Sensitivity to Instruction Strategies in Motor Learning Is Predicted by Anterior–Posterior TMS Motor Thresholds
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
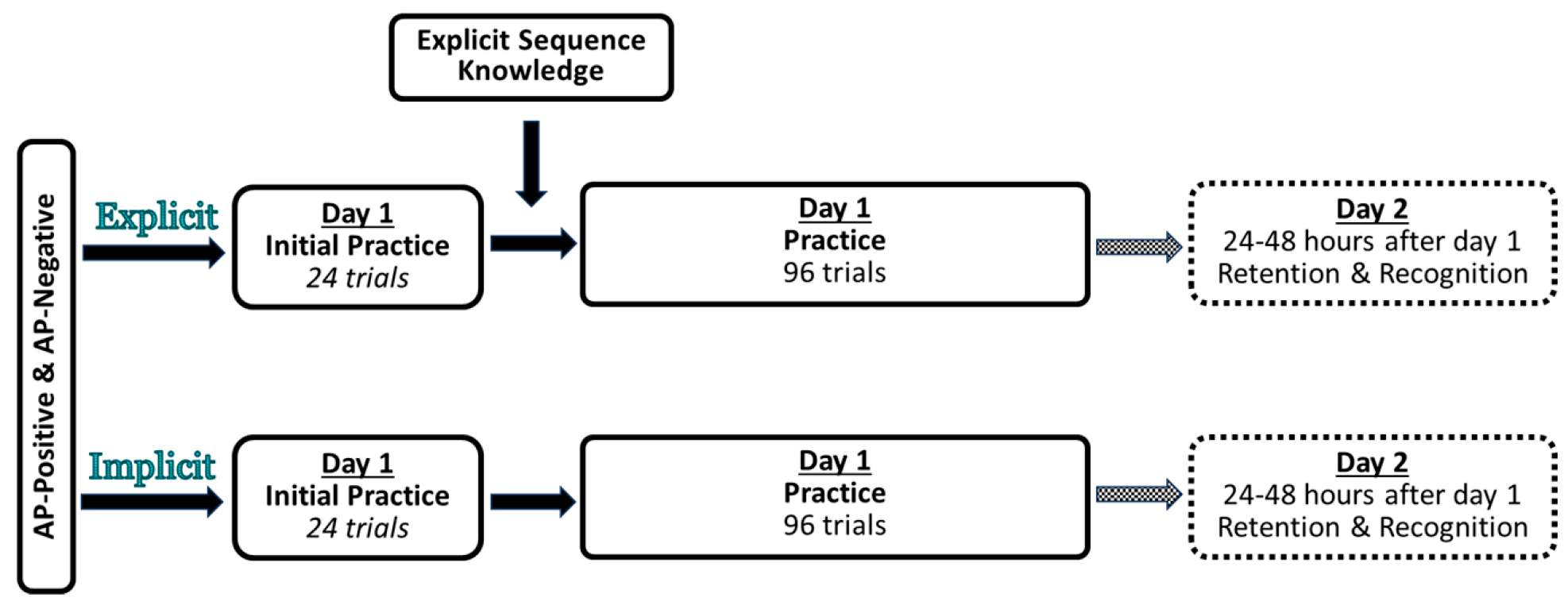
2.2. Experimental Design and Procedure
2.3. Continuous Tracking Task
2.4. Delayed Retention Test
2.5. Recognition Test
2.6. Controllable Pulse Parameter Transcranial Magnetic Stimulation (cTMS)
2.7. Data Analysis
3. Results
3.1. General Improvements in Motor Ability
3.1.1. Overall Performance
3.1.2. Online Learning
3.1.3. Offline Consolidation
3.2. Sequence Specific Learning
3.2.1. Overall Performance
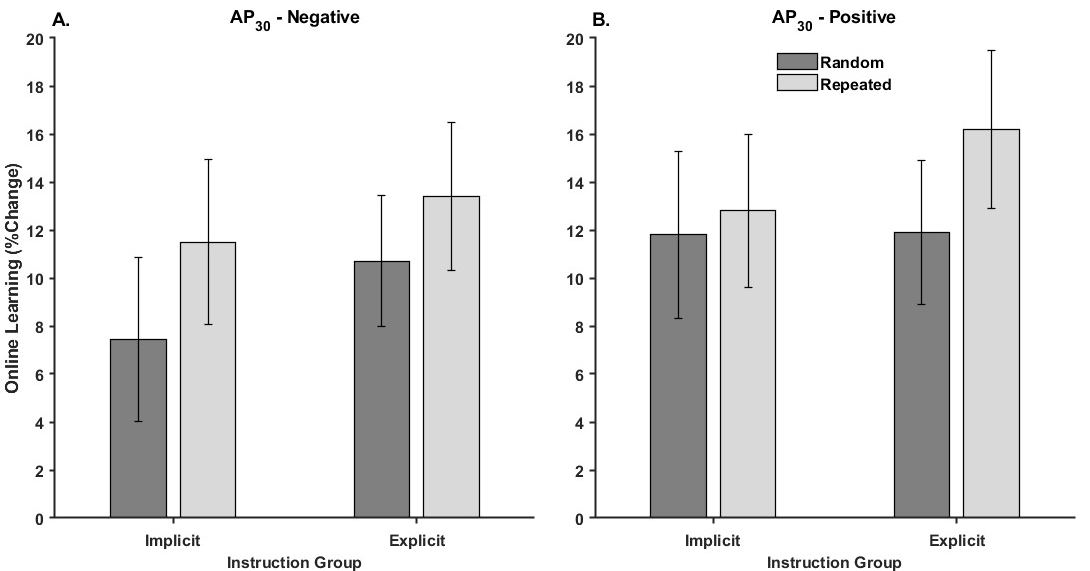
3.2.2. Online Learning
3.2.3. Offline Consolidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Squire, L.R. Memory and Brain; pp. xii, 315 p.-xii, 315 p; Oxford University Press: New York, NY, USA, 1987. [Google Scholar]
- Willingham, D.B. A neuropsychological theory of motor skill learning. Psychol. Rev. 1998, 105, 558–584. [Google Scholar] [CrossRef] [PubMed]
- Willingham, D.B.; Salidis, J.; Gabrieli, J.D. Direct comparison of neural systems mediating conscious and unconscious skill learning. J. Neurophysiol. 2002, 88, 1451–1460. [Google Scholar] [CrossRef]
- Squire, L.R. Memory systems of the brain: A brief history and current perspective. Neurobiol. Learn. Mem. 2004, 82, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Squire, L.R.; Dede, A.J. Conscious and unconscious memory systems. Cold Spring Harb. Perspect. Biol. 2015, 7, a021667. [Google Scholar] [CrossRef] [PubMed]
- Vidoni, E.D.; Boyd, L.A. Achieving enlightenment: What do we know about the implicit learning system and its interaction with explicit knowledge? J. Neurol. Phys. Ther. 2007, 31, 145–154. [Google Scholar] [CrossRef]
- Scoville, W.B.; Milner, B. Loss of recent memory after bilateral hippocampal lesions. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 103–113. [Google Scholar] [CrossRef]
- Poldrack, R.A.; Clark, J.; Pare-Blagoev, E.J.; Shohamy, D.; Creso Moyano, J.; Myers, C.; Gluck, M.A. Interactive memory systems in the human brain. Nature 2001, 414, 546–550. [Google Scholar] [CrossRef]
- Poldrack, R.A.; Packard, M.G. Competition among multiple memory systems: Converging evidence from animal and human brain studies. Neuropsychologia 2003, 41, 245–251. [Google Scholar] [CrossRef]
- Krakauer, J.W.; Hadjiosif, A.M.; Xu, J.; Wong, A.L.; Haith, A.M. Motor Learning. Compr. Physiol. 2019, 9, 613–663. [Google Scholar] [CrossRef]
- Green, T.D.; Flowers, J.H. Implicit versus explicit learning processes in a probabilistic, continuous fine-motor catching task. J. Mot. Behav. 1991, 23, 293–300. [Google Scholar] [CrossRef]
- Reber, A.S. Implicit learning of synthetic languages: The role of instructional set. J. Exp. Psychol. Human. Learn. Mem. 1976, 2, 88–94. [Google Scholar] [CrossRef]
- Wulf, G.; Weigelt, C. Instructions about physical principles in learning a complex motor skill: To tell or not to tell. Res. Q. Exerc. Sport 1997, 68, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Boyd, L.A.; Winstein, C.J. Impact of explicit information on implicit motor-sequence learning following middle cerebral artery stroke. Phys. Ther. 2003, 83, 976–989. [Google Scholar] [CrossRef]
- Boyd, L.A.; Winstein, C.J. Providing explicit information disrupts implicit motor learning after basal ganglia stroke. Learn. Mem. 2004, 11, 388–396. [Google Scholar] [CrossRef]
- Boyd, L.; Winstein, C. Explicit information interferes with implicit motor learning of both continuous and discrete movement tasks after stroke. J. Neurol. Phys. Ther. 2006, 30, 46–57; discussion 58–59. [Google Scholar] [CrossRef] [PubMed]
- Bizzi, E.; Ajemian, R. From motor planning to execution: A sensorimotor loop perspective. J. Neurophysiol. 2020, 124, 1815–1823. [Google Scholar] [CrossRef]
- Doyon, J.; Benali, H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr. Opin. Neurobiol. 2005, 15, 161–167. [Google Scholar] [CrossRef]
- Debaere, F.; Wenderoth, N.; Sunaert, S.; Van Hecke, P.; Swinnen, S.P. Changes in brain activation during the acquisition of a new bimanual coodination task. Neuropsychologia 2004, 42, 855–867. [Google Scholar] [CrossRef]
- Chen, R. Studies of human motor physiology with transcranial magnetic stimulation. Muscle Nerve Suppl. 2000, 9, S26–S32. [Google Scholar] [CrossRef]
- Siebner, H.R.; Funke, K.; Aberra, A.S.; Antal, A.; Bestmann, S.; Chen, R.; Classen, J.; Davare, M.; Di Lazzaro, V.; Fox, P.T.; et al. Transcranial magnetic stimulation of the brain: What is stimulated?—A consensus and critical position paper. Clin. Neurophysiol. 2022, 140, 59–97. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Profice, P.; Saturno, E.; Pilato, F.; Insola, A.; Mazzone, P.; Tonali, P.; Rothwell, J.C. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr. Clin. Neurophysiol/Electromyogr. Mot. Control 1998, 109, 397–401. [Google Scholar] [CrossRef] [PubMed]
- D’Ostilio, K.; Goetz, S.M.; Hannah, R.; Ciocca, M.; Chieffo, R.; Chen, J.A.; Peterchev, A.V.; Rothwell, J.C. Effect of coil orientation on strength-duration time constant and I-wave activation with controllable pulse parameter transcranial magnetic stimulation. Clin. Neurophysiol. 2016, 127, 675–683. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Saturno, E.; Pilato, F.; Insola, A.; Mazzone, P.; Profice, P.; Tonali, P.; Rothwell, J.C. The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Exp. Brain Res. 2001, 138, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Ziemann, U. The contribution of transcranial magnetic stimulation in the functional evaluation of microcircuits in human motor cortex. Front. Neural Circuits 2013, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M.; Murase, N.; Hasan, A.; Balaratnam, M.; Rothwell, J.C. The role of interneuron networks in driving human motor cortical plasticity. Cereb. Cortex 2013, 23, 1593–1605. [Google Scholar] [CrossRef]
- Guidali, G.; Zazio, A.; Lucarelli, D.; Marcantoni, E.; Stango, A.; Barchiesi, G.; Bortoletto, M. Effects of transcranial magnetic stimulation (TMS) current direction and pulse waveform on cortico-cortical connectivity: A registered report TMS-EEG study. Eur. J. Neurosci. 2023, 58, 3785–3809. [Google Scholar] [CrossRef]
- Lucarelli, D.; Guidali, G.; Sulcova, D.; Zazio, A.; Bonfiglio, N.S.; Stango, A.; Barchiesi, G.; Bortoletto, M. Stimulation Parameters Recruit Distinct Cortico-Cortical Pathways: Insights from Microstate Analysis on TMS-Evoked Potentials. Brain Topogr. 2025, 38, 39. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Profice, P.; Ranieri, F.; Capone, F.; Dileone, M.; Oliviero, A.; Pilato, F. I-wave origin and modulation. Brain Stimul. 2012, 5, 512–525. [Google Scholar] [CrossRef]
- Volz, L.J.; Hamada, M.; Rothwell, J.C.; Grefkes, C. What Makes the Muscle Twitch: Motor System Connectivity and TMS-Induced Activity. Cereb. Cortex 2014, 25, 2346–2353. [Google Scholar] [CrossRef]
- Groppa, S.; Schlaak, B.H.; Munchau, A.; Werner-Petroll, N.; Dunnweber, J.; Baumer, T.; van Nuenen, B.F.; Siebner, H.R. The human dorsal premotor cortex facilitates the excitability of ipsilateral primary motor cortex via a short latency cortico-cortical route. Hum. Brain Mapp. 2012, 33, 419–430. [Google Scholar] [CrossRef]
- Civardi, C.; Cantello, R.; Asselman, P.; Rothwell, J.C. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. NeuroImage 2001, 14, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Peterchev, A.V.; D’Ostilio, K.; Rothwell, J.C.; Murphy, D.L. Controllable pulse parameter transcranial magnetic stimulator with enhanced circuit topology and pulse shaping. J. Neural Eng. 2014, 11, 056023. [Google Scholar] [CrossRef]
- Hannah, R.; Rothwell, J.C. Pulse Duration as Well as Current Direction Determines the Specificity of Transcranial Magnetic Stimulation of Motor Cortex during Contraction. Brain Stimul. 2017, 10, 106–115. [Google Scholar] [CrossRef]
- Wulf, G.; Schmidt, R.A. Variability of practice and implicit motor learning. J. Exp. Psychol.-Learn. Mem. Cogn. 1997, 23, 987–1006. [Google Scholar] [CrossRef]
- Chambaron, S.; Ginhac, D.; Ferrel-Chapus, C.; Perruchet, P. Implicit learning of a repeated segment in continuous tracking: A reappraisal. Q. J. Exp. Psychol. 2006, 59, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Ewolds, H.E.; Bröker, L.; de Oliveira, R.F.; Raab, M.; Künzell, S. Implicit and Explicit Knowledge Both Improve Dual Task Performance in a Continuous Pursuit Tracking Task. Front. Psychol. 2017, 8, 2241. [Google Scholar] [CrossRef]
- Zhu, F.F.; Poolton, J.M.; Maxwell, J.P.; Fan, J.K.; Leung, G.K.; Masters, R.S. Refining the continuous tracking paradigm to investigate implicit motor learning. Exp. Psychol. 2014, 61, 196–204. [Google Scholar] [CrossRef][Green Version]
- Sommer, M.; Ciocca, M.; Chieffo, R.; Hammond, P.; Neef, A.; Paulus, W.; Rothwell, J.C.; Hannah, R. TMS of primary motor cortex with a biphasic pulse activates two independent sets of excitable neurones. Brain Stimul. 2018, 11, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Ugawa, Y.; Terao, Y.; Hanajima, R.; Furubayashi, T.; Kanazawa, I. Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp. Brain Res. 1997, 113, 24–32. [Google Scholar] [CrossRef]
- Silbert, B.I.; Patterson, H.I.; Pevcic, D.D.; Windnagel, K.A.; Thickbroom, G.W. A comparison of relative-frequency and threshold-hunting methods to determine stimulus intensity in transcranial magnetic stimulation. Clin. Neurophysiol. 2013, 124, 708–712. [Google Scholar] [CrossRef]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2019. Available online: https://cran.r-project.org/web/packages/rstatix/index.html (accessed on 1 January 2025).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; D’Agostino, L. Welcome to the {tidyverse}. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Kassambara, A. Ggpubr: ‘Ggplot2’ Based Publication Ready Plots. 2020. Available online: https://cran.r-project.org/web/packages/ggpubr/ggpubr.pdf (accessed on 1 January 2025).
- Reber, P.J.; Squire, L.R. Encapsulation of implicit and explicit memory in sequence learning. J. Cogn. Neurosci. 1998, 10, 248–263. [Google Scholar] [CrossRef]
- Aberra, A.S.; Wang, B.; Grill, W.M.; Peterchev, A.V. Simulation of transcranial magnetic stimulation in head model with morphologically-realistic cortical neurons. Brain Stimul. 2020, 13, 175–189. [Google Scholar] [CrossRef]
- Mirdamadi, J.L.; Suzuki, L.Y.; Meehan, S.K. Attention modulates specific motor cortical circuits recruited by transcranial magnetic stimulation. Neuroscience 2017, 359, 151–158. [Google Scholar] [CrossRef]
- Gaser, C.; Schlaug, G. Brain structures differ between musicians and non-musicians. J. Neurosci. 2003, 23, 9240–9245. [Google Scholar] [CrossRef] [PubMed]
- Shenker, J.J.; Steele, C.J.; Chakravarty, M.M.; Zatorre, R.J.; Penhune, V.B. Early musical training shapes cortico-cerebellar structural covariation. Brain Struct. Funct. 2022, 227, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, S.L.; Nagy, Z.; Skare, S.; Forsman, L.; Forssberg, H.; Ullén, F. Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci. 2005, 8, 1148–1150. [Google Scholar] [CrossRef]
- Tan, X.Y.; Pi, Y.L.; Wang, J.; Li, X.P.; Zhang, L.L.; Dai, W.; Zhu, H.; Ni, Z.; Zhang, J.; Wu, Y. Morphological and Functional Differences between Athletes and Novices in Cortical Neuronal Networks. Front. Hum. Neurosci. 2016, 10, 660. [Google Scholar] [CrossRef]
- Zhang, J.P.; Zhu, P.; Cai, Z.L.; Xing, X.X.; Wu, J.J.; Zheng, M.X.; Hua, X.Y.; Gong, B.M.; Xu, J.G. Sports promote brain evolution: A resting-state fMRI study of volleyball athlete. Front. Sports Act. Living 2024, 6, 1393988. [Google Scholar] [CrossRef]
- Mirdamadi, J.L.; Meehan, S.K. Specific sensorimotor interneuron circuits are sensitive to cerebellar-attention interactions. Front. Human. Neurosci. 2022, 16, 920526. [Google Scholar] [CrossRef]
- Pavlides, C.; Miyashita, E.; Asanuma, H. Projection from the sensory to the motor cortex is important in learning motor skills in the monkey. J. Neurophysiol. 1993, 70, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, H.; Pavlides, C. Neurobiological basis of motor learning in mammals. Neuroreport 1997, 8, i–vi. [Google Scholar]
- Vidoni, E.D.; Acerra, N.E.; Dao, E.; Meehan, S.K.; Boyd, L.A. Role of the primary somatosensory cortex in motor learning: An rTMS study. Neurobiol. Learn. Mem. 2010, 93, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, J.E.; Cheron, G. Non-cephalic reference recording of early somatosensory potentials to finger stimulation in adult or aging normal man: Differentiation of widespread N18 and contralateral N20 from the prerolandic P22 and N30 components. Electroencephalogr. Clin. Neurophysiol. 1981, 52, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, D.A.; Block, H.J.; Celnik, P.A. Cerebellar-M1 Connectivity Changes Associated with Motor Learning Are Somatotopic Specific. J. Neurosci. 2017, 37, 2377–2386. [Google Scholar] [CrossRef]
- Voller, B.; St Clair Gibson, A.; Dambrosia, J.; Pirio Richardson, S.; Lomarev, M.; Dang, N.; Hallett, M. Short-latency afferent inhibition during selective finger movement. Exp. Brain Res. 2006, 169, 226–231. [Google Scholar] [CrossRef][Green Version]
- Asmussen, M.J.; Zapallow, C.M.; Jacobs, M.F.; Lee, K.G.H.; Tsang, P.; Nelson, A.J. Modulation of Short-Latency Afferent Inhibition Depends on Digit and Task-Relevance. PLoS ONE 2014, 9, e104807. [Google Scholar] [CrossRef]
- Suzuki, L.Y.; Meehan, S.K. Attention focus modulates afferent input to motor cortex during skilled action. Hum. Mov. Sci. 2020, 74, 102716. [Google Scholar] [CrossRef]
- Beck, S.; Hallett, M. Surround inhibition in the motor system. Exp. Brain Res. 2011, 210, 165–172. [Google Scholar] [CrossRef]
- Seki, K.; Fetz, E.E. Gating of sensory input at spinal and cortical levels during preparation and execution of voluntary movement. J. Neurosci. 2012, 32, 890–902. [Google Scholar] [CrossRef]
- Bailey, A.Z.; Asmussen, M.J.; Nelson, A.J. Short-latency afferent inhibition determined by the sensory afferent volley. J. Neurophysiol. 2016, 116, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Staines, W.R.; Graham, S.J.; Black, S.E.; McIlroy, W.E. Task-relevant modulation of contralateral and ipsilateral primary somatosensory cortex and the role of a prefrontal-cortical sensory gating system. NeuroImage 2002, 15, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.D.; Khan, M.E.R.; Graham, K.R.; Staines, W.R.; Meehan, S.K. Persistent adaptations in sensorimotor interneuron circuits in the motor cortex with a history of sport-related concussion. Exp. Brain Res. 2024, 243, 5. [Google Scholar] [CrossRef] [PubMed]




| Group 1 | N | Sex | Age | AP30 TMS Threshold | PA120 TMS Threshold |
|---|---|---|---|---|---|
| AP30-positive | |||||
| Implicit | 18 | 6 M, 12 F | 22.3 ± 4.9 | 82.6 ± 11.0 | - |
| Explicit | 18 | 8 M, 10 F | 24.8 ± 4.9 | 80.3 ± 10.3 | - |
| AP30-negative | |||||
| Implicit | 18 | 8 M, 10 F | 22.6 ± 4.4 | - | 31.0 ± 10.0 |
| Explicit | 18 | 10 M, 8 F | 23.0 ± 3.7 | - | 33.2 ± 5.1 |
| Effect | dfn | dfd | F | p | p < 0.05 | ηp2 |
|---|---|---|---|---|---|---|
| Threshold response | 1 | 68 | 0.97 | 0.33 | 0.01 | |
| Instruction | 1 | 68 | 0.48 | 0.49 | 0.01 | |
| Waveform | 1 | 68 | 8.40 | 0.01 | * | 0.11 |
| Threshold response × Instruction | 1 | 68 | 6.84 | 0.01 | * | 0.09 |
| Threshold response × Waveform | 1 | 68 | 0.47 | 0.49 | 0.01 | |
| Instruction × Waveform | 1 | 68 | 0.55 | 0.46 | 0.01 | |
| Threshold response × Instruction × Waveform | 1 | 68 | 4.24 | 0.04 | * | 0.06 |
| Effect | dfn | dfd | F | p | p < 0.05 | ηp2 |
|---|---|---|---|---|---|---|
| Threshold response | 1 | 68 | 0.60 | 0.44 | 0.01 | |
| Instruction | 1 | 68 | 0.48 | 0.49 | 0.01 | |
| Waveform | 1 | 68 | 13.47 | <0.001 | * | 0.17 |
| Threshold response × Instruction | 1 | 68 | 0.02 | 0.89 | <0.001 | |
| Threshold response × Waveform | 1 | 68 | 0.21 | 0.65 | 0.003 | |
| Instruction × Waveform | 1 | 68 | 0.39 | 0.54 | <0.001 | |
| Threshold response × Instruction × Waveform | 1 | 68 | 1.99 | 0.16 | 0.043 |
| Effect | dfn | dfd | F | p | p < 0.05 | ηp2 |
|---|---|---|---|---|---|---|
| Threshold response | 1 | 68 | 0.05 | 0.82 | 0.001 | |
| Instruction | 1 | 68 | 2.06 | 0.16 | 0.029 | |
| Waveform | 1 | 68 | 0.39 | 0.54 | 0.006 | |
| Threshold response × Instruction | 1 | 68 | 9.05 | 0.004 | * | 0.12 |
| Threshold response × Waveform | 1 | 68 | <0.001 | 0.10 | <0.001 | |
| Instruction × Waveform | 1 | 68 | 0.004 | 0.95 | <0.001 | |
| Threshold response × Instruction × Waveform | 1 | 68 | 0.41 | 0.53 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrier, M.L.; Graham, K.R.; Vander Vaart, J.E.; Staines, W.R.; Meehan, S.K. Sensitivity to Instruction Strategies in Motor Learning Is Predicted by Anterior–Posterior TMS Motor Thresholds. Brain Sci. 2025, 15, 645. https://doi.org/10.3390/brainsci15060645
Perrier ML, Graham KR, Vander Vaart JE, Staines WR, Meehan SK. Sensitivity to Instruction Strategies in Motor Learning Is Predicted by Anterior–Posterior TMS Motor Thresholds. Brain Sciences. 2025; 15(6):645. https://doi.org/10.3390/brainsci15060645
Chicago/Turabian StylePerrier, Michael L., Kylee R. Graham, Jessica E. Vander Vaart, W. Richard Staines, and Sean K. Meehan. 2025. "Sensitivity to Instruction Strategies in Motor Learning Is Predicted by Anterior–Posterior TMS Motor Thresholds" Brain Sciences 15, no. 6: 645. https://doi.org/10.3390/brainsci15060645
APA StylePerrier, M. L., Graham, K. R., Vander Vaart, J. E., Staines, W. R., & Meehan, S. K. (2025). Sensitivity to Instruction Strategies in Motor Learning Is Predicted by Anterior–Posterior TMS Motor Thresholds. Brain Sciences, 15(6), 645. https://doi.org/10.3390/brainsci15060645






