Self, Me, or I? Unravelling the Triumvirate of Selfhood in Pathological Consciousness
Abstract
1. Introduction
- (i)
- Self-consciousness appears to be generally more resilient to brain damage than specialized cognitive functions [25]. For example, consider cases where self-consciousness was preserved despite (a) severe hydrocephaly, where massive ventricular enlargement left only a thin cortical mantle [26], (b) extensive brain damage, including the destruction of nearly one-third of the brain, encompassing the insula, anterior cingulate cortex, and medial prefrontal cortex [27], (c) widespread neural damage caused by herpes simplex encephalitis [28,29], (d) hemispherectomy, a radical surgical procedure removing an entire brain hemisphere, leaving the patient with the remaining hemisphere intact and functioning [30], and (e) ‘split-brain’ surgery [31,32,33].
- (ii)
- In many cases, self-consciousness plays a critical role in enabling cognitive resilience and facilitating cognitive function recovery from impairments [25]. It appears that rebooting self-consciousness is often the first critical step in cognitive rehabilitation, preceding and enabling the recovery (along the regaining autonomy) of more specialized functions [7]. These observations are further supported by a condition called anosognosia, where individuals’ lack of self-awareness of their own deficits is negatively correlated with resilience [34].
- (iii)
- Even in severe memory disorders, such as amnesia or Alzheimer’s disease, patients retain aspects of self-knowledge [23]. For example, despite losing access to their recent autobiographical memories, such patients can still describe aspects of their identity, such as their appearance, personality, and social relations, that are particularly important to their self-concepts [28,35,36,37,38].
- (iv)
- Additionally, differences in self-perception influence how individuals interpret ambiguous situations, potentially contributing to the onset and persistence of emotional symptoms [39]. Furthermore, self-esteem (a component of self-concept) has a formative and sustaining function in individuals’ mental health [40,41].
- (v)
- Moreover, self-consciousness alterations predominate the patient’s phenomenological experiences and may be either long-lasting or permanent [42]. See for example Kean’s own description [43] (p. 1034): “The clinical symptoms come and go, but this nothingness of the self is permanently there […] By nothingness, I mean a sense of emptiness, a painful void of existence that only I can feel. My thoughts, my emotions, and my actions, none of them belong to me anymore. This omnipotent and omnipresent emptiness has taken control of everything. I am an automaton, but nothing is working inside me.”
- (vi)
- Finally, self-consciousness is foundational to an individual’s ability to have preferences regarding how their life goes and make meaningful choices about it [44].
2. Self-Consciousness in Sickness: A Methodological Remark
- (i)
- Most research on self-disorders has been limited to schizophrenia spectrum disorders [52,53,54] (just to mention a few). However, alterations in self-consciousness have also been observed in other conditions, for example, autism [55], cognitive disorders [56], vestibular disorders [57], and internalizing disorders (descriptive label uniting depression and anxiety disorders; see [58]).
- (ii)
- Many studies treat self-consciousness as a singular, unitary phenomenon or equate it with only one of its aspects, such as the ‘minimal self’ or ‘narrative self’ [59], the ‘core self’ [28], the ‘pre-reflective self’ [60], or the ‘bodily-self’ [61]. However, using an oversimplified unitary or one-component conceptualization of self-consciousness makes it difficult to adequately describe/explain the variety of phenomenological manifestations within and between different neuropsychopathologies. A more productive approach conceptualizes self-consciousness as a dynamic, multi-component phenomenon, where self is a complex pattern emerging from the dynamic interactions of characteristic aspects, features, qualities, and components—none of which, on their own, are entirely sufficient or specific to define the self; only jointly do they constitute it [62]. Such a multi-component conceptualization of self-consciousness is more appropriate for capturing the rich multitude of phenomenological manifestations within and between different neuropsychopathologies. Additionally, it can achieve the following:
- Explain cases where a person is aware of their unfolding experience yet lacks a sense of ego.
- Differentiate between the experiencer (‘I’) and the object of awareness (‘Me’).
- Capture the internal dialogue between the ‘I’ and ‘Me’.
- Distinguish between the experience of ‘pure awareness’, ‘minimal phenomenal experience’, and ‘content-free’ awareness (along different features that characterize such states).
- Provide insight into loss-of-self experiences, hyper-reflexivity, or an enhanced sense of self.
- Recognize the phenomenological complexity of patients’ subjective realities.
However, this pattern-based approach carries the risk of incorporating an open-ended list of aspects, features, qualities, and components which may arise ad hoc and ultimately diminish explanatory effectiveness. To prevent such vagueness, we propose that Selfhood can be meaningfully understood through a limited set of higher-order aspects that structure its complexity. Specifically, we identify three integrative aspects—phenomenal first-person agency, embodiment, and reflection/narration—each encompassing a range of more granular features. This structured, multi-component approach (as outlined in the following section) provides a balance between conceptual richness and analytical clarity.
3. Experiential Selfhood: The Selfhood Triumvirate Model
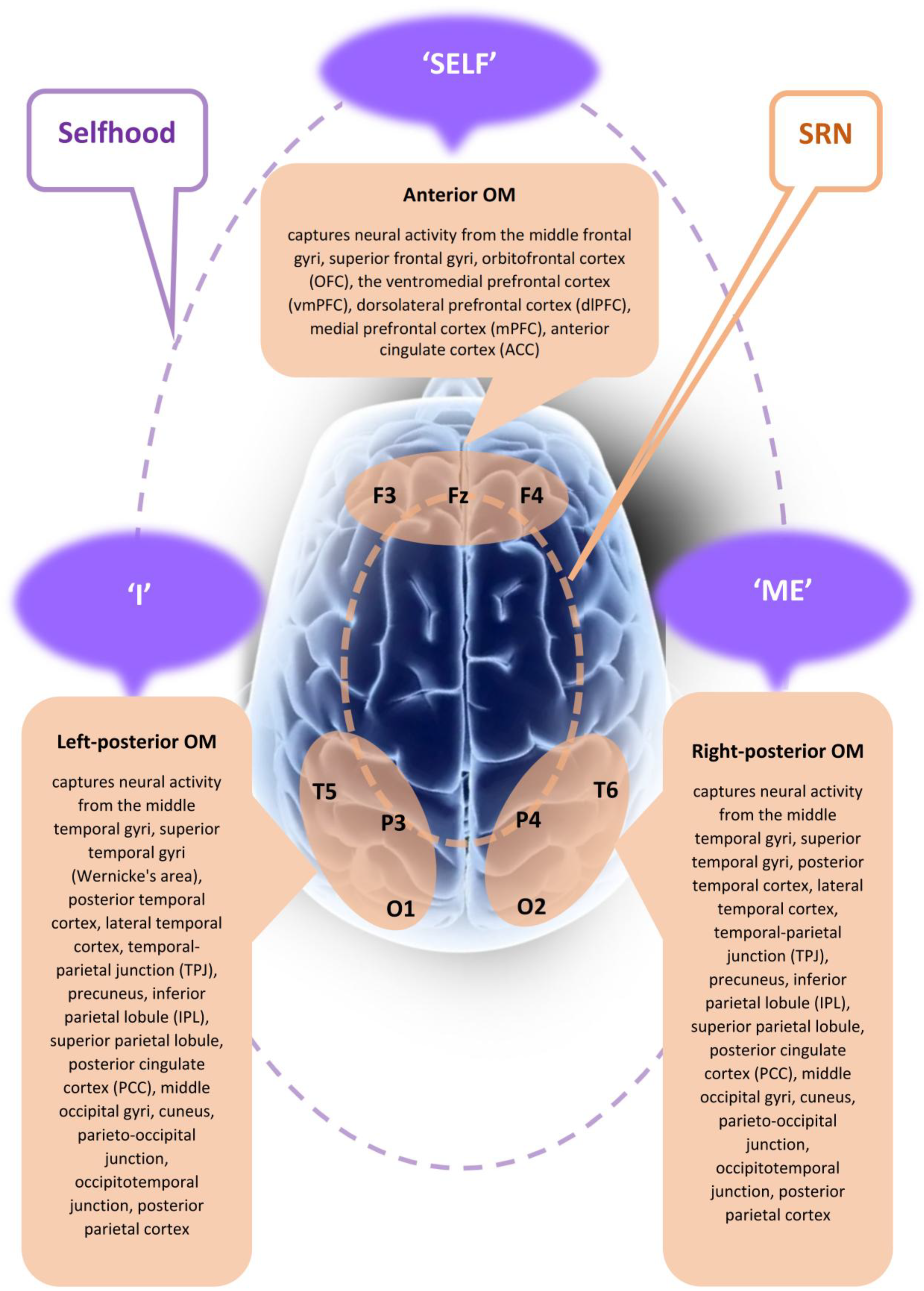
- Neurophysiological evidence: The existence of three major spatially distinct yet functionally interconnected brain subnetworks—also known as operational modules (OMs)—that together constitute the larger brain’s self-referential network (SRN) [46,63,64,66]. These OMs are estimated using quantitative electroencephalography (qEEG) as explained below.
- Phenomenological distinctions: The differentiation of three fundamental aspects of Selfhood, each associated with one of the three SRN’s OMs—phenomenal first-person agency, embodiment, and reflection/narration—all of which are commensurate with each other [62,67,68,69] and thus reflect the multi-faceted nature of self-consciousness [70].
- (i)
- Present (albeit asynchronously) since early childhood [92]: Embodiment (‘Me’) as a pre-reflective, non-conceptual, and pre-linguistic sense of one’s body begins to form the earliest, already in utero [93,94], and by the age of 2, infants can construct a body image of themselves as an entire object while also considering this image as a subject, i.e., as an active source of self-representation and with the capacity to internalize discrete emotions [85] long before they internalize cultural standards and knowledge. Phenomenal point of view (‘Self’), and thus first-person perspective, first starts to develop when a child is about 2 years old and is completed around the age of 4–5 years [95], allowing the child to represent him/herself not only as a core/center but also as a holistic entity in the act of knowing [71]—a prerequisite for awareness of the subjectivity of one’s own experiences. Finally, narration (‘I’) begins when the child becomes a fluent internalized language user, around the age of 4–5 years [96,97]; at that time, the concept of being a subject of experience is formed, allowing for deep self-reflection and identity formation.
- (ii)
- Expressed along the entire continuum of functioning, from health to pathology [65].
- (iii)
- Transdiagnostic [65], which means that changes in the ‘Self,’ ‘Me’, and ‘I’ are observed in multiple disorders across the spectrum of neuropsychopathology.
- (iv)
- Reflected in the features of qEEG phenotypes. qEEG is a digitally recorded and algorithmically analyzed electrical activity generated by the brain. On one hand, qEEG reflects the brain’s inherent functional organization and dynamic activity structure, which are intra-individually stable traits, as demonstrated by test–retest reliability and genetic studies. On the other hand, because intrinsic brain activity shapes and conditions cognitive processes, information processing, self-regulation, decision-making, behavior, and consciousness, qEEG also serves as a reflection of neurophysiological predispositions underlying cognition, personality, temperament, and character. In this way, it captures an individual’s psychological and behavioral traits (for relevant references, see [98]). In this context, qEEG functions as an ‘interface’ between neural activity, personal experience, and behavior, making it a reliable indicator of neurocognitive efficiency and overall well-being. To measure three aspects of the experiential Selfhood (‘Self’, ‘Me’, and ‘I’), the qEEG operational synchrony analysis is used (see [99,100]). Operational synchrony in qEEG refers to a measure of the functional connectivity between different brain regions, based on the simultaneous temporal synchronization of local EEG signal segments (i.e., naturally existing quasi-stable microstates). It quantifies how often and how reliably these discrete EEG patterns occur in synchrony across spatially distinct electrodes (OMs), reflecting the coordination of functional neuronal assemblies underlying integrated cognitive or conscious processes [99,100]. Spatial resolution is often regarded as a limitation of qEEG. However, local qEEG is understood to represent a functional source—defined as the brain region(s) contributing to the activity recorded by a single sensor. A functional source is an operational concept that does not necessarily align with a distinct anatomical brain structure. It remains neutral regarding challenges related to primary source localization and volume conduction (for references, see [98]).
4. The Dynamics of the Experiential Selfhood Triumvirate in Neuropsychopathology
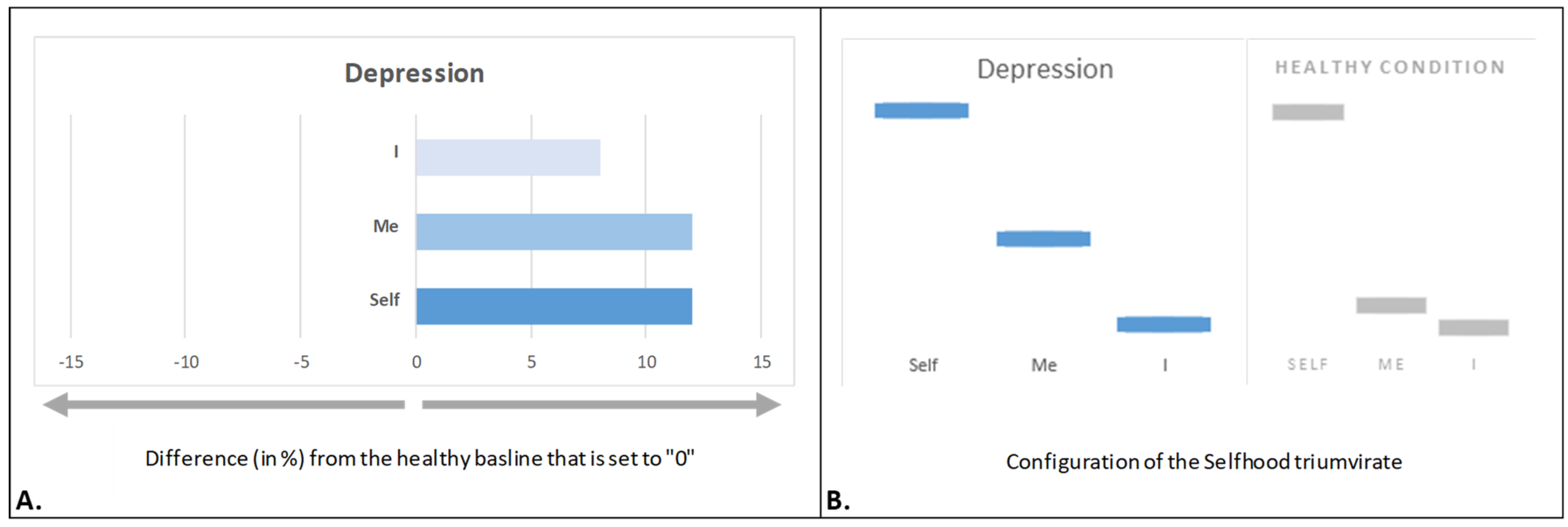
4.1. Depressed Selfhood
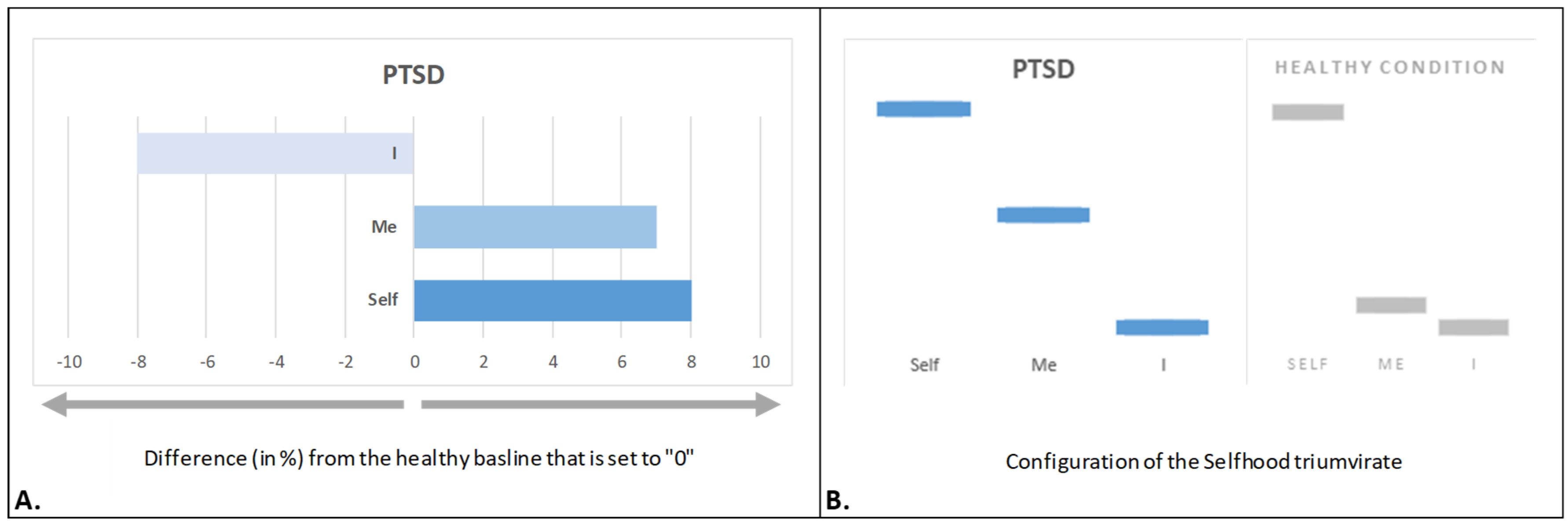
4.2. Traumatized Selfhood
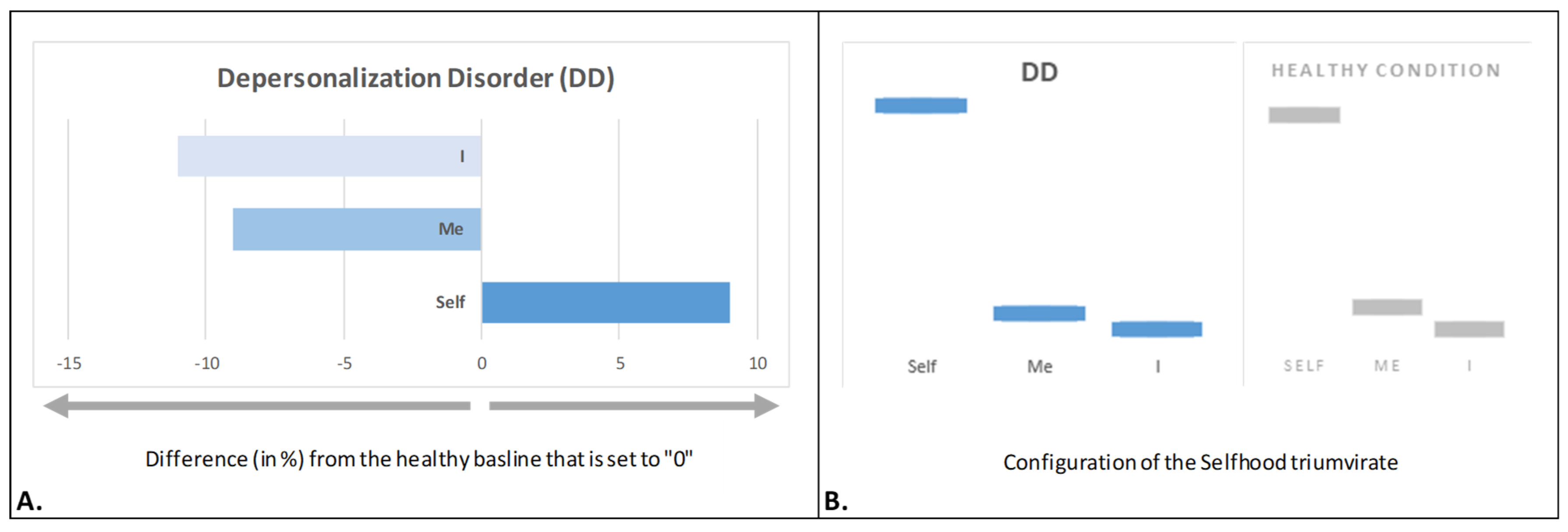
4.3. Depersonalized Selfhood
4.4. Psychotic Selfhood
4.5. Unconscious Selfhood
- (i)
- Unconscious individuals are unresponsive and unable to describe/report their phenomenological experience;
- (ii)
- When Selfhood is unconscious, it can be in either a subconscious, unconscious, or even nonconscious state—each with distinct implications for comprehending personhood and the moral significance of what makes life worth living (for a detailed description and analysis, see [47]).
- (i)
- Witnessing without content (experience of absence, nothingness, or emptiness): This state is characterized by the presence of normal or heightened ‘Self’ expression, with a simultaneous loss of both the ‘Me’ and ‘I’ aspects of the triumvirate. It is suggested [47] (pp. 14–15) that this configuration of the Selfhood triumvirate is associated with the experience of “being a phenomenal spatial-temporal (and often dimensionless) point, that observes and witnesses itself and the world” [64] (p. 264) due to the intact ‘Self’ aspect. This is accompanied by a total absence of content due to the loss of automatic and immediate sense of physical agency (the sense of disembodiment), as well as a decrease in the first-order experiential sense of ownership and emotionality [28,59,157,158] linked to a disintegrated ‘Me’ aspect and the lack of thinking, self-reflection, and personal narrative [159,160,161] associated with the disintegrated ‘I’ aspect of the triumvirate. Additionally, since it has been shown that the phenomenal sense of time arises from the embodiment sense maintained over time [162,163], one should expect a significant change in time perception (feeling of timelessness) when the sense of body is lost [64]. Despite being a ‘thin’ or ‘nonexplicit’ phenomenal experience [73,164], this ‘witnessing without content’ experience is nevertheless “... sufficient for creating a phenomenological centre of gravity and self-identification that is tied to an individual phenomenological first-personal givenness...” [64] (p. 266) (see also [165,166], and for recent empirical evidence, see [46,64]). Therefore, a patient in this state would maintain personhood with a distinct individual first-person perspective in accordance with Levy’s ‘full moral status’ postulate [49]. However, there would be a loss of awareness of being the same person extended over time. This is due to the absence of intact self-narration and autobiographical memory, typically supported by the ‘I’ aspect, which is disintegrated in this state. As such, based on Levy’s definition [49], this state only confers a partial moral status because it lacks the experience of a ‘life worth living’ [49,167].
- (ii)
- Observing agency is not present: This state is distinguished by a significant loss of the ‘Self’ aspect of the Selfhood triumvirate, despite normal levels of the ‘Me’ and ‘I’ aspects. This Selfhood triumvirate configuration may imply [47] (p. 15) that there is phenomenal ‘emptiness’ or ‘nothingness’ because there is not anyone to whom the experience is occurring, not even the unextended point capable of epistemic self-identification [165,166]. Since the other two aspects of the triumvirate (‘Me’ and ‘I’) are functioning normally, there will be phenomenal states related to stimuli originating from both the outside and within the organism that are stored as memory traces; however, they will not be integrated within the first-person meaningful perspective [46]. In such a circumstance, when the sense of ‘Self’ has collapsed, binding fragmented representations of the internal and external world into unified, lived experiences is not possible. Reinterpreting Baars et al. [125], in this state, the phenomenal objects are not blocked from consciousness; rather, the observing subject is absent. Furthermore, in light of Levy’s ‘full moral status’ postulate [49], it is reasonable to expect that the patient will not have full moral status while being in this state. Although autobiographical memory events are phenomenally present, they are not present to any observing agent, as there is no witnessing entity capable of perceiving them from a phenomenal first-person perspective and to which the experiences are occurring [46,64].
- (iii)
- Complete dissolution of experiential Selfhood: This state is characterized by a profound loss (complete disintegration) of all three aspects of the Selfhood triumvirate: ‘Self’, ‘Me’, and ‘I’. Such a state would represent [47] (p. 15) the total absence of all self-relevant phenomenological contents, characterized by “selfless, objectless, and timeless presence” [64] (p. 272), where the self-referential mechanisms for forming phenomenological events are suspended [166]. This state is generally marked by a significant lack of individual first-person perspective, a sense of witnessing agency, and ownership [46,64]. Additionally, subjective time (a sense of presence, past, or future) ceases to exist [46,64]. In this state, there is no phenomenality related to Selfhood, and therefore, patients in this condition lack a “locus of experience and self-ascription” [46] (p. 23), rendering moral considerations regarding personhood irrelevant [49].
4.6. Other Pathological Conditions of Selfhood
‘Dead’ Selfhood
5. A Selfhood Triumvirate Neurophenomenological Perspective on Pathological Consciousness
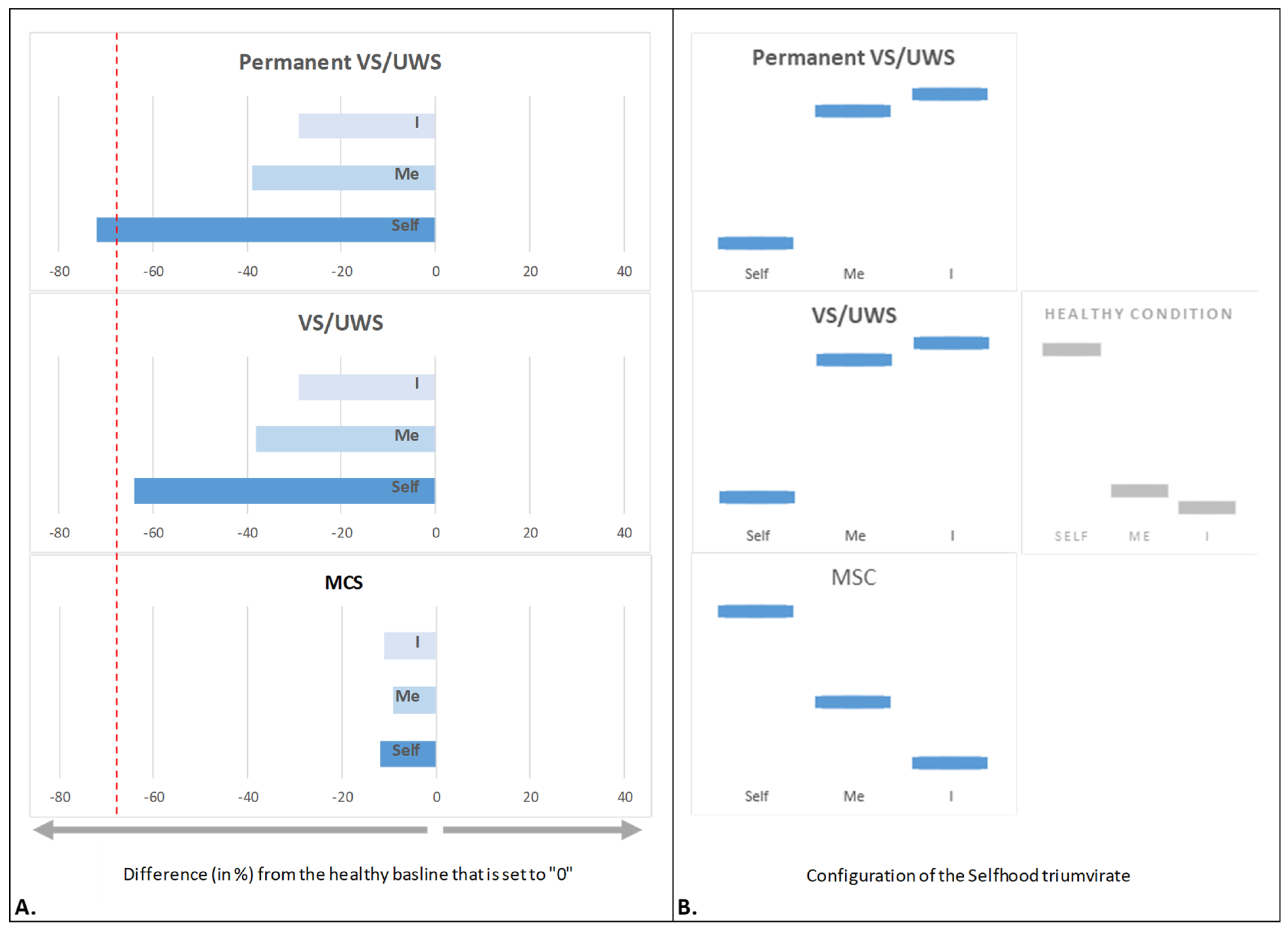
The Resilience of Selfhood Configuration
6. Summary and Concluding Remarks
7. Future Directions
- (i)
- To deepen our understanding of the variability and the variation limits of the Selfhood triumvirate aspects (‘Self’, ‘Me’, and ‘I’), a wider range of neuropsychopathological conditions should be examined using this neurophenomenological approach.
- (ii)
- In order to effectively assess the variability in the Selfhood triumvirate associated with neuropsychopathological conditions, it is important to determine the trait-like properties of the ‘Self’, ‘Me’, and ‘I’ aspects. This can be achieved by investigating their within-subject stability and reliability in healthy populations.
- (iii)
- To better grasp the lived experiences of patients—beyond classical symptomatology—neuropsychopathologies should be examined through the lens of the Selfhood triumvirate framework.
- (iv)
- Understanding the distressing nature of altered states of Selfhood in neuropsychopathological conditions could benefit from comparisons with non-distressing altered states experienced during contemplative practices, such as meditation.
- (v)
- Examining the discrepancy between the actual Selfhood and ideal Selfhood could enhance our understanding of the self-esteem construct. Evidence suggests that self-esteem has a causal role in the development of some mental health conditions [40] and that people with mental health conditions are found to experience a greater prevalence of discrepancies between the actual self and ideal self [41].
- (vi)
- Further research is needed to identify thresholds in the variations in the ‘Self’, ‘Me’, and ‘I’ aspects of the Selfhood triumvirate that signal the transition from one functioning condition to another along the health–pathology continuum.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CS | Cotard syndrome |
| DD | depersonalization disorder |
| DoC | disorder of consciousness |
| DSM | Diagnostic and Statistical Manual of Mental Disorders |
| GNW | Global Neuronal Workspace |
| HAM | Hamilton depression rating scale |
| MCS | minimally conscious state |
| OM | operational module |
| PTSD | post-traumatic stress disorder |
| qEEG | quantitative electroencephalography |
| SRN | self-referential network |
| UWS | unresponsive wakefulness syndrome |
| VS | vegetative state |
References
- Pedhazur, E.J.; Pedhazur Schmelkin, L. Measurement, Design, and Analysis: An Integrated Approach; Psychology Press: New York, NY, USA, 1991; p. 840. [Google Scholar]
- Williams, G. What is it like to be nonconscious? A defense of Julian Jaynes. Phenom. Cogn. Sci. 2011, 10, 217–239. [Google Scholar] [CrossRef]
- Poznanski, R.R.; Holmgren, J.; Cacha, L.A.; Alemdar, E.; Brändas, E.J. The act of understanding certainty is consciousness. J. Multiscale Neurosci. 2023, 2, 280–291. [Google Scholar] [CrossRef]
- Poznanski, R.R. The dynamic organicity theory of consciousness: How consciousness arises from the functionality of multiscale complexity in the material brain. J. Multiscale Neurosci. 2024, 3, 68–87. [Google Scholar] [CrossRef]
- Lane, T.J. Somebody is home. Cogn. Neuropsychol. 2020, 37, 193–196. [Google Scholar] [CrossRef]
- Boly, M.; Massimini, M.; Tononi, G. Theoretical approaches to the diagnosis of altered states of consciousness. Prog. Brain. Res. 2009, 177, 383–398. [Google Scholar] [CrossRef]
- Tononi, G.; Boly, M.; Gosseries, O.; Laureys, S. The neurology of consciousness: An overview, Chapter 25. In The Neurology of Consciousness: Cognitive Neuroscience and Neuropathology, 2nd ed.; Laureys, S., Tononi, G., Gosseries, O., Eds.; Elsevier Academic Press: Oxford, UK, 2016; pp. 407–461. [Google Scholar]
- Jaynes, J. The Origin of Consciousness in the Breakdown of the Bicameral Mind; Houghton Mifflin, Mariner Books: Boston, MA, USA, 1976. [Google Scholar]
- Neisser, J.U. Unconscious subjectivity. Psyche 2006, 12, 1–14. Available online: https://journalpsyche.org/files/0xaaf7.pdf (accessed on 23 March 2025).
- Revonsuo, A. Inner Presence. Consciousness as a Biological Phenomenon; MIT Press: Cambridge, MA, USA, 2006; p. 473. [Google Scholar]
- Kriegel, U. Consciousness: Phenomenal consciousness, access consciousness, and scientific practice. In Handbook of the Philosophy of Science, Philosophy of Psychology and Cognitive Science; Thagard, P., Ed.; Elsevier, North-Holland: Amsterdam, The Netherlands, 2007; pp. 195–217. [Google Scholar]
- Mashour, G.A.; Hudetz, A.G. Neural correlates of unconsciousness in large-scale brain networks. Trends Neurosci. 2018, 41, 150–160. [Google Scholar] [CrossRef]
- Fingelkurts, A.A.; Fingelkurts, A.A. The deep essence of consciousness: In search of definition. Review of: “The Study of Consciousness Is Mired in Complexities and Difficulties: Can They Be Resolved?”. Qeios 2024, PHF7IA. [Google Scholar] [CrossRef]
- Fingelkurts, A.A.; Fingelkurts, A.A.; Neves, C.F.H. Natural world physical, brain operational, and mind phenomenal space–time. Phys. Life Rev. 2010, 7, 195–249. [Google Scholar] [CrossRef]
- Fingelkurts, A.A.; Fingelkurts, A.A.; Neves, C.F.H. Consciousness as a phenomenon in the operational architectonics of brain organization: Criticality and self-organization considerations. Chaos Solitons Fractals 2013, 55, 13–31. [Google Scholar] [CrossRef]
- Fichte, J.G. Science of Knowledge: With the First and Second Introductions [1794]; Heath, P., Lachs, J., Eds. and Translators; Cambridge University Press: Cambridge, UK, 1982; p. 324. [Google Scholar]
- Lane, T.J.; Caleb, L. Self-Consciousness and Immunity. J. Philos. 2011, 108, 78–99. Available online: http://www.jstor.org/stable/23039007 (accessed on 6 June 2025). [CrossRef][Green Version]
- Lane, T.J. Toward an explanatory framework for mental ownership. Phenom. Cogn. Sci. 2012, 11, 251–286. [Google Scholar] [CrossRef]
- Lane, T.J. The minimal self hypothesis. Conscious. Cogn. 2020, 85, 103029. [Google Scholar] [CrossRef] [PubMed]
- Humpston, C.S. Isolated by oneself: Ontologically impossible experiences in schizophrenia. Philos. Psychiatry Psychol. 2022, 29, 5–15. [Google Scholar] [CrossRef]
- Kihlstrom, J.F.; Cantor, N. Mental representations of the self. In Advances in Experimental Social Psychology; Berkowitz, L., Ed.; Elsevier, Academic Press: New York, NY, USA, 1984; Volume 17, pp. 1–47. [Google Scholar]
- Kihlstrom, J.F.; Klein, S.B. Self-knowledge and self-awareness. Ann. N. Y. Acad. Sci. 1997, 18, 4–17. [Google Scholar] [CrossRef]
- Kihlstrom, J.F. The psychological unconscious. In Handbook of Personality: Theory and Research, 3rd ed.; John, O., Robins, R., Pervin, L., Eds.; Guilford: New York, NY, USA, 2008; pp. 583–602. [Google Scholar]
- Poznanski, R.R.; Alemdar, E. The ‘hidden’ structure of uncertainties unfolding through poststructural dynamics of the entropic brain. J. Multiscale Neurosci. 2024, 3, 264–273. [Google Scholar] [CrossRef]
- Rudrauf, D. Structure-function relationships behind the phenomenon of cognitive resilience in neurology: Insights for neuroscience and medicine. Adv. Neurosc. 2014, 1, 462765. [Google Scholar] [CrossRef]
- Feuillet, L.; Dufour, H.; Pelletier, J. Brain of a white-collar worker. Lancet 2007, 370, 262. [Google Scholar] [CrossRef]
- Philippi, C.L.; Feinstein, J.S.; Khalsa, S.S.; Damasio, A.; Tranel, D.; Landini, G.; Williford, K.; Rudrauf, D. Preserved self-awareness following extensive bilateral brain damage to the insula, anterior cingulate, and medial prefrontal cortices. PLoS ONE 2012, 7, e38413. [Google Scholar] [CrossRef]
- Damasio, A. The Feeling of What Happens: Body and Emotion in the Making of Consciousness; Harcourt Brace: Orlando, FL, USA, 1999; p. 386. [Google Scholar]
- Damasio, A. Self Comes to Mind: Constructing the Conscious Brain; Knopf Doubleday Publishing Group: New York, NY, USA, 2010; p. 336. [Google Scholar]
- Blackmon, J. Hemispherectomies and independently conscious brain regions. J. Cogn. Neuroethics 2016, 3, 1–26. [Google Scholar]
- Pinto, Y.; Neville, D.A.; Otten, M.; Corballis, P.M.; Lamme, V.A.F.; de Haan, E.H.F.; Foschi, N.; Fabri, M. Split brain: Divided perception but undivided consciousness. Brain 2017, 140, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Y.; de Haan, E.H.F.; Lamme, V.A.F. The split-brain phenomenon revisited: A single conscious agent with split perception. Trends Cogn. Sci. 2017, 21, 835–851. [Google Scholar] [CrossRef] [PubMed]
- de Haan, E.H.F.; Corballis, P.M.; Hillyard, S.A.; Marzi, C.A.; Seth, A.; Lamme, V.A.F.; Volz, L.; Fabri, M.; Schechter, E.; Bayne, T.; et al. Split-brain: What we know now and why this is important for understanding consciousness. Neuropsychol. Rev. 2020, 30, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Spitznagel, M.B.; Tremont, G. Cognitive reserve and anosognosia in questionable and mild dementia. Arch. Clin. Neuropsychol. 2005, 20, 505–515. [Google Scholar] [CrossRef]
- Tulving, E. Self-knowledge of an amnesic individual is represented abstractly. In The Mental Representation of Trait and Autobiographical Knowledge About the Self; Srull, T.K., Wyer, R.S., Eds.; Lawrence Erlbaum Associates Inc.: Hillsdale, NJ, USA, 1993; pp. 147–156. [Google Scholar]
- Klein, S.B.; Loftus, J.; Kihlstrom, J.F. Self-knowledge of an amnesic patient: Toward a neuropsychology of personality and social psychology. J. Exp. Psychol. Gen. 1996, 125, 250–260. [Google Scholar] [CrossRef]
- Klein, S.B.; Cosmides, L.; Costabile, K.A. Preserved knowledge of self in a case of Alzheimer’s Dementia. Soc. Cogn. 2003, 21, 157–165. [Google Scholar] [CrossRef]
- Wilson, B.A.; Kopelman, M.; Kapur, N. Prominent and persistent loss of past awareness in amnesia: Delusion, impaired consciousness or coping strategy? Neuropsychol. Rehabil. 2008, 18, 527–540. [Google Scholar] [CrossRef]
- Martin-Garcia, O.; Blanco, I.; Sanchez-Lopez, A. Do we interpret ambiguity and feel according to how we define ourselves? Relationships between self-perception, interpretation biases, and their role on emotional symptoms. Front. Psychiatry 2024, 15, 1502130. [Google Scholar] [CrossRef]
- Henriksen, I.O.; Ranøyen, I.; Indredavik, M.S.; Stenseng, F. The role of self-esteem in the development of psychiatric problems: A three-year prospective study in a clinical sample of adolescents. Child Adolesc. Psychiatry Ment. Health 2017, 11, 68. [Google Scholar] [CrossRef]
- Mason, T.B.; Smith, K.E.; Engwall, A.; Lass, A.; Mead, M.; Sorby, M.; Bjorlie, K.; Strauman, T.J.; Wonderlich, S.l. Self-discrepancy theory as a transdiagnostic framework: A meta-analysis of self-discrepancy and psychopathology. Psychol. Bull. 2019, 145, 372–389. [Google Scholar] [CrossRef]
- Parnas, J.; Møller, P.; Kircher, T.; Thalbitzer, J.; Jansson, L.; Handest, P.; Zahavi, D. EASE: Examination of anomalous self-experience. Psychopathology 2005, 38, 236–258. [Google Scholar] [CrossRef] [PubMed]
- Kean, C. Silencing the self: Schizophrenia as a self-disturbance. Schizophr. Bull. 2009, 35, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
- Levy, N. What difference does consciousness make? Monash. Bioeth. Rev. 2009, 28, 13–25. [Google Scholar] [CrossRef]
- Fingelkurts, A.A.; Fingelkurts, A.A. Depersonalization puzzle: A new view from the neurophenomenological Selfhood perspective. J. Nphi. 2022, 1, 181–202. [Google Scholar] [CrossRef]
- Fingelkurts, A.A.; Fingelkurts, A.A.; Kallio-Tamminen, T. Selfhood triumvirate: From phenomenology to brain activity and back again. Conscious. Cogn. 2020, 86, 103031. [Google Scholar] [CrossRef]
- Fingelkurts, A.A.; Fingelkurts, A.A. Patients with disorders of consciousness: Are they nonconscious, unconscious, or subconscious? Expanding the Discussion. Brain Sci. 2023, 13, 814. [Google Scholar] [CrossRef]
- Kihlstrom, J.F. Dissociative disorders. Annu. Rev. Clin. Psychol. 2005, 1, 227–253. [Google Scholar] [CrossRef]
- Levy, N.; Savulescu, J. Moral significance of phenomenal consciousness. Prog. Brain Res. 2009, 177, 361–370. [Google Scholar] [CrossRef]
- Fingelkurts, A.A.; Fingelkurts, A.A. Contemplating on the nature of Selfhood in DoC patients: Neurophenomenological perspective. J. Integr. Neurosci. 2023, 22, 23. [Google Scholar] [CrossRef]
- Gallagher, S. The Self and its Disorders. Oxford University Press: Oxford, UK, 2024; p. 368. [Google Scholar]
- Rasmussen, A.R.; Raballo, A.; Preti, A.; Sæbye, D.; Parnas, J. Anomalies of imagination, self-disorders, and schizophrenia spectrum psychopathology: A network analysis. Front. Psychiatry 2022, 12, 808009. [Google Scholar] [CrossRef]
- Raballo, A.; Poletti, M.; Preti, A.; Parnas, J. The self in the spectrum: A meta-analysis of the evidence linking basic self-disorders and schizophrenia. Schizophr. Bull. 2021, 47, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Feyaerts, J.; Nelson, B.; Sass, L. Self-disorders in schizophrenia as disorders of transparency: An exploratory account. Philos. Psychol. 2024, 38, 49–76. [Google Scholar] [CrossRef]
- Tordjman, S.; Celume, M.P.; Denis, L.; Motillon, T.; Keromnes, G. Reframing schizophrenia and autism as self-consciousness disorders associating a deficit of theory of mind and empathy with social communication impairments. Neurosci. Biobehav. Rev. 2019, 103, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Burraco, A.; Adornetti, I.; Ferretti, F.; Progovac, L. An evolutionary account of impairment of self in cognitive disorders. Cogn. Process. 2023, 24, 107–127. [Google Scholar] [CrossRef]
- Lopez, C. A neuroscientific account of how vestibular disorders impair bodily self-consciousness. Front. Integr. Neurosci. 2013, 7, 91. [Google Scholar] [CrossRef]
- Sabbah, S.G.; Northoff, G. The self in depression and anxiety as a transdiagnostic and differential-diagnostic neural marker: A systematic review. Neurosci. Biobehav. Rev. 2025, 169, 106034. [Google Scholar] [CrossRef]
- Gallagher, S. Philosophical conceptions of the self: Implications for cognitive science. Trends Cogn. Sci. 2000, 4, 14–21. [Google Scholar] [CrossRef]
- Legrand, D. Pre-reflective self-as-subject from experiential and empirical perspectives. Conscious. Cogn. 2007, 16, 583–599. [Google Scholar] [CrossRef]
- Gwyther, M.P.D.; Lenggenhager, B.; Windt, J.M.; Aspell, J.E.; Ciaunica, A. Examining the association between depersonalisation traits and the bodily self in waking and dreaming. Sci. Rep. 2024, 14, 6107. [Google Scholar] [CrossRef]
- Gallagher, S. A pattern theory of self. Front. Hum. Neurosci. 2013, 7, 443. [Google Scholar] [CrossRef]
- Fingelkurts, A.A.; Fingelkurts, A.A. Persistent operational synchrony within brain default-mode network and self-processing operations in healthy subjects. Brain Cogn. 2011, 75, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Fingelkurts, A.A.; Fingelkurts, A.A.; Kallio-Tamminen, T. Self, Me and I in the repertoire of spontaneously occurring altered states of Selfhood: Eight neurophenomenological case study reports. Cogn. Neurodyn. 2022, 16, 255–282. [Google Scholar] [CrossRef] [PubMed]
- Fingelkurts, A.A.; Fingelkurts, A.A.; Kallio-Tamminen, T. The selfhood-components dynamics in the spectrum of discrete normotypical and pathological modes. J. NPhi. 2023, 2, 402–431. [Google Scholar] [CrossRef]
- Northoff, G.; Heinzel, A.; de Greck, M.; Bermpohl, F.; Dobrowolny, H.; Panksepp, J. Self-referential processing in our brain. A meta-analysis of imaging studies on the self. NeuroImage 2006, 31, 440–457. [Google Scholar] [CrossRef]
- Zahavi, D. First-person thoughts and embodied self-awareness: Some reflections on the relation between recent analytic philosophy and phenomenology. Phenomenol. Cogn. Sci. 2002, 1, 7–26. [Google Scholar] [CrossRef]
- Hohwy, J. The sense of self in the phenomenology of agency and perception. Psyche 2007, 13, 1–20. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwi1tLmm8-2NAxXCIBAIHXi0AKAQFnoECB4QAQ&url=https%3A%2F%2Fjournalpsyche.org%2Ffiles%2F0xab11.pdf&usg=AOvVaw2GL71vc5iD4qzqIsT--UZK&opi=89978449 (accessed on 23 March 2025).
- Gallagher, S.; Daly, A. Dynamical relations in the self-pattern. Front. Psychol. 2018, 9, 664. [Google Scholar] [CrossRef]
- Klein, S.B.; Gangi, C.E. The multiplicity of self: Neuropsychological evidence and its implications for the self as a construct in psychological research. Ann. N. Y. Acad. Sci. 2010, 1191, 1–15. [Google Scholar] [CrossRef]
- Blanke, O.; Metzinger, T. Full-body illusions and minimal phenomenal selfhood. Trends Cogn. Sci. 2009, 13, 7–13. [Google Scholar] [CrossRef]
- Dennett, D.C. Consciousness Explained; Little, Brown and Company: Boston, MA, USA, 1991. [Google Scholar]
- Velmans, M. Conscious agency and the preconscious/unconscious Self. In Interdisciplinary Perspectives on Consciousness and the Self; Menon, S., Sinha, A., Sreekantan, B.V., Eds.; Springer: New Delhi, India, 2014; pp. 11–25. [Google Scholar]
- Metzinger, T. Being no One: The Self-Model Theory of Subjectivity; MIT Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Metzinger, T. Empirical perspectives from the self-model theory of subjectivity: A brief summary with examples. In Progress in Brain Research; Banerjee, R., Chakrabarti, B.K., Eds.; Elsevier B.V.: Boston, MA, USA, 2008; pp. 215–245. [Google Scholar]
- Trehub, A. Space, self, and the theater of consciousness. Conscious. Cogn. 2007, 16, 310–330. [Google Scholar] [CrossRef]
- Solms, M.; Turnbull, O. The Brain and the Inner World. An Introduction to the Neuroscience of Subjective Experience; Karnac: London, UK, 2002. [Google Scholar]
- Varela, F.J.; Thompson, E.; Rosch, E. The Embodied Mind: Cognitive Science and Human Experience; The MIT Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Gallagher, S. How the Body Shapes the Mind; Clarendon Press: Oxford, UK, 2005. [Google Scholar]
- Legrand, D. The bodily self: The sensori-motor roots of pre-reflective self-consciousness. Phenomenol. Cogn. Sci. 2006, 5, 89–118. [Google Scholar] [CrossRef]
- Apps, M.A.; Tsakiris, M. The free-energy self: A predictive coding account of self-recognition. Neurosci. Biobehav. Rev. 2014, 41, 85–97. [Google Scholar] [CrossRef] [PubMed]
- McAdams, D.P.; Olson, B.D. Personality development: Continuity and change over the life course. Ann. Rev. Psychol. 2010, 61, 517–542. [Google Scholar] [CrossRef] [PubMed]
- Gazzaniga, M. The Social Brain. Discovering the Networks of the Mind; Basic Books: New York, NY, USA, 1985. [Google Scholar]
- McAdams, D.P.; Cox, K.S. Self and identity across the life span. In The Handbook of Life-Span Development, Volume II; Lerner, R.M., Ed.; Wiley: New York, NY, USA, 2010; pp. 158–207. [Google Scholar]
- Marraffa, M.; Meini, C. Self-consciousness as a construction all the way down. Behav. Sci. 2024, 14, 200. [Google Scholar] [CrossRef]
- Frewen, P.; Schroeter, M.L.; Riva, G.; Cipresso, P.; Fairfield, B.; Padulo, C.; Kemp, A.H.; Palaniyappan, L.; Owolabi, M.; Kusi-Mensah, K.; et al. Neuroimaging the consciousness of self: Review, and conceptual-methodological framework. Neurosci. Biobehav. Rev. 2020, 112, 164–212. [Google Scholar] [CrossRef]
- Koessler, L.; Maillard, L.; Benhadid, A.; Vignal, J.P.; Felblinger, J.; Vespignani, H.; Braun, M. Automated cortical projection of EEG sensors: Anatomical correlation via the international 10-10 system. NeuroImage 2009, 46, 64–72. [Google Scholar] [CrossRef]
- Gusnard, D.A.; Raichle, M.E. Searching for a baseline: Functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001, 2, 685–694. [Google Scholar] [CrossRef]
- Mantini, D.; Perrucci, M.G.; Del Gratta, C.; Romani, G.L.; Corbetta, M. Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. USA 2007, 104, 13170–13175. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Thermenos, H.W.; Milanovic, S.; Tsuang, M.T.; Faraone, S.V.; McCarley, R.W.; Shenton, M.E.; Green, A.I.; Nieto-Castanon, A.; LaViolette, P.; et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. USA 2009, 106, 1279–1284. [Google Scholar] [CrossRef]
- Herold, D.; Spengler, S.; Sajonz, B.; Usnich, T.; Bermpohl, F. Common and distinct networks for self-referential and social stimulus processing in the human brain. Brain Struct. Funct. 2016, 221, 3475–3485. [Google Scholar] [CrossRef]
- Woźniak, M. How to grow a self: Development of self-representation in the Bayesian brain. Front. Hum. Neurosci. 2024, 18, 1441931. [Google Scholar] [CrossRef] [PubMed]
- Ciaunica, A.; Constant, A.; Preissl, H.; Fotopoulou, K. The first prior: From co-embodiment to co-homeostasis in early life. Conscious. Cogn. 2021, 91, 103117. [Google Scholar] [CrossRef] [PubMed]
- Delafield-Butt, J.; Ciaunica, A. Sensorimotor foundations of self-consciousness in utero. Curr. Opin. Behav. Sci. 2024, 59, 101428. [Google Scholar] [CrossRef]
- Butterworth, G. An ecological perspective on the self and its development. In Exploring the Self. Philosophical and Psychopathological Perspectives on Self-Experience; Zahavi, D., Ed.; John Benjamins: Amsterdam, The Netherlands, 2000; pp. 19–38. [Google Scholar]
- Vygotsky, L.S. The Collected Works of Vygotsky; Plenum Press: New York, NY, USA, 1987. [Google Scholar]
- Geva, S.; Fernyhough, C. A penny for your thoughts: Children’s inner speech and its neuro-development. Front. Psychol. 2019, 10, 1708. [Google Scholar] [CrossRef] [PubMed]
- Fingelkurts, A.A.; Fingelkurts, A.A. Quantitative electroencephalogram (qEEG) as a natural and non-invasive window into living brain and mind in the functional continuum of healthy and pathological conditions. Appl. Sci. 2022, 12, 9560. [Google Scholar] [CrossRef]
- Fingelkurts, A.A.; Fingelkurts, A.A. Brain-mind operational architectonics imaging: Technical and methodological aspects. Open Neuroimag. J. 2008, 2, 73–93. [Google Scholar] [CrossRef]
- Fingelkurts, A.A.; Fingelkurts, A.A. Operational architectonics methodology for EEG analysis: Theory and results. In Modern Electroencephalographic Assessment Techniques. Neuromethods; Sakkalis, V., Ed.; Humana Press: New York, NY, USA, 2015; Volume 91, pp. 1–59. [Google Scholar] [CrossRef]
- Fingelkurts, A.A.; Fingelkurts, A.A.; Kallio-Tamminen, T. Trait lasting alteration of the brain default mode network in experienced meditators and the experiential selfhood. Self Identity 2016, 15, 381–393. [Google Scholar] [CrossRef]
- Hartmann, E.; Harrison, R.; Zborowski, M. Boundaries in the mind: Past research and future directions. N. Am. J. Psychol. 2001, 3, 347–368. [Google Scholar]
- Hanley, A.W.; Nakamura, Y.; Garland, E.L. The nondual awareness dimensional assessment (NADA): New tools to assess nondual traits and states of consciousness occurring within and beyond the context of meditation. Psychol. Assess. 2018, 30, 1625–1639. [Google Scholar] [CrossRef]
- Martin, J.A. Clusters of individuals experiences form a continuum of persistent non-symbolic experiences in adults. Conscious. Ideas Res. Twenty-First Century 2020, 8, 1. Available online: https://digitalcommons.ciis.edu/conscjournal/vol8/iss8/1 (accessed on 6 June 2025).
- Lindström, L.M.; Goldin, P.; Mårtensson, J.; Cardeña, E. Nonlinear brain correlates of trait self-boundarylessness. Neurosci. Conscious. 2023, 1, niad006. [Google Scholar] [CrossRef]
- Thatcher, R.W.; Krause, P.; Hrybyk, M. Cortico-cortical associations and EEG coherence: A two compartmental model. Electroencephalogr. Clin. Neurophysiol. 1986, 64, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Gasser, T.; Jennen-Steinmetz, C.; Verleger, R. EEG coherence at rest and during a visual task in two groups of children. Electroencephalogr. Clin. Neurophysiol. 1987, 67, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Corsi-Cabrera, M.; Solís-Ortiz, S.; Guevara, M.A. Stability of EEG inter-and intrahemispheric correlation in women. Electroencephalogr. Clin. Neurophysiol. 1997, 102, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Corsi-Cabrera, M.; Galindo-Vilchis, L.; del-Río-Portilla, Y.; Arce, C.; Ramos-Loyo, J. Within-subject reliability and inter-session stability of EEG power and coherent activity in women evaluated monthly over nine months. Clin. Neurophysiol. 2007, 118, 9–21. [Google Scholar] [CrossRef]
- Roberts, A.M.; Fillmore, P.T.; Decker, S.L. Clinical applicability of the test-retest reliability of qEEG coherence. NeuroRegulation 2016, 3, 7–22. [Google Scholar] [CrossRef]
- van Baal, G.C.; de Geus, E.J.; Boomsma, D.I. Genetic influences on EEG coherence in 5-year-old twins. Behav. Genet. 1998, 28, 9–19. [Google Scholar] [CrossRef]
- van Baal, G.C.; Boomsma, D.I.; de Geus, E.J. Longitudinal genetic analysis of EEG coherence in young twins. Behav. Genet. 2001, 31, 637–651. [Google Scholar] [CrossRef]
- van Beijsterveldt, C.E.; Molenaar, P.C.; de Geus, E.J.; Boomsma, D.I. Genetic and environmental influences on EEG coherence. Behav. Genet. 1998, 28, 443–453. [Google Scholar] [CrossRef]
- Chorlian, D.B.; Tang, Y.; Rangaswamy, M.; O’Connor, S.; Rohrbaugh, J.; Taylor, R.; Porjesz, B. Heritability of EEG coherence in a large sib-pair population. Biol. Psychol. 2007, 75, 260–266. [Google Scholar] [CrossRef]
- Cuthbert, B.N.; Insel, T.R. Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Med. 2013, 11, 126. [Google Scholar] [CrossRef]
- Lindström, L.; Stålhammar, S.; Cardeña, E. The core of me is that which observes: A mixed-methods study of trait-level sense of self. Psychol. Conscious. Theory Res. Pract. 2023, 12, 1–18. [Google Scholar] [CrossRef]
- Cole, C.; Oetting, E.R.; Hinkle, J. Non-linearity of self-concept discrepancy: The value dimension. Psychol. Rep. 1967, 21, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Hellhammer, D.; Meinlschmidt, G.; Pruessner, J.C. Conceptual endophenotypes: A strategy to advance the impact of psychoneuroendocrinology in precision medicine. Psychoneuroendocrinology 2018, 89, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Fingelkurts, A.A.; Fingelkurts, A.A. Three-dimensional components of selfhood in treatment-naive patients with major depressive disorder: A resting-state qEEG imaging study. Neuropsychologia 2017, 99, 30–36. [Google Scholar] [CrossRef]
- Beck, A.T. Cognitive models of depression. J. Cogn. Psychother. 1987, 1, 5–37. [Google Scholar]
- Rimes, K.A.; Watkins, E. The effects of self-focused rumination on global negative self-judgements in depression. Behav. Res. Ther. 2005, 43, 1673–1681. [Google Scholar] [CrossRef]
- Northoff, G. Psychopathology and pathophysiology of the self in depression—Neuropsychiatric hypothesis. J. Affect. Disord. 2007, 104, 1–14. [Google Scholar] [CrossRef]
- Nolen-Hoeksema, S.; Wisco, B.E.; Lyubomirsky, S. Rethinking rumination. Perspect. Psychol. Sci. 2008, 3, 400–424. [Google Scholar] [CrossRef]
- Paulus, M.P.; Stein, M.B. Interoception in anxiety and depression. Brain Struct. Funct. 2010, 214, 451–463. [Google Scholar] [CrossRef]
- Baars, B.J.; Ramsøy, T.Z.; Laureys, S. Brain, conscious experience and the observing self. Trends Neurosci. 2003, 26, 671–675. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Furman, D.J.; Chang, C.; Thomason, M.E.; Dennis, E.; Gotlib, I.H. Default-mode and task-positive network activity in major depressive disorder: Implications for maladaptive rumination. Biol. Psychiatry 2011, 70, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Nejad, A.B.; Fossati, P.; Lemogne, C. Self-referential processing, rumination, and cortical midline structures in major depression. Front. Hum. Neurosci. 2013, 7, 666. [Google Scholar] [CrossRef] [PubMed]
- Veale, D.; Kinderman, P.; Riley, S.; Lambrou, C. Self-discrepancy in body dysmorphic disorder. Br. J. Clin. Psychol. 2003, 42, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. Human feelings: Why are some more aware than others? Trends Cogn. Sci. 2004, 8, 239–241. [Google Scholar] [CrossRef]
- Lane, T.J.; Northoff, G. Is depressive rumination rational? In Rationality: Constraints and Contexts; Lane, T.J., Tzu-Wei Hung, T.-W., Eds.; Elsevier Academic Press: London, UK, 2016; pp. 121–145. [Google Scholar]
- Fingelkurts, A.A.; Fingelkurts, A.A. Alterations in the three components of Selfhood in persons with post-traumatic stress disorder symptoms: A pilot qEEG neuroimaging study. Open Neuroimag. J. 2018, 12, 42–54. [Google Scholar] [CrossRef][Green Version]
- American Psychiatric Association, DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5TM, 5th ed.; The American Psychiatric Publishing Inc.: Arlington, VA, USA, 2013. [Google Scholar]
- van der Kolk, B.A. The body keeps the score: Memory and the evolving psychobiology of posttraumatic stress. Harv. Rev. Psychiatry. 1994, 1, 253–265. [Google Scholar] [CrossRef]
- McNally, R.J. Remembering Trauma; Belknap Press/Harvard University Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Ataria, Y. Traumatic memories as black holes: A qualitative-phenomenological approach. Qual. Psychol. 2014, 1, 123–140. [Google Scholar] [CrossRef]
- van der Kolk, B.A.; Fisler, R. Dissociation and the fragmentary nature of traumatic memories: Overview and exploratory study. J. Trauma Stress. 1995, 8, 505–525. [Google Scholar] [CrossRef]
- Zepinic, V. Hidden Scars: Understanding and Treating Complex Trauma; Xlibris Corp.: London, UK, 2011; p. 716. [Google Scholar]
- Mucci, C. Implicit memory, unrepressed unconscious and trauma theory: The turn of the screw between contemporary Psychoanalysis and Neuroscience. In Unrepressed Unconscious, Implicit memory, and Clinical Work; Craparo, G., Mucci, C., Eds.; Karnac: London, UK, 2017; pp. 99–129. [Google Scholar]
- Laub, D.; Auerhahn, N.C. Knowing and not knowing massive psychic trauma: Forms of traumatic memory. Int. J. Psychoanal. 1993, 74, 287–302. [Google Scholar]
- Sierra, M. Depersonalization: A New Look at a Neglected Syndrome; Cambridge University Press: New York, NY, USA, 2009. [Google Scholar]
- Fuchs, T. The temporal structure of intentionality and its disturbance in schizophrenia. Psychopathology 2007, 40, 229–235. [Google Scholar] [CrossRef]
- Klar, P.; Northoff, G. When the world breaks down: A 3-stage existential model of nihilism in Schizophrenia. Psychopathology 2021, 54, 169–192. [Google Scholar] [CrossRef] [PubMed]
- Cermolacce, M.; Naudin, J.; Parnas, J. The “minimal self” in psychopathology: Re-examining the self-disorders in the schizophrenia spectrum. Conscious. Cog. 2007, 16, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Sass, L.A. Self-disturbance in schizophrenia: Hyperreflexivity and diminished self-affection. In The Self in Neuroscience and Psychiatry; Kircher, T., David, A., Eds.; Cambridge University Press: New York, NY, USA, 2003; pp. 242–271. [Google Scholar]
- Jennett, B.; Plum, F. Persistent vegetative state after brain damage. A syndrome in search of a name. Lancet 1972, 1, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Laureys, S.; Celesia, G.G.; Cohadon, F.; Lavrijsen, J.; León-Carrión, J.; Sannita, W.G.; Sazbon, L.; Schmutzhard, E.; von Wild, K.R.; Zeman, A.; et al. Unresponsive wakefulness syndrome: A new name for the vegetative state or apallic syndrome. BMC Med. 2010, 8, 68. [Google Scholar] [CrossRef]
- Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state (1). N. Engl. J. Med. 1994, 330, 1499–1508. [Google Scholar] [CrossRef]
- Giacino, J.T.; Ashwal, S.; Childs, N.; Cranford, R.; Jennett, B.; Katz, D.I.; Kelly, J.P.; Rosenberg, J.H.; Whyte, J.; Zafonte, R.D.; et al. The minimally conscious state: Definition and diagnostic criteria. Neurology 2002, 58, 349–353. [Google Scholar] [CrossRef]
- Fingelkurts, A.A.; Fingelkurts, A.A.; Bagnato, S.; Boccagni, C.; Galardi, G. DMN Operational synchrony relates to self-consciousness: Evidence from patients in vegetative and minimally conscious states. Open Neuroimag. J. 2012, 6, 55–68. [Google Scholar] [CrossRef]
- Kinsbourne, M. A continuum of self-consciousness that emerges in phylogeny and ontogeny. In The Missing Link in Cognition: Origins of Self-Reflective Consciousness; Terrace, H.S., Metcalfe, J., Eds.; Oxford University Press: New York, NY, USA, 2005; pp. 142–156. [Google Scholar]
- Augusto, L.M. Unconscious knowledge: A survey. Adv. Cogn. Psychol. 2010, 6, 116–141. [Google Scholar] [CrossRef]
- Dresp-Langley, B. Why the brain knows more than we do: Non-conscious representations and their role in the construction of conscious experience. Brain Sci. 2011, 2, 1–21. [Google Scholar] [CrossRef]
- Searle, J.R. The Rediscovery of The Mind; MIT Press: Cambridge, MA, USA, 1992. [Google Scholar]
- Oakley, D.A.; Halligan, P.W. Chasing the rainbow: The non-conscious nature of being. Front. Psychol. 2017, 8, 1924. [Google Scholar] [CrossRef]
- Allegrini, P.; Paradisi, P.; Menicucci, D.; Laurino, M.; Bedini, R.; Piarulli, A.; Gemignani, A. Sleep unconsciousness and breakdown of serial critical intermittency: New vistas on the global workspace. Chaos Solit. Fractals 2013, 55, 32–43. [Google Scholar] [CrossRef]
- Fingelkurts, A.A.; Fingelkurts, A.A.; Bagnato, S.; Boccagni, C.; Galardi, G. The chief role of frontal operational module of the brain default mode network in the potential recovery of consciousness from the vegetative state: A preliminary comparison of three case reports. Open Neuroimag. J. 2016, 10 (Suppl. S1), 41–51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seth, A.K. Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci. 2013, 17, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, M. My body in the brain: A neurocognitive model of body-ownership. Neuropsychologia 2010, 48, 703–712. [Google Scholar] [CrossRef]
- Millière, R. Looking for the self: Phenomenology, neurophysiology and philosophical significance of drug-induced ego dissolution. Front. Hum. Neurosci. 2017, 11, 245. [Google Scholar] [CrossRef]
- Millière, R. The varieties of selflessness. Phi. Mi. Sci. 2020, 1, 8. [Google Scholar] [CrossRef]
- Girn, M.; Christoff, K. Expanding the scientific study of self-experience with psychedelics. J. Conscious. Stud. 2018, 25, 131–154. [Google Scholar]
- Wittmann, M. The inner sense of time: How the brain creates a representation of duration. Nat. Rev. Neurosci. 2013, 14, 217–223. [Google Scholar] [CrossRef]
- Friston, K. Am I self-conscious? (Or does self-organization entail self-consciousness?). Front. Psychol. 2018, 9, 579. [Google Scholar] [CrossRef]
- Zahavi, D. Subjectivity and Selfhood; The MIT Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Metzinger, T. Why are dreams interesting for philosophers? The example of minimal phenomenal selfhood, plus an agenda for future research. Conscious. Res. 2013, 4, 746. [Google Scholar] [CrossRef]
- Metzinger, T. Minimal phenomenal experience: Meditation, tonic alertness, and the phenomenology of “pure” consciousness. Phi.Mi.Sci. 2020, 1, 7. [Google Scholar] [CrossRef]
- Singer, P. Practical Ethics; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Fingelkurts, A.A.; Fingelkurts, A.A. Longitudinal dynamics of 3-dimensional components of selfhood after severe traumatic brain injury: A qEEG case study. Clin. EEG Neurosci. 2017, 48, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.; Donnan, G.; Cameron, I.; Williams, J.H.G. Emotional self-awareness in autism: A meta-analysis of group differences and developmental effects. Autism 2021, 25, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Carls-Diamante, S. Know thyself: Bipolar disorder and self-concept. Philoso. Explor. 2022, 26, 110–126. [Google Scholar] [CrossRef]
- Ponticiello, M.N.; Chang, A.L.; Chang, R.J.; Mirza, S.; Martin, A. On being and having: A qualitative study of self-perceptions in bipolar disorder. Front. Psychiatry 2025, 15, 1509979. [Google Scholar] [CrossRef]
- Radovic, F. The sense of death and non-existence in nihilistic delusions. Phenomenol. Cogn. Sci. 2017, 16, 679–699. [Google Scholar] [CrossRef]
- Billon, A. Making sense of the Cotard Syndrome: Insights from the study of depersonalisation. Mind Lang. 2016, 31, 356–391. [Google Scholar] [CrossRef]
- Billon, A. Basic self-awareness: Lessons from the real world. Eur. J. Philos. 2017, 25, 732–763. [Google Scholar] [CrossRef]
- Newen, A. The embodied self, the pattern theory of self, and the predictive mind. Front. Psychol. 2018, 9, 2270. [Google Scholar] [CrossRef]
- Danilova, N.N. Psychophysiological Diagnostics of Functional States; Publishing House of Moscow State University: Moscow, Russia, 1992. [Google Scholar]
- Northoff, G.; Tumati, S. ‘Average is good, extremes are bad’—Non-linear inverted U-shaped relationship between neural mechanisms and functionality of mental features. Neurosci. Biobehav. Rev. 2019, 104, 11–25. [Google Scholar] [CrossRef]
- Daglish, M.R.; Nutt, D.J. Brain imaging studies in human addicts. Eur. Neuropsychopharmacol. 2003, 13, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Kiyatkin, E.A. Brain hyperthermia during physiological and pathological conditions: Causes, mechanisms, and functional implications. Curr. Neurovasc. Res. 2004, 1, 77–90. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Stress, adaptation, and disease: Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Le Moal, M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 2001, 24, 97–129. [Google Scholar] [CrossRef]
- Kelso, J.A.S. Dynamic Patterns: The Self-Organization of Brain and Behavior; MIT Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Kelso, J.A.S.; Engstrøm, D. The Complementary Nature; MIT Press: Cambridge, MA, USA, 2006. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fingelkurts, A.A.; Fingelkurts, A.A. Self, Me, or I? Unravelling the Triumvirate of Selfhood in Pathological Consciousness. Brain Sci. 2025, 15, 640. https://doi.org/10.3390/brainsci15060640
Fingelkurts AA, Fingelkurts AA. Self, Me, or I? Unravelling the Triumvirate of Selfhood in Pathological Consciousness. Brain Sciences. 2025; 15(6):640. https://doi.org/10.3390/brainsci15060640
Chicago/Turabian StyleFingelkurts, Alexander A., and Andrew A. Fingelkurts. 2025. "Self, Me, or I? Unravelling the Triumvirate of Selfhood in Pathological Consciousness" Brain Sciences 15, no. 6: 640. https://doi.org/10.3390/brainsci15060640
APA StyleFingelkurts, A. A., & Fingelkurts, A. A. (2025). Self, Me, or I? Unravelling the Triumvirate of Selfhood in Pathological Consciousness. Brain Sciences, 15(6), 640. https://doi.org/10.3390/brainsci15060640





