Perfecting Sensory Restoration and the Unmet Need for Personalized Medicine in Cochlear Implant Users: A Narrative Review
Abstract
1. Introduction
2. Methods
3. Sound Perception
4. Cochlear Implant Design and Technical Limitations
5. Music Perception in Cochlear Implant Users
6. Integrating Personalized Medicine into Cochlear Implantation
6.1. Hearing Loss Prevention
6.2. Pre-Implantation
6.3. Surgery
6.4. Post-Implantation
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | cochlear implant |
| CIS | continuous interleaved sampling |
| FSP | fine structure processing |
| DF | default fitting |
| ABF | Anatomy-based fitting |
| HHA | Hearing Handicap Inventory for Adults |
| NIOSH | National Institute for Occupational Safety and Health |
| AID | angular insertion depth |
| CT | computed tomography |
| ECAP | evoked compound action potential |
| MAPLaw | Mapping Law |
References
- Podury, A.; Jiam, N.T.; Kim, M.; Donnenfield, J.I.; Dhand, A. Hearing and sociality: The implications of hearing loss on social life. Front. Neurosci. 2023, 17, 1245434. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.J.A.; Lemos, S.M.A.; Goulart, L.M.H.F. Language development and its relation to social behavior and family and school environments: A systematic review. Codas 2016, 28, 470–479. [Google Scholar] [CrossRef]
- Shukla, A.; Harper, M.; Pedersen, E.; Goman, A.; Suen, J.J.; Price, C.; Reed, N.S. Hearing Loss, Loneliness, and Social Isolation: A Systematic Review. Otolaryngol. Head Neck Surg. 2020, 162, 622–633. [Google Scholar] [CrossRef]
- Viola, E.; Martorana, M.; Airoldi, C.; Meini, C.; Ceriotti, D.; De Vito, M.; De Ambrosi, D.; Faggiano, F. The role of music in promoting health and wellbeing: A systematic review and meta-analysis. Eur. J. Public Health 2023, 33, 738–745. [Google Scholar] [CrossRef]
- Cunningham, L.L.; Tucci, D.L. Hearing loss in adults. N. Engl. J. Med. 2017, 377, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Michels, T.C.; Duffy, M.Y.; Rogers, D.J. Hearing Loss in Adults: Differential Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 98–108. [Google Scholar]
- Davis, A.C.; Hoffman, H.J. Hearing loss: Rising prevalence and impact. Bull. World Health Organ. 2019, 97, 646. [Google Scholar] [CrossRef]
- GBD 2019 Hearing Loss Collaborators. Hearing loss prevalence and years lived with disability, 1990–2019: Findings from the global burden of disease study 2019. Lancet 2021, 397, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Jiam, N.T.; Li, C.; Agrawal, Y. Hearing loss and falls: A systematic review and meta-analysis. Laryngoscope 2016, 126, 2587–2596. [Google Scholar] [CrossRef]
- Griffiths, T.D.; Lad, M.; Kumar, S.; Holmes, E.; McMurray, B.; Maguire, E.A.; Billig, A.J.; Sedley, W. How Can Hearing Loss Cause Dementia? Neuron 2020, 108, 401–412. [Google Scholar] [CrossRef]
- Yeo, B.S.Y.; Song, H.J.J.M.D.; Toh, E.M.S.; Ng, L.S.; Ho, C.S.H.; Ho, R.; Merchant, R.A.; Tan, B.K.J.; Loh, W.S. Association of Hearing Aids and Cochlear Implants with Cognitive Decline and dementia: A systematic review and Meta-analysis. JAMA Neurol. 2022, 80, 134. [Google Scholar] [CrossRef] [PubMed]
- Niazi, Y.; Ejaz, B.; Muazzam, A. Impact of hearing impairment on psychological distress and subjective well-being in older adults. Pak. J. Med. Sci. 2020, 36, 1210–1215. [Google Scholar]
- Lye, J.; Delaney, D.S.; Leith, F.K.; Sardesai, V.S.; McLenachan, S.; Chen, F.K.; Atlas, M.D.; Wong, E.Y.M. Recent Therapeutic Progress and Future Perspectives for the Treatment of Hearing Loss. Biomedicines 2023, 11, 3347. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.C.; Reddy, V.; Launico, M.V.; Hamel, R.N. Neuroanatomy, Auditory Pathway; StatPearls [Internet]: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Oxenham, A.J. How We Hear: The Perception and Neural Encoding of Sound. Anny. Rev. Psychol. 2017, 4, 27–50. [Google Scholar]
- Doman, A.D.; Lin, F.R. Prevalence of Hearing Loss by Severity in the United States. Am. J. Public Health 2016, 106, 1820–1822. [Google Scholar]
- Sutton, A.E.; Krogmann, R.J.; Khalili, Y.A. Cochlear Implants; StatPearls: 2025.
- Crowson, M.G.; Semenov, Y.R.; Tucci, D.L.; Niparko, J.K. Quality of Life and Cost-Effectiveness os Cochlear Implants: A Narrative Review. Audiol. Neurootol. 2017, 22, 236–258. [Google Scholar] [CrossRef]
- Daher, G.S.; Kocharyan, A.; Dillon, M.T.; Carlson, M.L. Cochlear Implantation Outcomes in Adults with Single-Sided Deafness: A Systematic Review and Meta-Analysis. Otol. Neurotol. 2023, 44, 297–309. [Google Scholar] [CrossRef]
- Roy, A.T.; Jiradejvong, R.; Carver, C.; Limb, C.J. Assessment of sound quality perception in implant users during music listening. Otol. Neurotol. 2012, 33, 319–327. [Google Scholar] [CrossRef]
- McDermott, J.H. Music perception with cochlear implants: A review. Trends Amplif. 2004, 8, 49–82. [Google Scholar] [CrossRef]
- Limb, C.J.; Roy, A.T. Technological, biological, and acoustical constraints to music perception in cochlear implant users. Hear. Res. 2014, 308, 13–26. [Google Scholar] [CrossRef]
- Casale, J.; Kandkle, P.F.; Murray, I.V.; Murr, N.I. Physiology, Cochlear Function; StatPearls [Internet]: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Sheikh, A.; Bint-e-Zainab; Shabbir, K.; Imtiaz, A. Structure and Physiology of Human Ear Involved in Hearing; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Fettiplace, R. Hair cell transduction, tuning and synaptic transmission in the mammalian cochlea. Compr. Physiol. 2017, 7, 1197–1227. [Google Scholar] [CrossRef]
- Zeng, G.J.; Shannon, R.V. Loudness-coding mechanisms inferred from stimulation of the human auditory system. Science 1994, 264, 564–566. [Google Scholar] [CrossRef]
- Rohl, M.; Uppenkamp, S. Neural Coding of Sound Intensity and Loudness in the Human Auditory System. J. Assoc. Res. Otolaryngol. 2012, 13, 369–379. [Google Scholar] [CrossRef]
- Oxenham, A.J. Pitch Perception. J. Neurosci. 2012, 32, 13335–13338. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gan, L.; Huang, X. A Review of Research on Neurocognition for Timbre Perception. Front. Psychol. 2022, 13, 869475. [Google Scholar] [CrossRef]
- Oxenham, A.J. Revisiting place and temporal theories of pitch. Acoust. Sci. Technol. 2014, 34, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, D.D. A cochlear frequency-position function for several species—29 years later. J. Acoust. Soc. Am. 1990, 87, 2592–2605. [Google Scholar] [CrossRef] [PubMed]
- Dhanasingth, A. Cochlear duct length along the outer wall vs organ of corti: Which one is relevant for the electrode array length selection and frequency mapping using Greenwood function? World J. Otorhinolaryngol. Head Neck Surg. 2018, 5, 117–121. [Google Scholar] [CrossRef]
- Joris, P.X.; Smith, P.H. The volley theory and the spherical cell puzzle. Neuroscience 2008, 154, 65–76. [Google Scholar] [CrossRef]
- Palmer, A.R.; Russell, I.J. Phase-locking in the cochlear nerve of the guinea-pig and its relation to the receptor potential of inner hair-cells. Hear. Res. 1986, 24, 1–15. [Google Scholar] [CrossRef]
- Oxenham, A.J.; Micheyl, C.; Keebler, M.V.; Loper, A.; Santurette, S. Pitch perception beyond the traditional existence region of pitch. Proc. Natl. Acad. Sci. USA 2011, 108, 7629–7634. [Google Scholar] [CrossRef]
- Rosen, S. Temporal information in speech: Acoustic, auditory and linguistic aspects. Philos. Trans. R Soc. Lond. B Biol. Sci. 1992, 336, 367–373. [Google Scholar] [PubMed]
- D’Alessandro, H.D.; Ballantyne, D.; Boyle, P.J.; De Seta, E.; DeVincentiis, M.; Mancini, P. Temporal Fine Structure Processing, Pitch, and Speech Perception in Adult Cochlear Implant Recipients. Ear Hear. 2018, 39, 679–686. [Google Scholar] [CrossRef]
- Adunka, O.; Kiefer, J. How Does a Cochlear Implant Speech Processor Work? LaryngoRhinoOtologie 2005, 84, 841–851. [Google Scholar] [CrossRef]
- Deep, J.L.; Dowling, E.M.; Jethanamest, D.; Carlon, M.L. Cochlear Implantation: An Overview. J. Neurol. Surg. B Skull Base. 2018, 80, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Gibron, P.; Boyd, P. Optimal electrode design: Straight versus perimodiolar. Eur. Ann. Otorhinolaryngol. HN Diseases 2016, 133, S63–S65. [Google Scholar]
- Dhanasingth, A.; Jolly, C. An overview of cochlear implant electrode designs. Hear. Res. 2017, 356, 93–103. [Google Scholar] [CrossRef]
- Spahr, A.J.; Dorman, M.F. Performance of subjects fit with the Advanced Bionics CII and Nucleus 3G cochlear implant devices. Arch. Otolaryngol. Head Neck Surg. 2024, 130, 624–628. [Google Scholar] [CrossRef]
- Curtis, D.P.; Baumann, A.N.; Jeyakumar, A. Variation in cochlear size: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2023, 171, 111659. [Google Scholar] [CrossRef]
- Efterkharian, A.; Eftekharian, K.; Mokari, N.; Fazel, M. Cochlear implantation in incomplete partition type I. Eur. Arch. Otorhinolaryngol. 2019, 276, 2763–2768. [Google Scholar] [CrossRef]
- Swords, C.; Geerardyn, A.; Zhu, M.; O’Malley, J.T.; Wu, P.; Arenberg, J.G.; Podury, A.; Brassett, C.; Bance, M.; Quesnel, A.M. Incomplete Partition Type II Cochlear Malformations: Delineating the Three-Dimensional Structure from Digitized Human Histopathological Specimens. Otol. Neurotol. 2023, 44, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Jiam, N.T.; Podury, A.; Quesnel, A.M.; Handzel, O. Worldwide differences in surgeon intraoperative practices for cochlear implantation. Cochlear Implants Int. 2024, 25, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.S.; Finney, C.C.; Lawson, D.T.; Wolford, R.D.; Zerbi, M. Design and evaluation of a continuous interleaved sampling (CIS) processing strategy for multichannel cochlear implants. J. Rehabil. Res. Dev. 1993, 30, 110–116. [Google Scholar] [PubMed]
- Magnusson, L. Comparison of the fine structure processing (FS) strategy and the CIS strategy used in the MED-EL cochlear implant system: Speech intelligibility and music sound quality. Int. J. Audio. 2011, 50, 278–287. [Google Scholar] [CrossRef]
- Fu, Q.; Shannon, R.V. Frequency mapping in cochlear implants. Ear Hear. 2002, 23, 339–348. [Google Scholar] [CrossRef]
- Perreau, A.; Tyler, R.S.; Witt, S.A. The Effect of Reducing the Number of Electrodes on Spatial Hearing Tasks for Bilateral Cochlear Implant Recipients. J. Am. Acad. Audio. 2010, 21, 110–120. [Google Scholar] [CrossRef]
- Creff, G.; Liboux, N.B.; Coudert, P.; Bourdon, H.; Pean, V.; Wallaert, N.; Lamberg, C.; Godey, B. Tonotopic and Default Frequenfcy Fitting for Perception in Cochlear Implant Recipients: A Randomized Clinical Trial. JAMA Otolaryngol. Head Neck Surg. 2024, 150, 960–968. [Google Scholar] [CrossRef]
- Reiss, L.A.J.; Gantz, B.J.; Turner, C.W. Cochlear Implant Speech Processor Frequency Allocations May Influence Pitch Perception. Otol. Neurotol. 2008, 29, 160–167. [Google Scholar] [CrossRef]
- Nie, K.; Zeng, F.G.; Barco, A. Spectral and temporal cues in cochlear implant speech perception. Ear Hear. 2005, 102, 2293–2298. [Google Scholar] [CrossRef]
- Carroll, J.; Zeng, F.G. Fundamental frequency discrimination and speech perception in noise in cochlear implant simulations. Hear. Res. 2007, 231, 42–53. [Google Scholar] [CrossRef]
- Rosen, S.; Faulkner, A.; Wilkinson, L. Adaptation by normal listeners to upward spectral shifts of speech: Implications for cochlear implants. J. Acoust. Soc. Am. 1999, 106, 3629–3636. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.J.; Shannon, R.V. Effects of electrode configuration and frequency allocation on vowel recognition with the Nucleus-22 cochlear implant. Ear Hear. 1999, 20, 332–344. [Google Scholar] [CrossRef]
- Nimmons, G.L.; Kang, R.S.; Drennan, W.R.; Longnion, J.; Ruffin, C.; Worman, T.; Yueh, B.; Rubinstein, J.T. Clinical Assessment of Music Perception in Cochlear Implant Listeners. Otol. Neurotol. 2007, 29, 149–155. [Google Scholar] [CrossRef]
- Hwa, T.P.; Wen, C.Z.; Ruckenstein, M.J. Assessment of music experience after cochlear implantation: A review of currrent tools and their utilization. World J. OHNS 2021, 7, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Nimmons, G.L.; Drennan, W.; Longnion, J.; Ruffin, C.; Nie, K.; Won, J.H.; Worman, T.; Yueh, B.; Rubinstein, J. Development and validation of the University of Washington Clinical Assessment of Music Perception test. Ear Hear. 2009, 30, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, J.B.; Mancuso, D.; Cheng, M.Y. Development of a clinical test of musical perception: Appreciation of music in cochlear implantees (AMICI). J. Am. Acad. Audiol. 2008, 19, 56–81. [Google Scholar] [CrossRef]
- Shafiro, V.; Hebb, M.; Walker, C.; Oh, J.; Hsiao, Y.; Brown, K.; Sheft, S.; Li, Y.; Vasil, K.; Moberly, A.C. Development of the Basic Auditory Skills Evaluation Battery for Online Testing of Cochlear Implant Listeners. Am. J. Audiol. 2020, 29, 577–590. [Google Scholar] [CrossRef]
- Brockmeier, S.J.; Fitzgerald, D.; Searle, O. The MuSIC perception test: A novel battery for testing music perception of cochlear implant users. Cochlear Implants Int. 2011, 12, 10–20. [Google Scholar] [CrossRef]
- Kong, Y.Y.; Cruz, R.; Jones, J.A.; Zeng, F.G. Music perception with temporal cues in acoustic and electric hearing. Ear Hear. 2004, 25, 173–185. [Google Scholar] [CrossRef]
- Bigand, E.; Parncutt, R.; Lerdahl, F. Perception of musical tension in short chord sequences: The influence of harmonic function, sensory dissonance, horizontal motion, and musical training. Percept. Psychophys. 1996, 58, 121–141. [Google Scholar] [CrossRef]
- Spangmose, S.; Hjortkjaer, J.; Marozeau, J. Perception of Musical Tension in Cochlear Implant Listeners. Front. Neurosci. 2019, 13, 987. [Google Scholar] [CrossRef]
- Hopyan, T.; Peretz, I.; Chan, L.P.; Papsin, B.C.; Gorgon, K.A. Children Using Cochlear Implants Capitalize on Acoustical hearing for Music Perception. Front. Psychol. 2012, 3, 425. [Google Scholar] [CrossRef] [PubMed]
- Walia, A.; Ortmann, A.J.; Lefler, S.; Holden, T.A.; Puram, S.V.; Herzog, J.A.; Buchman, C.A. Electrocochleography-Based Tonotopic Map: I. Place Coding of the Human Cochlea with Hearing Loss. Ear Hear. 2025, 46, 253–264. [Google Scholar] [CrossRef]
- Dristakis, G.; Frosolini, A.; Lam, C. Thr Music-Related Quality of Life Measure (MuRQoL):A Scoping Review of Its Validation and Application. Audiol. Res. 2025, 15, 26. [Google Scholar]
- Looi, V.; Gfeller, K.; Driscoli, V.D. Music Appreciation and Training for Cochlear Implant Recipients: A Review. Semin. Hear. 2012, 33, 307–334. [Google Scholar]
- Preoteau, C.; Chen, S.Y.; Lalwani, A.K. Music enjoyment with cochlear implantation. Aurix Nasus Larynx 2018, 45, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Bruns, L.; Murbe, D.; Hanhe, A. Understanding music with cochlear implants. Sci. Rep. 2016, 6, 32026. [Google Scholar] [CrossRef]
- Jiam, N.T.; Caldwell, M.T.; Limb, C.J. What Does Music Sound Like for a Cochlear Implant User? Otol. Neurotol. 2017, 38, e240–e247. [Google Scholar] [CrossRef]
- Hassanzadeh, S.; Farhadi, M.; Daneshi, A.; Emamdjomeh, H. The effects of age on auditory speech perception development in cochlear-implanted prelingually deaf children. Otolaryngol. Head Neck Surg. 2002, 126, 524–527. [Google Scholar] [CrossRef]
- Nemer, J.S.; Kohlberg, G.D.; Mancuso, D.M.; Griffin, B.M.; Certo, M.V.; Chen, S.Y.; Chun, M.B.; Spitzer, J.B.; Lalwani, A.K. Reduction of the Harmonic Series Influences Musical Enjoyment with Cochlear Implants. Otol. Neurotol. 2018, 38, 31–37. [Google Scholar] [CrossRef]
- Wright, R.; Uchanski, R.M. Music Perception and Appraisal: Cochlear Implant Users and Simulated DI Listening. J. Am. Acad. Audiol. 2012, 23, 350–379. [Google Scholar] [CrossRef] [PubMed]
- Cartocci, G.; Maria Serena Inguscio, B.; Giorgi, A.; Vozzi, A.; Leone, C.A.; Grassia, R.; Di Nardo, W.; Di Cesare, T.; Fetoni, A.R.; Freni, F.; et al. Music in noise recognition: An EEG study of listening effort in cochlear implant users and normal hearing controls. PLoS ONE 2023, 18, e0288461. [Google Scholar] [CrossRef] [PubMed]
- Matziorinis, A.M.; Koelsch, S. The promise of music therapy for Alzheimer’s disease: A review. Ann. N. Y. Acad. Sci. 2022, 1516, 11–17. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, Z.; Zhou, H.; Ye, P. Effects of music theory on depression: A meta-analysis of randomized controlled trials. PLoS ONE 2020, 15, e0240862. [Google Scholar] [CrossRef] [PubMed]
- Sharda, M.; Tuerk, C.; Chowdhury, R.; Jamey, K.; Foster, N.; Custo-Blanch, M.; Tan, M.; Nadig, A.; Hyde, K. Music improves social communication and auditory-motor connectivity in children with autism. Transl. Psychiatry 2018, 8, 231. [Google Scholar] [CrossRef]
- Ivanova, E.; Panayotova, T.; Grechenliev, I.; Peshev, B.; Kolchakova, P.; Milanova, V. A Complex Combination Therapy for a Complex Disease-Neuroimaging Evidence for the Effect of Music Therapy in Schizophrenia. Front. Psychiatry 2022, 13, 795344. [Google Scholar] [CrossRef]
- Riedl, H.; Else, B.A.; Grunhaus, C.; Holck, U. Economic Evaluations of Music Theory and Other Music-Based Interventions: A Scoping Review. J. Music. Ther. 2025, 62, thae023. [Google Scholar] [CrossRef]
- Thomson, R.S.; Auduong, P.; Miller, A.T. Hearing loss as a risk factor for dementia: A systematic review. Laryngoscope Investig. Otolaryngol. 2017, 2, 69–79. [Google Scholar] [CrossRef]
- Blazer, D.G. Hearing Loss: The Silent Risk for Psychiatric Disorders in Late Life. Clin. Geriatr. Med. 2020, 36, 201–209. [Google Scholar] [CrossRef]
- Dingle, G.A.; Sharman, L.S.; Bauer, Z.; Beckman, E.; Broughton, M.; Bunzli, E.; Davidson, R.; Draper, G.; Fairley, S.; Farrell, C.; et al. How Do Music Activities Affect Health and Well-Being? A Scoping Review of Studies Examining Psychosocial Mechanisms. Front Psychol. 2021, 12, 1713818. [Google Scholar] [CrossRef]
- Prasad, K.; Borre, E.D.; Dillard, L.K.; Dillard, L.K.; Ayer, A.; Der, C.; Bainbridge, K.E.; McMahon, C.M.; Tucci, D.L.; Wilson, B.S.; et al. Priorities for hearing loss prevention and estimates of global cause-specific burdens of hearing loss: A systematic rapid review. Lancet Glob. Health. 2024, 12, e217–e225. [Google Scholar] [CrossRef] [PubMed]
- Dehankar, S.S.; Gaurkar, S.S. Impact on Hearing Due to Prolonged Use of Audio Devices: A Literature Review. Cureus 2022, 12, e31425. [Google Scholar] [CrossRef] [PubMed]
- Themann, C.L.; Masterson, E.A.; Peterson, J.S.; Murphy, W.J. Preventing Occupational Hearing Loss: 50 Years of Research and Recommendations from the National Institute for Occupational Safety and Health. Semin. Hear. 2023, 44, 351–393. [Google Scholar] [CrossRef]
- Zhu, D.T. Encouraging Hearing Loss Prevention in Music Listeners Using Personalized Technology: Questionnaire Study. JMIR Form. Res. 2022, 6, e24903. [Google Scholar] [CrossRef]
- Cha, J.; Smukler, S.R.; Chung, Y.; House, R.; Bogoch, I.I. Increase in use of protective earplugs by Rock and Roll concert attendees when provided for free at concert venues. Int. J. Audiol. 2015, 54, 984–986. [Google Scholar] [CrossRef]
- Bennett, R.J.; Conway, N.; Fletcher, S.; Barr, C. The role of the general practitioner in managing age-related hearing loss: A scoping review. Am. J. Audiol. 2020, 29, 265–289. [Google Scholar] [CrossRef]
- Wallhagen, M.I.; Pettengill, E. Hearing impairment: Significant but underassessed in primary care settings. J. Gerontol. Nurs. 2008, 34, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.L.; Nassiri, A.M.; Marinelli, J.P.; Lohse, C.M.; Sydlowski, S.A. Hearing Health Collaborative. Awareness, perceptions, and literacy surrounding hearing loss and hearing rehabilitation among the adult population in the United States. Otol. Neurotol. 2020, 43, e323–e330. [Google Scholar] [CrossRef]
- Cohen, S.M.; Labadie, R.F.; Haynes, D.S. Primary care approach to hearing loss: The hidden disability. Ear Nose Throat J. 2005, 84, 26–44. [Google Scholar] [CrossRef]
- Sydlowski, S.S.; Marinelli, J.P.; Lohse, C.M.; Carlson, M.L. Hearing Health Perceptions and Literacy Among Primary Healthcare Providers in the United States: A National Cross-Sectional Survey. Otol. Neurotol. 2002, 43, 894–899. [Google Scholar] [CrossRef]
- Ritter, C.R.; Barker, B.A.; Scharp, K.M. Using attribution theory to explore the reasons adults with hearing loss do not use their hearing aids. PLoS ONE 2020, 15, e0238468. [Google Scholar] [CrossRef] [PubMed]
- Zazove, P.; Plegue, M.A.; McKee, M.M.; DeJonckheere, M.; Kileny, P.R.; Schleicher, L.S.; Green, L.A.; Sen, A.; Rapai, M.E.; Mulhem, E. Effective Hearing Loss Screening in Primary Care: The Early Auditory Referral-Primary Care Study. Ann. Fam. Med. 2020, 1896, 520–527. [Google Scholar] [CrossRef]
- Nassiri, A.M.; Sorkin, D.L.; Carlson, M.L. Current estimates of cochlear implant utilization in the United States. Otol. Neurotol. 2022, 43, e558–e562. [Google Scholar] [CrossRef]
- Hay-McCutcheon, M.J.; Hyams, A.; Yang, X.; Parton, J. Hearing loss and social support in urban and rural communities. Int. J. Audiol. 2018, 57, 610–617. [Google Scholar] [CrossRef]
- Shan, A.; Ting, J.S.; Price, C.; Goman, A.M.; Willink, A.; Reed, N.S.; Nieman, C.L. Hearing loss and employment: A systematic review of the association between hearing loss and employment among adults. J. Laryngol. Otol. 2020, 134, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.M.; Beer, J.; Bergeson, T.R.; Chin, S.B.; Pisoni, D.B.; Miyamoto, R.T. The ear is connected to the brain: Some new directions in the study of children with cochlear implants at Indiana University. J. Am. Acad. Audiol. 2012, 23, 446–463. [Google Scholar] [CrossRef]
- Friedmann, N.; Rusou, D. Critical period for first language: The crucial role of language input during the first year of life. Curr. Opin. Neurob. 2015, 35, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Wiefferink, C.H.; Rieffe, C.; Ketelaar, L.; Frijns, J.H.M. Predicting social functioning in children with a cochlear implant and in normal-hearing children: The role of emotion regulation. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Polat, F. Factors affecting psychosocial adjustment of deaf students. J. Deaf Stud. 2003, 8, 325–339. [Google Scholar] [CrossRef]
- Powell, W.; Jacobs, J.A.; Noble, W.; Bush, M.L.; Snell-Rood, C. Rural Adult Perspectives on Impact of Hearing Loss and Barriers to Care. J. Community Health 2019, 44, 668–674. [Google Scholar] [CrossRef]
- Tan, L.; Holland, S.K.; Deshpande, A.K.; Chen, Y.; Choo, D.I.; Lu, L.J. A semi-supervised Support Vector Machine model for predicting the language outcomes following cochlear implantation based on pre-implant brain fMRI imaging. Brain Behav. 2015, 5, e000391. [Google Scholar] [CrossRef] [PubMed]
- Patro, A.; Perken, E.L.; Ortega, C.A.; Lindquist, N.R.; Dawant, B.M.; Gifford, R.; Haynes, D.S.; Chowdhury, N. Machine Learning Approach for Screening Cochlear Implant Candidates: Comparing with the 60/60 Guideline. Otol. Neurotol. 2023, 44, e486–e491. [Google Scholar] [CrossRef]
- Machine Learning to Predict Adult Cochlear Implant Candidacy. Available online: https://link.springer.com/article/10.1007/s40136-024-00511-7 (accessed on 1 March 2025).
- Kalkman, R.K.; Briaire, J.J.; Dekker, D.M.T.; Frijns, J.H.M. Place Pitch versus Electrode Location in a Realistic Computational Model of the Implanted Human Cochlea. Hear. Res. 2014, 315, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Kurz, A.; Muller-Graff, F.; Hagen, R.; Rak, K. One Click Is Not Enough: Anatomy-Based Fitting in Experienced Cochlear Implant Users. Otol. NeurOtol. 2022, 43, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Gatto, A.; Tofanelli, M.; Costariol, L.; Rizzo, S.; Borsetto, D.; Gardenal, N.; Uderzo, F.; Boscolo-Rizzo, P.; Tirelli, G. Otological Planning Software—OTOPLAN: A Narrative Literature Review. Audiol. Res. 2023, 13, 791–801. [Google Scholar] [CrossRef]
- Paouris, D.; Kunzo, S.; Goljerova, I. Validation of Automatic Cochlear Measurements Using OTOPLAN Software. J. Pers. Med. 2023, 13, 805. [Google Scholar] [CrossRef]
- Breitsprecer, T.; Mlynski, R.; Volter, C.; Van de Heyning, P.; Van Rompaey, V.; Dazert, S.; Weiss, N.M. Acuracy of Preoperative Cochlear Duct Length Estimation and Angular Insertion Depth Prediction. Otol. Neurotol. 2023, 44, e566–e571. [Google Scholar] [CrossRef]
- Weller, T.; Timm, M.E.; Lenarz, T. Cochlear coverage with lateral wall cochlear electrode arrays affects post-operative speech recognition. PLoS ONE 2023, 18, e0287450. [Google Scholar] [CrossRef]
- Kurz, A.; Herrmann, D.; Muller-Graff, F.; Voelker, J.; Hackenberg, S.; Rak, K. Anatomy-based fitting improves speech perception in noise for cochlear implant recipients with single-sided deafness. Eur. Arch. Otorhinolaryngol. 2024, 282, 467–479. [Google Scholar] [CrossRef]
- Kurz, A.; Herrmann, D.; Hagen, R.; Rak, K. Using Anatomy-Based Fitting to Reduce Frequency-to-Place Mismatch in Experienced Bilateral Cochlear Implant Users: A Promising Concept. J. Pers. 2023, 13, 1109. [Google Scholar] [CrossRef]
- Heitkotter, F.S.; Kramer, B.A.; Beule, A.G.; Ruack, C. Influence of Anatomy-Based Fitting in Cochlear Implant Users on Music Perception Using the Montreal Battery of Evaluation of Amusia. Otol. Neurotol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Shin, Y.; Chung, Y. Cochlear Implant Surgery Through Round Window Approach is Always Possible. Ann. Otol. Rhinol. Larynol. 2019, 128 (Suppl S6), 38S–44S. [Google Scholar] [CrossRef] [PubMed]
- Colletti, V.; Fiorino, F.G.; Carner, M.; Sacchetto, L.; Giarbini, N. New approach for cochlear implantation: Cochleostomy through the middle fossa. Otolaryngol. Head Neck Surg. 2000, 123, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Fayad, J.N.; Doherty, K.; Linthicum, F.H., Jr. Round Window versus Cochleostomy Technique in Cochlear Implantation: Histological Findings. Otol. Neurotol. 2012, 33, 1181–1187. [Google Scholar] [CrossRef]
- Jiam, N.T.; Limb, C.J. The impact of round window s cochleostomy surgical approaches on interscalar excursion in the cochlea: Preliminary results from a flat-panel computed tomography study. World J. Otorhinolaryngol. Head Neck Surg. 2016, 2, 142–147. [Google Scholar] [CrossRef]
- Jiam, N.T.; Jiradejvong, P.; Pearl, M.S.; Limb, C.J. The Effect of Round Widnwo s Cochleostomy Surgical Approaches on Cochlear Implant Electrode Position: A Flat-Panel Computed Tomography Study. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 873–880. [Google Scholar] [CrossRef]
- Van Wermeskerken, G.K.; Van Oplhen, A.F.; Graamans, K. Imaging of electrode position in relation to electrode functioning after cochlear implantation. Eur. Arch. Otorhinolaryngol. 2009, 266, 1527–1531. [Google Scholar] [CrossRef]
- Macias, A.R.; Zaballos, M.T.P.; De Miguel, A.R.; Paz, J.C. Importance of Perimodiolar Electrode Position for Psychoacoustic Discrimination in Cochlear Implantation. Otol. Neurotol. 2017, 38, e429–e437. [Google Scholar] [CrossRef]
- Peters, J.P.M.; Bennik, E.; Van Zanten, G.A. Comparison of Place-versus-Pitch Mismatch between a Perimodiolar and Lateral Wall Cochelar Implant Electrode Array in Patients with Single Sided Deafness and a Cochlear Implant. Audiol. Neurootol. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Jiam, N.T.; Gilbert, M.; Cooke, D.; Jiradejvong, P.; Barrett, K.; Caldwell, M.; Limb, C.J. Association Between Flat-Panel Computed Tomography Imaging-Guided Place-Pitch Mapping and Speech and Pitch Perception in Cochlear Implant Users. JAMA Otolaryngol. Head Neck Surg. 2018, 145, 109–116. [Google Scholar] [CrossRef]
- Arnolder, C.; Riss, D.; Brunner, M.; Durisin, M.; Baumgartner, W.-D.; Hamzavi, J.-S. Speech and music perception with new fine structure speech coding strategy: Preliminary results. Acta Otolarngol. 2007, 127, 1298–1303. [Google Scholar] [CrossRef]
- Berenstein, C.K.; Mens, L.H.M.; Mulder, J.S.J.; Vanpoucke, F.J. Current steering and current focusing in cochlear implants: Comparison of monopolar, tripolar, and vritual channel electrode configurations. Ear Hear. 2008, 29, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.H.; Tsao, Y.; Lu, X.; Chen, F.; Su, Y.T.; Chen, K.C.; Lee, C.H. Deep-learning based noise reduction approach to improve speech intelligibility for cochlear implant recipients. Ear Hear. 2018, 39, 795–809. [Google Scholar] [CrossRef]
- Henry, F.; Glavin, M.; Jones, E. Noise Reduction in Cochlear Implant Signal Processing: A Review and Recent Developments. IEEE Rev. Biomed. Eng. 2023, 16, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Kashio, A.; Yamasoba, T. Prediction of Cochlear Implant Fitting by Machine Learning Techniques. Otol. Neurotol. 2024, 45, 643–650. [Google Scholar] [CrossRef]
- Vaarenberg, B.; Smits, C.; De Ceulaer, G.; Zir, E.; Harman, S.; Jaspers, N.; Tam, Y.; Dillon, M.; Wesarg, T.; Martin-Bonniot, D.; et al. Cochlear Implant Programming: A Global Survey on the State of the Art. Sci. World J. 2014, 2014, 501738. [Google Scholar] [CrossRef] [PubMed]
- Boyd, P.J. Effects of programming threshold and maplaw settings on acoustic thresholds and speech discrimination with the MED-EL COMBI 40+ cochlear implant. Ear Hear. 2006, 27, 608–618. [Google Scholar] [CrossRef]
- Gilbert, M.L.; Deroche, M.L.D.; Jiradejvong, P.; Barrett, K.C.; Limb, C.J. Cochlear Implant Compression Optimization for Sound Quality in MED-EL Users. Ear Hear. 2022, 43, 862–873. [Google Scholar] [CrossRef]
- Boyer, J.; Stohl, J. MELUDIA–Online music training for cochlear implant users. Cochlear Implants Int. 2022, 23, 257–269. [Google Scholar] [CrossRef]
- Jiam, N.T.; Deroche, M.L.; Jiradevjong, P.; Limb, C.J. A Randomized Controlled Crossover Stud on the Impact of Online Music Training on Pitch and Timbre Perception in Cochlear Implant Users. J. Assoc. Res. Otolaryngol. 2019, 20, 247–262. [Google Scholar] [CrossRef]
- Smith, L.; Bartel, L.; Joglekar, S.; Chen, S. Musical Rehabilitation in Adult Cochlear Implant Recipients with a Self-administered Software. Otol. Neurogol. 2017, 38, e262–e267. [Google Scholar] [CrossRef] [PubMed]
- Lerousseau, J.P.; Hidalgo, C.; Schol, D. Musical Training for Auditory Rehabilitation in Heaing Loss. J. Clin. Med. 2010, 9, 1058. [Google Scholar] [CrossRef] [PubMed]
- Frosolini, A.; Franz, L.; Badin, G.; Mancuso, A.; de Filippis, C.; Marioni, G. Quality of life improvement in Cochlear implant outpatients: A non-randomized clinical trial of an auditory music training program. Int. J. Audiol. 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
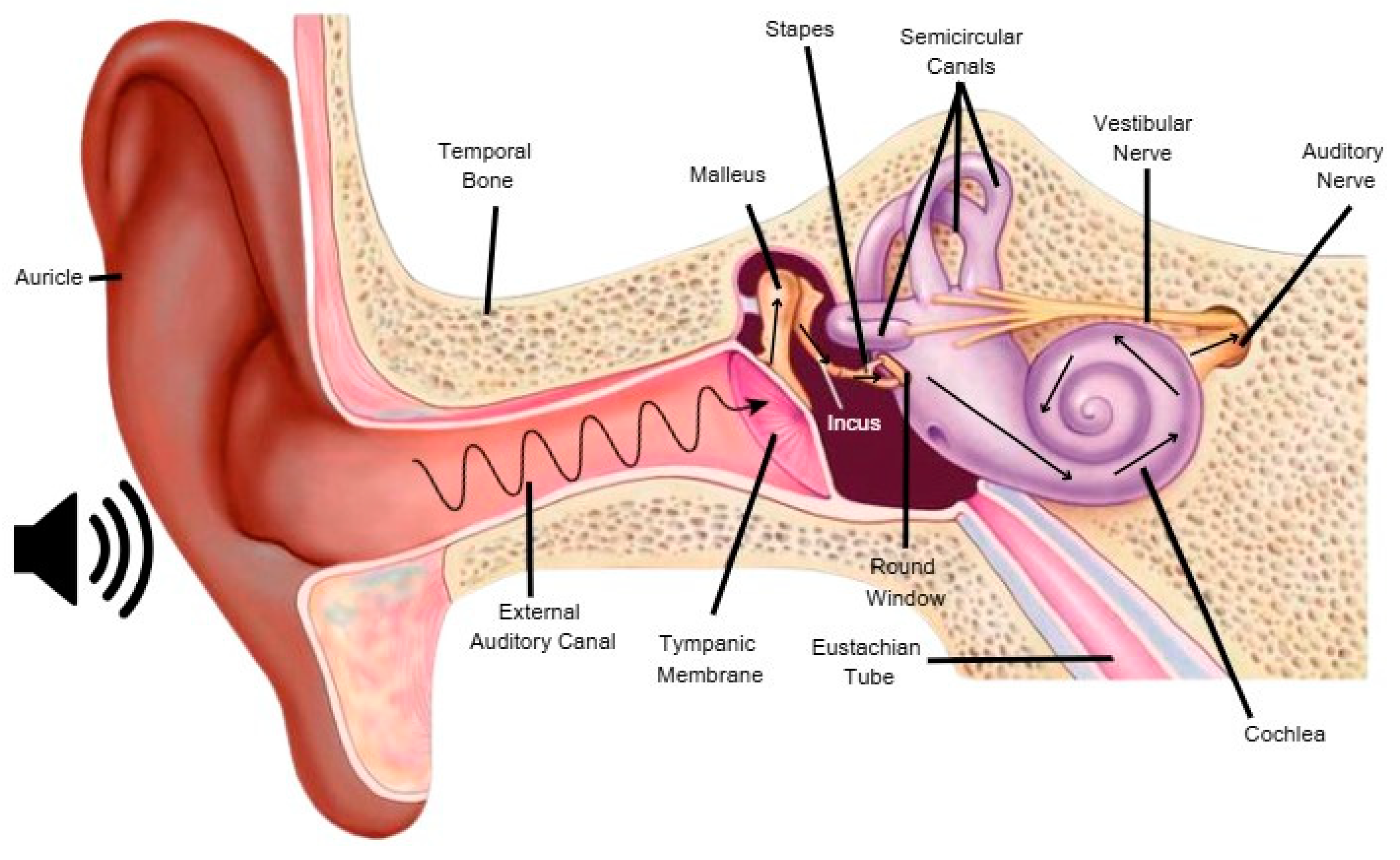
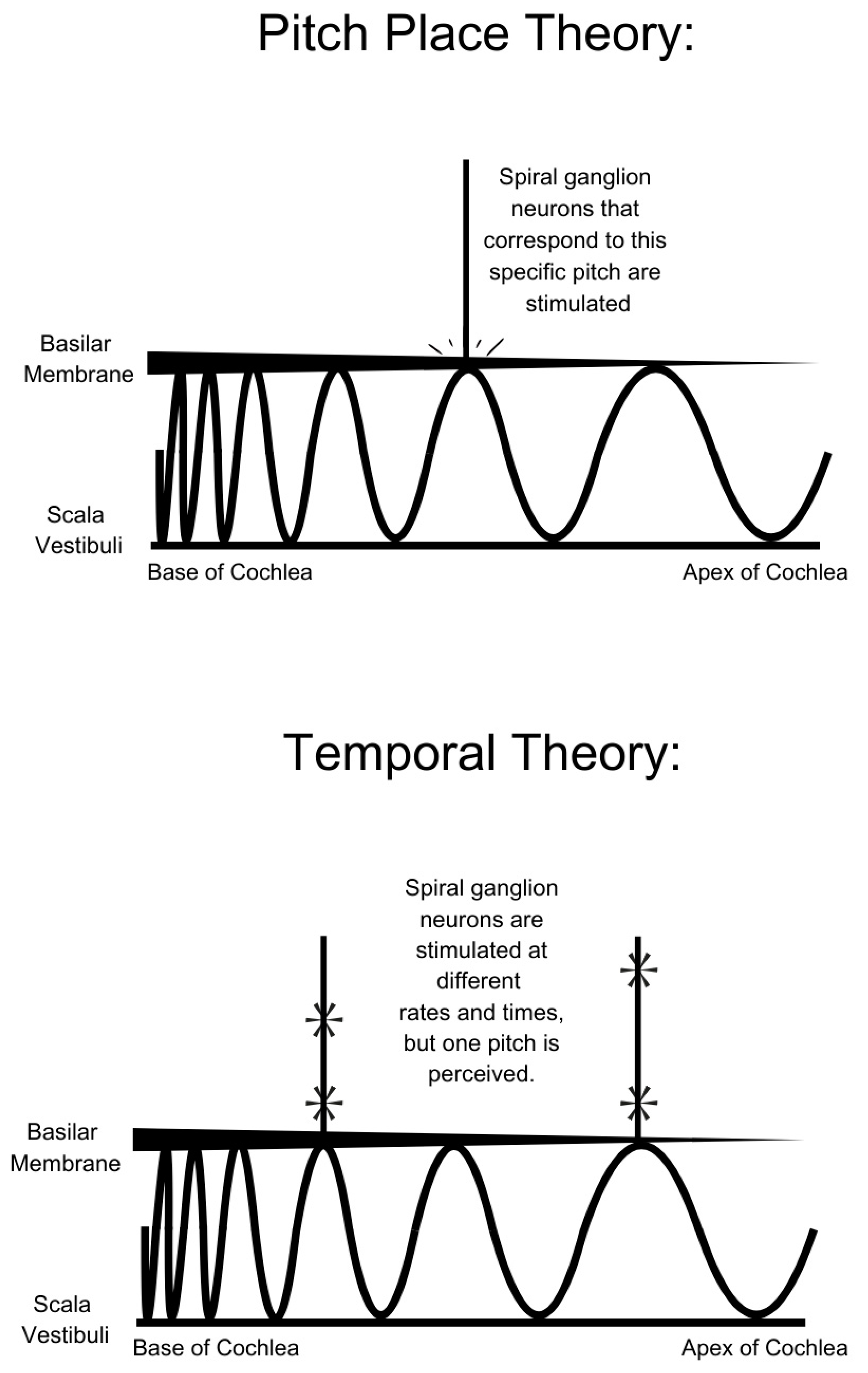
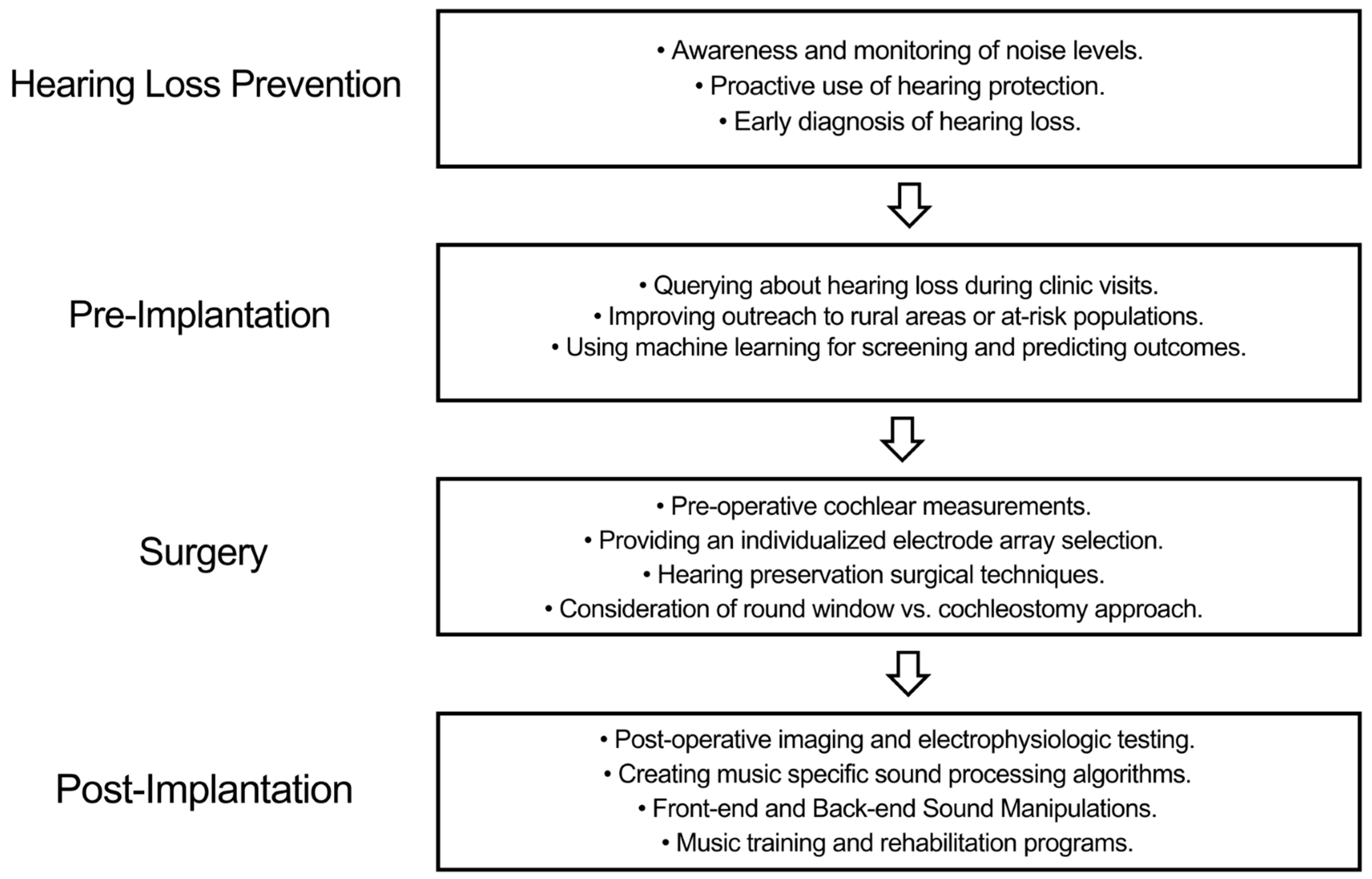
| Factor | Variability and Impact on Outcomes |
|---|---|
| CI Manufacturer | MED-EL, Advanced Bionics Corporation, Cochlear Corporation; comparable performance |
| Electrode Shape | Straight: against lateral wall of scala tympani; include shorter and atraumatic designs for hybrid stimulation Perimodiolar: curve along cochlear axis; may improve pitch discrimination |
| Electrode Count | Ranges from 16 to 22 electrodes; higher counts allow for finer pitch discrimination but may increase interference |
| Standard vs. Hybrid Design | Standard: bypass hair cells to directly electrically stimulate the auditory nerve Hybrid: both electric stimulation and acoustic amplification for patients with residual hearing |
| CI Sound Processing Algorithm | CIS: focuses on temporal envelop to optimize speech outcomes FSP: partially includes temporal fine structure to improve pitch perception |
| CI Frequency Mapping Method | DF: default frequency allocation across electrodes based on average cochlear size ABF: selects electrode type, insertion depth, and frequency allocation based on each patient’s cochlear duct length |
| Patient Factors | Pre- or post-lingually deafened, duration and severity of hearing loss, cochlear anatomy, long-term goals |
| Acoustic Feature | Physiology in Functional Cochlea | Function in CI | Implications for CI Users |
|---|---|---|---|
| Frequency range | 5 to 15,000 Hz | 70 to 8500 Hz | Loss of high-frequency information and difficulty perceiving base pitches |
| Frequency resolution | 3500 inner hair cells, each captures a ~20 Hz frequency band | 12–22 electrodes, each captures a frequency band of hundreds to thousands of Hz | Challenges with precise frequency discrimination (e.g., pitch changes such as semitones, speech in noisy environments) |
| Temporal processing | Detects both temporal envelope and temporal fine structure | Transmits temporal envelope, omits or partially transmits temporal fine structure | Challenges with precise pitch discrimination and timbre perception |
| Frequency mapping | Tonotopic organization of cochlea (Pitch Placement theory) and temporal code of action potentials in the auditory nerve (Temporal Pitch theory) | Assignment of a frequency band to each electrode based on location along the cochlea DF: uses the average cochlear tonotopic map ABF: tonotopic map developed for each patients’ cochlea | Pitch mismatch (e.g., hearing tones shifted by 1–2 octaves), loss of high or low frequency information |
| Dynamic range | 120-dB dynamic range | 20 to 30-dB dynamic range | Less contrast between loud and soft sounds, challenges with emotional prosody, soft speech music dynamics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podury, A.; Barry, B.; Barrett, K.C.; Jiam, N.T. Perfecting Sensory Restoration and the Unmet Need for Personalized Medicine in Cochlear Implant Users: A Narrative Review. Brain Sci. 2025, 15, 479. https://doi.org/10.3390/brainsci15050479
Podury A, Barry B, Barrett KC, Jiam NT. Perfecting Sensory Restoration and the Unmet Need for Personalized Medicine in Cochlear Implant Users: A Narrative Review. Brain Sciences. 2025; 15(5):479. https://doi.org/10.3390/brainsci15050479
Chicago/Turabian StylePodury, Archana, Brooke Barry, Karen C. Barrett, and Nicole T. Jiam. 2025. "Perfecting Sensory Restoration and the Unmet Need for Personalized Medicine in Cochlear Implant Users: A Narrative Review" Brain Sciences 15, no. 5: 479. https://doi.org/10.3390/brainsci15050479
APA StylePodury, A., Barry, B., Barrett, K. C., & Jiam, N. T. (2025). Perfecting Sensory Restoration and the Unmet Need for Personalized Medicine in Cochlear Implant Users: A Narrative Review. Brain Sciences, 15(5), 479. https://doi.org/10.3390/brainsci15050479






