurosurgery 2018, 83, 137–145. [Google Scholar] [CrossRef]
- Frontera, J.A.; Claassen, J.; Schmidt, J.M.; Wartenberg, K.E.; Temes, R.; Connolly, E.S., Jr.; MacDonald, R.L.; Mayer, S.A. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: The modified Fisher scale. Neurosurgery 2006, 59, 21–27. [Google Scholar] [PubMed]
- Covidence. Available online: https://www.covidence.org (accessed on 19 June 2025).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 19 June 2025).
- Feng, X.; Zhang, T.; Wang, N.; Qu, X.; Qi, M.; Zhao, H.; Zhang, H.; Xu, Y. Safety and efficacy of glibenclamide on cerebral oedema following aneurysmal subarachnoid haemorrhage: A randomised, double-blind, placebo-controlled clinical trial. Stroke Vasc. Neurol. 2024, 9, 530–540. [Google Scholar] [CrossRef]
- Hunt, W.E.; Hess, R.M. Surgical risk as related to time of intervention in the repair of intracranial aneurysm. J. Neurosurg. 1968, 28, 14–20. [Google Scholar] [CrossRef]
- Lin, Q.; Zhou, D.; Ma, J.; Zhao, J.; Chen, G.; Wu, L.; Li, T.; Zhao, S.; Wen, H.; Yu, H.; et al. Efficacy and safety of early treatment with glibenclamide in patients with aneurysmal subarachnoid hemorrhage: A randomized controlled trial. Neurocrit. Care. 2024, 41, 838–839. [Google Scholar] [CrossRef]
- Drake, C. Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. J. Neurosurg. 1988, 68, 985–986. [Google Scholar]
- Falcão, L.; Lopes Gomes, P.A.; Sampaio Silva, R.A.; Ogasawara, K.; Pereira Gonzalez, J.V.; Nishizima, A.; Ohannesian, V.A.; Souza Magalhães, L.; Fontoura Solla, D.J. Assessing Glibenclamide’s efficacy on functional recovery in aneurysmal subarachnoid hemorrhage: A meta-analysis of randomized controlled trials. Clin. Neurol. Neurosurg. 2025, 252, 108847. [Google Scholar] [CrossRef]
- Costola Windlin, I.; Sisnando da Costa, B.B.; Mota Telles, J.P.; Oliveira, L.B.; Koterba, E.; Nagai Yamaki, V.; Nunes Rabelo, N.; Fontoura Solla, C.J.; Jacobsen Texeira, M.; Gadelha Figueiredo, E. The effects of glibenclamide on cognitive performance, quality of life, and emotional aspects among patients with aneurysmal subarachnoid hemorrhage: A randomized controlled trial. World Neurosurg. 2025, 193, 345–352. [Google Scholar] [CrossRef]
- Sheth, K.N.; Petersen, N.H.; Cheung, K.; Elm, J.J.; Hinson, H.E.; Molyneaux, B.J.; Beslow, L.A.; Sze, G.K.; Simard, J.M.; Kimberly, W.T. Long-term outcomes in patients aged ≤ 70 years with intravenous glyburide from the Phase II GAMES-RP Study of Large Hemispheric Infarction: An exploratory analysis. Stroke 2018, 49, 1457–1463. [Google Scholar] [CrossRef]

| Database | Search Terms | Search Field | Search Results |
|---|---|---|---|
| Pubmed | (Glibenclamide OR Glyburide OR “KATP channel blocker” OR Sulphonylurea) AND (“Subarachnoid Hemorrhage” OR “Subarachnoid Haemorrhage” OR SAH OR “Aneurysmal Subarachnoid Hemorrhage” OR “Aneurysmal Subarachnoid Haemorrhage”) | All Field | 32 |
| Cochrane | (Glibenclamide OR Glyburide OR “KATP channel blocker” OR Sulphonylurea) AND (“Subarachnoid Hemorrhage” OR “Subarachnoid Haemorrhage” OR SAH OR “Aneurysmal Subarachnoid Hemorrhage” OR “Aneurysmal Subarachnoid Haemorrhage”) | All Text | 8 |
| WOS | (Glibenclamide OR Glyburide OR “KATP channel blocker” OR Sulphonylurea) AND (“Subarachnoid Hemorrhage” OR “Subarachnoid Haemorrhage” OR SAH OR “Aneurysmal Subarachnoid Hemorrhage” OR “Aneurysmal Subarachnoid Haemorrhage”) | All Fields | 57 |
| SCOPUS | “glibenclamide OR glyburide OR “KATP channel blocker” AND “Subarachnoid Hemorrhage” OR “Subarachnoid Haemorrhage” OR SAH OR “Aneurysmal Subarachnoid Hemorrhage” OR “Aneurysmal Subarachnoid Haemorrhage” | Title, Abstract, Keywords | 60 |
| First Author/ Year | Study Design | Country | Centers | Total Participants | Control | Main Inclusion Criteria | Primary Outcome | Follow-up Duration |
|---|---|---|---|---|---|---|---|---|
| da Costa et al., 2022 [18] | Randomized, double-blind and prospective clinical trial. | 1 country (Brazil) | Single center (Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, at São Paulo) | 78 | Placebo | Inclusion: SAH confirmed radiologically, aneurysmal origin verified, age 18–70, and treatment (clipping/coiling) within 96 h | 6-month mRS score distribution | 6 months |
| Feng et al., 2024 [27] | Randomized, double-blind, placebo controlled clinical trial. | 1 country (China) | Single center (Xuanwu Hospital Capital Medical University, Beijing, China) | 56 | Placebo | Inclusion: Radiological aSAH, age ≥ 18, surgery within 72 h, Hunt–Hess grade ≥ 2 | Proportion of patients with SAH Early Brain Edema Score 0–2 at 10 days post-medication. | 3 and 6 months |
| Lin et al., 2024 [29] | Randomized, controlled, open-label, blinded- endpoint clinical trial. | 1 country (China) | Multicenter (Beijing Tiantan Hospital) | 111 | Neither glibenclamide tablets nor placebo | Inclusion: Radiological aSAH within 48 h, age 18–74 (older age due to lower hypoglycemia tolerance) | Difference in serum NSE and S100B levels with or without glibenclamide | 3 months |
| Data | Number of Patients in Each Group | Age (Years) Mean (SD) | Male n (%) | BMI (kg/m²) Mean (SD) | Related Grading Median (IQR) | Medical History n (%) | Surgery n (%) | Time from Onset to Enrolment (h), Median (IQR) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hunt-Hess grade [28] | WFNS grade [30] | mFS [23] | SEBES [22] | mRS [21] | Hypertension | Diabetes | Coiling | Clipping | ||||||||||||||||||||
| G | P | G | P | G | P | G | P | G | P | G | P | G | P | G | P | G | P | G | P | G | P | G | P | G | P | G | P | |
| da Costa et al., 2022 [18] | 38 | 40 | 53.6 (11.6) | 52.7 (11.3) | 6 (15.8) | 13 (32.5) | NA | NA | 3 (2–4) | 3 (2–4) | 3 (1–4) | 2.5 (1–4) | 3 (3–4) | 3 (3–4) | NA | NA | NA | NA | NA | NA | NA | NA | 18 (47.4) | 10 (25.0) | 20 (52.6) | 30 (75.0) | 60 (24–96) | 72 (48–96) |
| Feng et al., 2024 [27] | 28 | 28 | 61.8 (11.6) | 59.1 (12.6) | 12 (42.9) | 17 (60.7) | 24.3 (3.6) | 25.6 (4.7) | 3 (3–4) | 3 (3–4) | 5 (4–5) | 4 (4–5) | 4 (3–4) | 4 (3–4) | 4 (3–4) | 4 (2–4) | 5 (4.3–5) | 4 (2–5) | 23 (82.1) | 18 (64.3) | 6 (21.4) | 2 (7.1) | NA | NA | NA | NA | NA | NA |
| Lin et al., 2024 [29] | 57 | 54 | 57 (11.4) | 55.3 (11.04) | 28 (49) | 25 (46) | 24.5 (2.5) | 24.3 (3.02) | 3 (3–4) | 3 (2–4) | 4 (2–4) | 4 (2–4) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 2 (2–3) | NA | NA | 35 (61) | 27 (50) | 6 (11) | 4 (7) | 3 (5) | 2 (4) | 54 (95) | 52 (96) | 24 (24, 28) | 26 (20, 40.75) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlBarakat, M.M.; Altawalbeh, R.B.; Hamam, K.M.; Lashin, A.A.; Wadaa-Allah, A.; Alkrarha, A.J.; Abuelazm, M.; Brašić, J.R. The Safety and Efficacy of Glibenclamide in Managing Cerebral Edema After Aneurysmal Subarachnoid Hemorrhage: A Systematic Review and Meta-Analysis. Brain Sci. 2025, 15, 677. https://doi.org/10.3390/brainsci15070677
AlBarakat MM, Altawalbeh RB, Hamam KM, Lashin AA, Wadaa-Allah A, Alkrarha AJ, Abuelazm M, Brašić JR. The Safety and Efficacy of Glibenclamide in Managing Cerebral Edema After Aneurysmal Subarachnoid Hemorrhage: A Systematic Review and Meta-Analysis. Brain Sciences. 2025; 15(7):677. https://doi.org/10.3390/brainsci15070677
Chicago/Turabian StyleAlBarakat, Majd M., Rana B. Altawalbeh, Khaled Mohamed Hamam, Ahmed A. Lashin, Ahmed Wadaa-Allah, Ayah J. Alkrarha, Mohamed Abuelazm, and James Robert Brašić. 2025. "The Safety and Efficacy of Glibenclamide in Managing Cerebral Edema After Aneurysmal Subarachnoid Hemorrhage: A Systematic Review and Meta-Analysis" Brain Sciences 15, no. 7: 677. https://doi.org/10.3390/brainsci15070677
APA StyleAlBarakat, M. M., Altawalbeh, R. B., Hamam, K. M., Lashin, A. A., Wadaa-Allah, A., Alkrarha, A. J., Abuelazm, M., & Brašić, J. R. (2025). The Safety and Efficacy of Glibenclamide in Managing Cerebral Edema After Aneurysmal Subarachnoid Hemorrhage: A Systematic Review and Meta-Analysis. Brain Sciences, 15(7), 677. https://doi.org/10.3390/brainsci15070677









