Exercise Improves Alzheimer’s Disease Phenotype in the TgF344-AD Rat, a Behavioral Time Course Study of Males and Females
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Progressive Treadmill Training Protocol
2.3. Behavioral Testing
2.3.1. Grip Strength
2.3.2. Rotarod
2.3.3. Morris Water Maze
2.4. AD Histopathology
2.5. Statistical Analysis
3. Results
3.1. General Characteristics
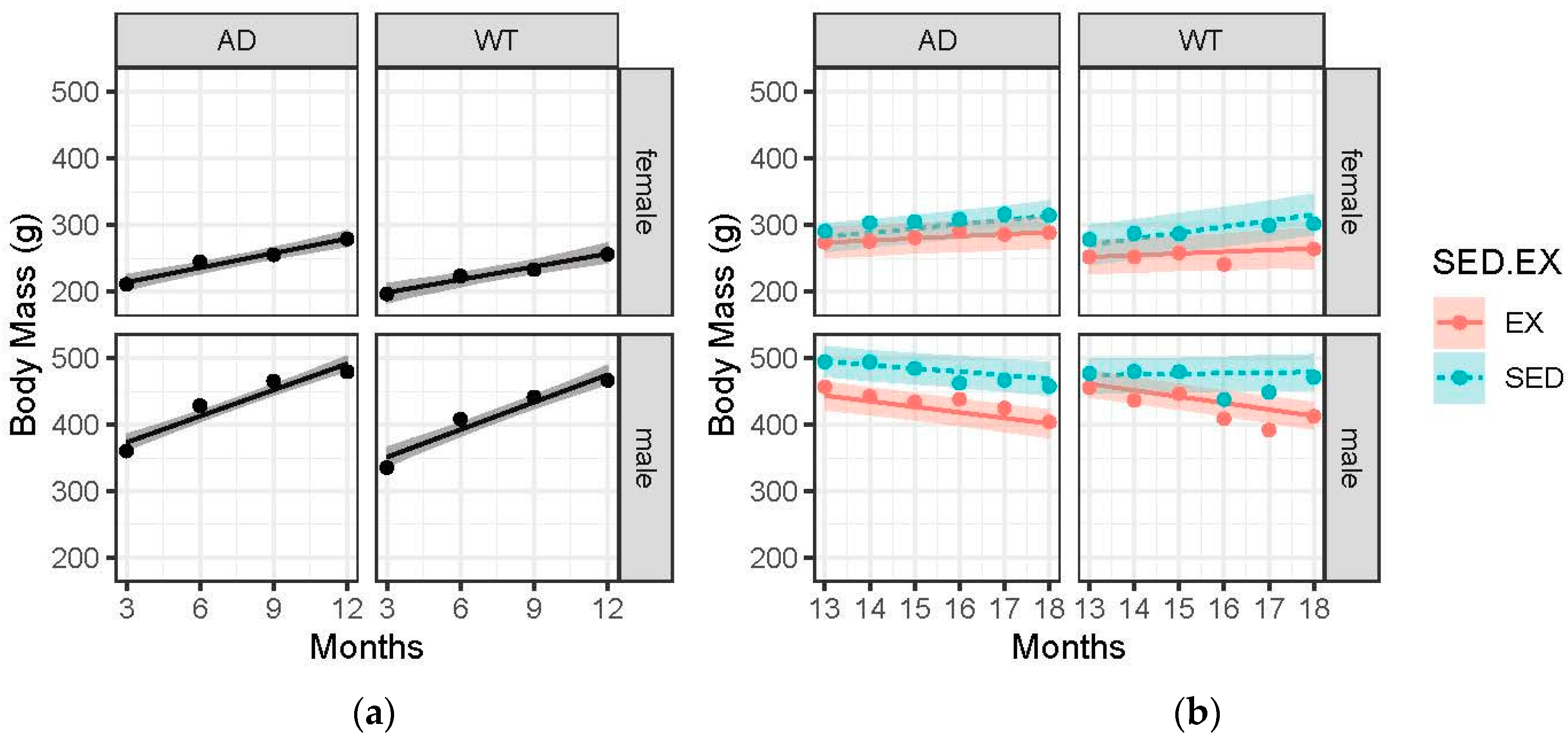
3.2. Body Mass Gains Are Blunted with Advanced Age and EX
3.3. Females Had Greater Grip Strength Relative to Body Mass Compared to Males, and Exercise Trended Towards Attenuating the Age-Related and AD-Induced Decline
3.4. Females (WT and AD) Performed Better on the Rotarod Compared to Males. Performance Pre-Intervention Declined with Age Across All Groups and Largely Reached an Asymptote in Post-Intervention Analysis
3.5. Memory in Female AD Animals Is More Likely to Be Positively Impacted by Exercise Compared to Male AD Animals
3.6. Exercise Significantly Decreased AD Pathology
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gustavsson, A.; Norton, N.; Fast, T.; Frölich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T.; et al. Global Estimates on the Number of Persons across the Alzheimer’s Disease Continuum. Alzheimers Dement. J. Alzheimers Assoc. 2023, 19, 658–670. [Google Scholar] [CrossRef] [PubMed]
- 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. J. Alzheimers Assoc. 2024, 20, 3708–3821. [CrossRef] [PubMed]
- Burns, J.M.; Cronk, B.B.; Anderson, H.S.; Donnelly, J.E.; Thomas, G.P.; Harsha, A.; Brooks, W.M.; Swerdlow, R.H. Cardiorespiratory Fitness and Brain Atrophy in Early Alzheimer Disease. Neurology 2008, 71, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Hu, L.; Morris, K.S.; White, S.M.; Wójcicki, T.R.; Mcauley, E.; Kramer, A.F. Aerobic Fitness Is Associated With Hippocampal Volume in Elderly Humans NIH Public Access. Hippocampus 2009, 19, 1030–1039. [Google Scholar] [CrossRef]
- Honea, R.A.; Thomas, G.P.; Harsha, A.; Anderson, H.S.; Donnelly, J.E.; Brooks, W.M.; Burns, J.M. Cardiorespiratory Fitness and Preserved Medial Temporal Lobe Volume in Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2009, 23, 188–197. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise Training Increases Size of Hippocampus and Improves Memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.-A. Exercise Builds Brain Health: Key Roles of Growth Factor Cascades and Inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef]
- Venturelli, M.; Scarsini, R.; Schena, F. Six-Month Walking Program Changes Cognitive and ADL Performance in Patients With Alzheimer. Am. J. Alzheimers Dis. Dementiasr 2011, 26, 381–388. [Google Scholar] [CrossRef]
- Bäckman, L.; Jones, S.; Berger, A.K.; Laukka, E.J.; Small, B.J. Cognitive Impairment in Preclinical Alzheimer’s Disease: A Meta-Analysis. Neuropsychology 2005, 19, 520–531. [Google Scholar] [CrossRef]
- Schroeter, M.L.; Stein, T.; Maslowski, N.; Neumann, J. Neural Correlates of Alzheimer’s Disease and Mild Cognitive Impairment: A Systematic and Quantitative Meta-Analysis Involving 1351 Patients. NeuroImage 2009, 47, 1196–1206. [Google Scholar] [CrossRef]
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean Diet and Risk for Alzheimer’s Disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Foster, P.P.; Rosenblatt, K.P.; Kuljiš, R.O. Exercise-Induced Cognitive Plasticity, Implications for Mild Cognitive Impairment and Alzheimer’s Disease. Front. Neurol. 2011, 2, 28. [Google Scholar] [CrossRef]
- Cheng, S.T.; Chow, P.K.; Song, Y.Q.; Yu, E.C.S.; Chan, A.C.M.; Lee, T.M.C.; Lam, J.H.M. Mental and Physical Activities Delay Cognitive Decline in Older Persons with Dementia. Am. J. Geriatr. Psychiatry 2014, 22, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for Primary Prevention of Alzheimer’s Disease: An Analysis of Population-Based Data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention CDC Behavioral Risk Factor Surveillance Survey. Available online: https://www.cdc.gov/brfss/data_documentation/index.htm (accessed on 4 September 2020).
- Eriksson, S.; Gustafson, Y.; Lundin-Olsson, L. Risk Factors for Falls in People with and without a Diagnose of Dementia Living in Residential Care Facilities: A Prospective Study. Arch. Gerontol. Geriatr. 2008, 46, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Suttanon, P.; Hill, K.D.; Said, C.M.; Williams, S.B.; Byrne, K.N.; LoGiudice, D.; Lautenschlager, N.T.; Dodd, K.J. Feasibility, Safety and Preliminary Evidence of the Effectiveness of a Home-Based Exercise Programme for Older People with Alzheimer’s Disease: A Pilot Randomized Controlled Trail. Clin. Rehabil. 2013, 27, 427–438. [Google Scholar] [CrossRef]
- Masliah, E.; Rockenstein, E.; Veinbergs, I.; Sagara, Y.; Mallory, M.; Hashimoto, M.; Mucke, L. β-Amyloid Peptides Enhance α-Synuclein Accumulation and Neuronal Deficits in a Transgenic Mouse Model Linking Alzheimer’s Disease and Parkinson’s Disease. Proc. Natl. Acad. Sci. USA 2001, 98, 12245–12250. [Google Scholar] [CrossRef]
- Buchman, A.S.; Wilson, R.S.; Boyle, P.A.; Bienias, J.L.; Bennett, D.A. Change in Motor Function and Risk of Mortality in Older Persons. J. Am. Geriatr. Soc. 2007, 55, 11–19. [Google Scholar] [CrossRef]
- Boyle, P.A.; Buchman, A.S.; Wilson, R.S.; Leurgans, S.E.; Bennett, D.A. Association of Muscle Strength With the Risk of Alzheimer Disease and the Rate of Cognitive Decline in Community-Dwelling Older Persons. Arch. Neurol. 2009, 66, 1339–1344. [Google Scholar] [CrossRef]
- Burns, J.M.; Johnson, D.K.; Watts, A.; Swerdlow, R.H.; Brooks, W.M. Reduced Lean Mass in Early Alzheimer Disease and Its Association With Brain Atrophy. Arch. Neurol. 2010, 67, 428–433. [Google Scholar] [CrossRef]
- Monteiro-Cardoso, V.F.; Castro, M.; Oliveira, M.M.; Moreira, P.I.; Peixoto, F.; A.Videira, R. Age-Dependent Biochemical Dysfunction in Skeletal Muscle of Triple-Transgenic Mouse Model of Alzheimer‘s Disease. Curr. Alzheimer Res. 2015, 12, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Sterniczuk, R.; Antle, M.C.; Laferla, F.M.; Dyck, R.H. Characterization of the 3xTg-AD Mouse Model of Alzheimer’s Disease: Part 2. Behavioral and Cognitive Changes. Brain Res. 2010, 1348, 149–155. [Google Scholar] [CrossRef]
- Cohen, R.M.; Rezai-Zadeh, K.; Weitz, T.M.; Rentsendorj, A.; Gate, D.; Spivak, I.; Bholat, Y.; Vasilevko, V.; Glabe, C.G.; Breunig, J.J.; et al. A Transgenic Alzheimer Rat with Plaques, Tau Pathology, Behavioral Impairment, Oligomeric Aβ, and Frank Neuronal Loss. J. Neurosci. 2013, 33, 6245–6256. [Google Scholar] [CrossRef]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid β-Protein Dimers Isolated Directly from Alzheimer Brains Impair Synaptic Plasticity and Memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Mcmillan, P.; Korvatska, E.; Poorkaj, P.; Evstafjeva, Z.; Robinson, L.; Greenup, L.; Leverenz, J.; Schellenberg, G.D.; Souza, I.D. Tau Isoform Regulation Is Region and Cell-Specific in Mouse Brain NIH Public Access. J. Comp. Neurol. 2008, 511, 788–803. [Google Scholar] [CrossRef] [PubMed]
- Hanes, J.; Zilka, N.; Bartkova, M.; Caletkova, M.; Dobrota, D.; Novak, M. Rat Tau Proteome Consists of Six Tau Isoforms: Implication for Animal Models of Human Tauopathies. J. Neurochem. 2009, 108, 1167–1176. [Google Scholar] [CrossRef]
- Rorabaugh, J.M.; Chalermpalanupap, T.; Botz-Zapp, C.A.; Fu, V.M.; Lembeck, N.A.; Cohen, R.M.; Weinshenker, D. Chemogenetic Locus Coeruleus Activation Restores Reversal Learning in a Rat Model of Alzheimer’s Disease. Brain J. Neurol. 2017, 140, 3023–3038. [Google Scholar] [CrossRef]
- Lopez, D.C.; White, Z.J.; Hall, S.E. Anxiety in Alzheimer’s Disease Rats Is Independent of Memory and Impacted by Genotype, Age, Sex, and Exercise. Alzheimers Dement. J. Alzheimers Assoc. 2024, 20, 3543–3550. [Google Scholar] [CrossRef]
- Morris, R. Morris Water Maze. Scholarpedia 2008, 3, 6315. [Google Scholar] [CrossRef]
- Rohn, T.T. Is Apolipoprotein E4 an Important Risk Factor for Vascular Dementia? Int. J. Clin. Exp. Pathol. 2014, 7, 3504–3511. [Google Scholar]
- Deacon, R.M.J. Measuring Motor Coordination in Mice. J. Vis. Exp. JoVE 2013, e2609. [Google Scholar] [CrossRef]
- Stover, K.R.; Campbell, M.A.; Van Winssen, C.M.; Brown, R.E. Analysis of Motor Function in 6-Month-Old Male and Female 3xTg-AD Mice. Behav. Brain Res. 2015, 281, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Garvock-de Montbrun, T.; Fertan, E.; Stover, K.; Brown, R.E. Motor Deficits in 16-Month-Old Male and Female 3xTg-AD Mice. Behav. Brain Res. 2019, 356, 305–313. [Google Scholar] [CrossRef]
- Nagase, T.; Tohda, C. Skeletal Muscle Atrophy-Induced Hemopexin Accelerates Onset of Cognitive Impairment in Alzheimer’s Disease. J. Cachexia Sarcopenia Muscle 2021, 12, 2199–2210. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Fang-Yu, L.; Hsiao, Y.-H. Myostatin Is Associated With Cognitive Decline in an Animal Model of Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 1984–1991. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kaneko, Y.; Sato, T.; Shimizu, S.; Kanetaka, H.; Hanyu, H. Sarcopenia and Muscle Functions at Various Stages of Alzheimer Disease. Front. Neurol. 2018, 9, 710. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.E.; Secher, M.; Gillette-Guyonnet, S.; van Kan, G.A.; Andrieu, S.; Nourhashemi, F.; Rolland, Y.; Vellas, B. Weight Loss and Rapid Cognitive Decline in Community-Dwelling Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2012, 28, 647–654. [Google Scholar] [CrossRef]
- Blanchard, J.; Wanka, L.; Tung, Y.C.; Cárdenas-Aguayo, M.D.C.; Laferla, F.M.; Iqbal, K.; Grundke-Iqbal, I. Pharmacologic Reversal of Neurogenic and Neuroplastic Abnormalities and Cognitive Impairments without Affecting Aβ and Tau Pathologies in 3xTg-AD Mice. Acta Neuropathol. 2010, 120, 605–621. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, Z.; Blanchard, J.; Dai, C.L.; Sun, S.; Lee, M.H.; Grundke-Iqbal, I.; Iqbal, K.; Liu, F.; Gong, C.X. A Non-Transgenic Mouse Model (Icv-STZ Mouse) of Alzheimer’s Disease: Similarities to and Differences from the Transgenic Model (3xTg-AD Mouse). Mol. Neurobiol. 2013, 47, 711–725. [Google Scholar] [CrossRef]
- Filali, M.; Lalonde, R.; Theriault, P.; Julien, C.; Calon, F.; Planel, E. Cognitive and Non-Cognitive Behaviors in the Triple Transgenic Mouse Model of Alzheimer’s Disease Expressing Mutated APP, PS1, and Mapt (3xTg-AD). Behav. Brain Res. 2012, 234, 334–342. [Google Scholar] [CrossRef]
- Andrade-Guerrero, J.; Martínez-Orozco, H.; Villegas-Rojas, M.M.; Santiago-Balmaseda, A.; Delgado-Minjares, K.M.; Pérez-Segura, I.; Baéz-Cortés, M.T.; Del Toro-Colin, M.A.; Guerra-Crespo, M.; Arias-Carrión, O.; et al. Alzheimer’s Disease: Understanding Motor Impairments. Brain Sci. 2024, 14, 1054. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yang, L.; Li, Y.; Dong, Y.A.N.; Yang, B.; Tucker, L.D.; Zong, X.; Zhang, Q. Effects of Exercise Training on Anxious-Depressive-like Behavior in Alzheimer Rat. Med. Sci. Sports Exerc. 2020, 52, 1456–1469. [Google Scholar] [CrossRef]
- Adlard, P.A.; Perreau, V.M.; Cotman, C.W. The Exercise-Induced Expression of BDNF within the Hippocampus Varies across Life-Span. Neurobiol. Aging 2005, 26, 511–520. [Google Scholar] [CrossRef]
- Um, H.-S.S.; Kang, E.-B.B.; Koo, J.-H.H.; Kim, H.-T.T.; Jin-Lee; Kim, E.-J.J.; Yang, C.-H.H.; An, G.-Y.Y.; Cho, I.-H.H.; Cho, J.-Y.Y. Treadmill Exercise Represses Neuronal Cell Death in an Aged Transgenic Mouse Model of Alzheimer’s Disease. Neurosci. Res. 2011, 69, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Cho, J.; Kang, H. Protective Effect of Exercise Training against the Progression of Alzheimer’s Disease in 3xTg-AD Mice. Behav. Brain Res. 2019, 374, 112105. [Google Scholar] [CrossRef]
- Hoveida, R.; Alaei, H.; Oryan, S.; Parivar, K.; Reisi, P. Treadmill Running Improves Spatial Memory in an Animal Model of Alzheimer’s Disease. Behav. Brain Res. 2011, 216, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Gaesser, G.A.; Hall, S.E.; Angadi, S.S.; Poole, D.C.; Racette, S.B. Increasing the Health Span: Unique Role for Exercise. J. Appl. Physiol. 2025, 138, 1285–1308. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.K.; Hohorst, A.A.; Mellert, S.M.; Loetz, E.C.; Baratta, M.V.; Greenwood, B.N. Female Rats Are More Responsive than Are Males to the Protective Effects of Voluntary Physical Activity against the Behavioral Consequences of Inescapable Stress. Stress Amst. Neth. 2023, 26, 2245492. [Google Scholar] [CrossRef]
- Tuscher, J.J.; Fortress, A.M.; Kim, J.; Frick, K.M. Regulation of Object Recognition and Object Placement by Ovarian Sex Steroid Hormones. Behav. Brain Res. 2015, 285, 140–157. [Google Scholar] [CrossRef]
- Guadagni, V.; Drogos, L.L.; Tyndall, A.V.; Davenport, M.H.; Anderson, T.J.; Eskes, G.A.; Longman, R.S.; Hill, M.D.; Hogan, D.B.; Poulin, M.J. Aerobic Exercise Improves Cognition and Cerebrovascular Regulation in Older Adults. Neurology 2020, 94, e2245–e2257. [Google Scholar] [CrossRef]
- Baker, L.D.; Frank, L.L.; Foster-Schubert, K.; Green, P.S.; Wilkinson, C.W.; McTiernan, A.; Plymate, S.R.; Fishel, M.A.; Watson, G.S.; Cholerton, B.A.; et al. Effects of Aerobic Exercise on Mild Cognitive Impairment: A Controlled Trial. Arch. Neurol. 2010, 67, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chu, J.M.T.; Yan, T.; Zhang, Y.; Chen, Y.; Chang, R.C.C.; Wong, G.T.C. Short-Term Resistance Exercise Inhibits Neuroinflammation and Attenuates Neuropathological Changes in 3xTg Alzheimer’s Disease Mice. J. Neuroinflammation 2020, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, G.; Shakerian, S.; Farbood, Y.; Ghanbarzadeh, M. Effects of Eight Weeks of Resistance Exercises on Neurotrophins and Trk Receptors in Alzheimer Model Male Wistar Rats. Basic Clin. Neurosci. 2021, 12, 349–359. [Google Scholar] [CrossRef]
- Hong, S.-G.; Kim, J.-H.; Jun, T.-W. Effects of 12-Week Resistance Exercise on Electroencephalogram Patterns and Cognitive Function in the Elderly With Mild Cognitive Impairment: A Randomized Controlled Trial. Clin. J. Sport Med. 2018, 28, 500. [Google Scholar] [CrossRef]
- Fiatarone Singh, M.A.; Gates, N.; Saigal, N.; Wilson, G.C.; Meiklejohn, J.; Brodaty, H.; Wen, W.; Singh, N.; Baune, B.T.; Suo, C.; et al. The Study of Mental and Resistance Training (SMART) Study—Resistance Training and/or Cognitive Training in Mild Cognitive Impairment: A Randomized, Double-Blind, Double-Sham Controlled Trial. J. Am. Med. Dir. Assoc. 2014, 15, 873–880. [Google Scholar] [CrossRef]
- Vital, T.M.; Hernández, S.S.S.; Pedroso, R.V.; Teixeira, C.V.L.; Garuffi, M.; Stein, A.M.; Costa, J.L.R.; Stella, F. Effects of Weight Training on Cognitive Functions in Elderly with Alzheimer’s Disease. Dement. Neuropsychol. 2012, 6, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Li, A.; Feng, T.; Liu, X.; You, Y.; Meng, F.; Wang, R.; Lu, J.; Zhang, C. The Benefits of Tai Chi and Brisk Walking for Cognitive Function and Fitness in Older Adults. PeerJ 2017, 5, e3943. [Google Scholar] [CrossRef]
- Saraulli, D.; Costanzi, M.; Mastrorilli, V.; Farioli-Vecchioli, S. The Long Run: Neuroprotective Effects of Physical Exercise on Adult Neurogenesis from Youth to Old Age. Curr. Neuropharmacol. 2017, 15, 519–533. [Google Scholar] [CrossRef]
- Peters, R. Ageing and the Brain. Postgrad. Med. J. 2006, 82, 84–88. [Google Scholar] [CrossRef]
- Hopkins, M.E.; Nitecki, R.; Bucci, D.J. Physical Exercise during Adolescence versus Adulthood: Differential Effects on Object Recognition Memory and Brain-Derived Neurotrophic Factor Levels. Neuroscience 2011, 194, 84–94. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, S.E.; White, Z.J.; Rohn, T.T.; Sudasinghe, K.H.; Young, M.E. Exercise Improves Alzheimer’s Disease Phenotype in the TgF344-AD Rat, a Behavioral Time Course Study of Males and Females. Brain Sci. 2025, 15, 631. https://doi.org/10.3390/brainsci15060631
Hall SE, White ZJ, Rohn TT, Sudasinghe KH, Young ME. Exercise Improves Alzheimer’s Disease Phenotype in the TgF344-AD Rat, a Behavioral Time Course Study of Males and Females. Brain Sciences. 2025; 15(6):631. https://doi.org/10.3390/brainsci15060631
Chicago/Turabian StyleHall, Stephanie E., Zachary J. White, Troy T. Rohn, Keshari H. Sudasinghe, and Michael E. Young. 2025. "Exercise Improves Alzheimer’s Disease Phenotype in the TgF344-AD Rat, a Behavioral Time Course Study of Males and Females" Brain Sciences 15, no. 6: 631. https://doi.org/10.3390/brainsci15060631
APA StyleHall, S. E., White, Z. J., Rohn, T. T., Sudasinghe, K. H., & Young, M. E. (2025). Exercise Improves Alzheimer’s Disease Phenotype in the TgF344-AD Rat, a Behavioral Time Course Study of Males and Females. Brain Sciences, 15(6), 631. https://doi.org/10.3390/brainsci15060631





