Assistive Artificial Intelligence in Epilepsy and Its Impact on Epilepsy Care in Low- and Middle-Income Countries
Abstract
1. Introduction
2. Current State of Epilepsy in LMICs
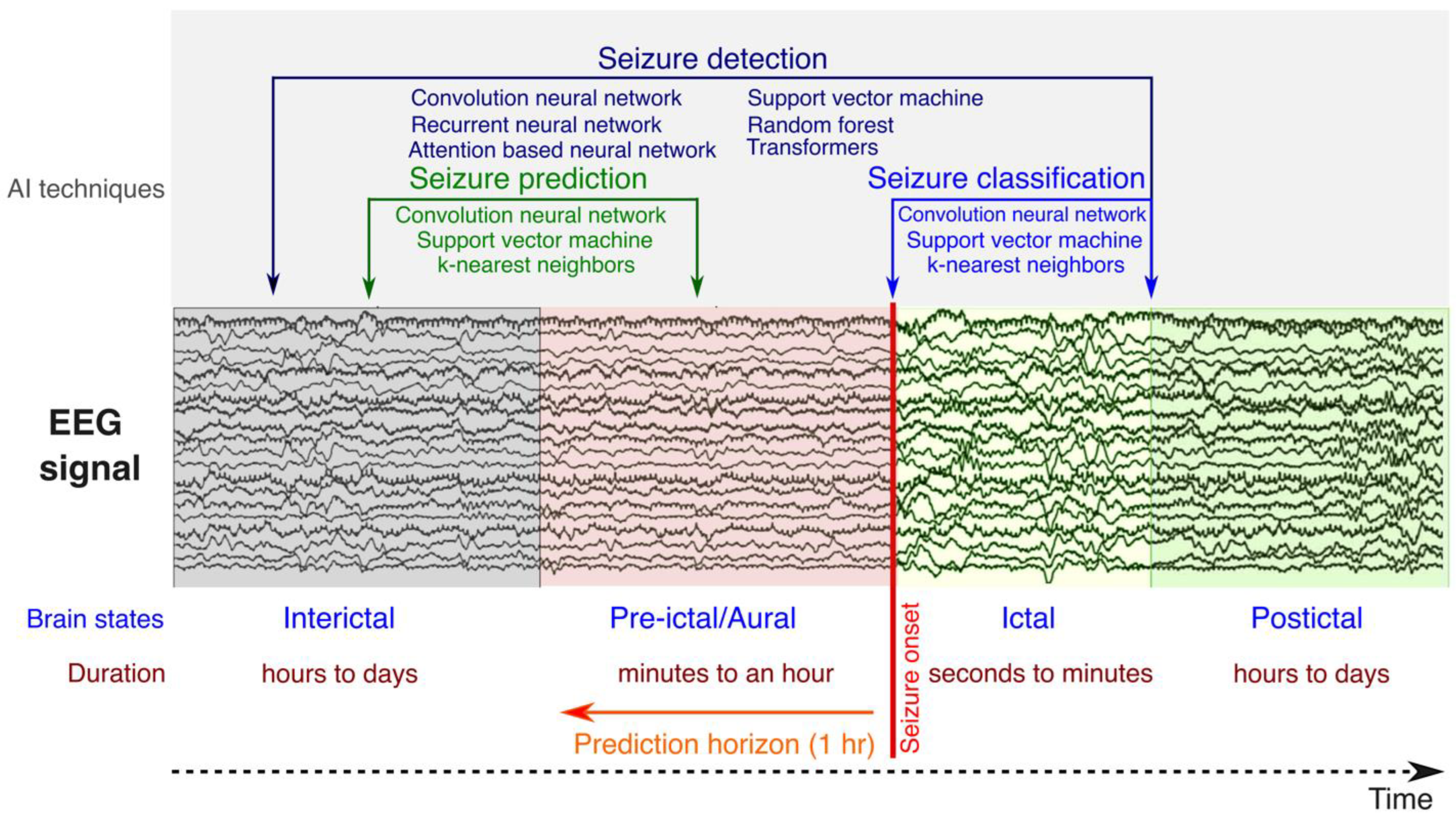
3. Artificial Intelligence in Epilepsy
- Variability of seizure patterns: Seizures can vary greatly in frequency, duration, and type, not only across individuals but also within the same individual over time [69]. This variability makes it challenging to identify universal predictors or markers that can reliably indicate an impending seizure.
- Identification of predictive biomarkers: Finding reliable biomarkers (physiological changes or patterns) that consistently precede seizures is crucial for prediction. These biomarkers can include changes in brain electrical activity, as measured by EEG, and other physiological signals [69].
- Data collection and analysis: Continuous monitoring of brain activity and other physiological signals generates large volumes of data. Analyzing these data requires high computational capacity, sophisticated data processing algorithms, and advanced machine learning techniques [70].
- Real-time prediction and intervention: For seizure prediction to be clinically relevant, it must operate in real time or near real time, providing timely alerts to patients or triggering interventions to prevent or mitigate the seizure [71]. This necessitates highly accurate prediction algorithms and user-friendly devices for monitoring and intervention.
- Individualized prediction models: Due to the individual variability in seizure patterns and physiological responses, seizure prediction models often need to be personalized by adding patient-specific information such as medical history and demographics [67]. Developing and tuning these individualized models adds an additional layer of complexity.
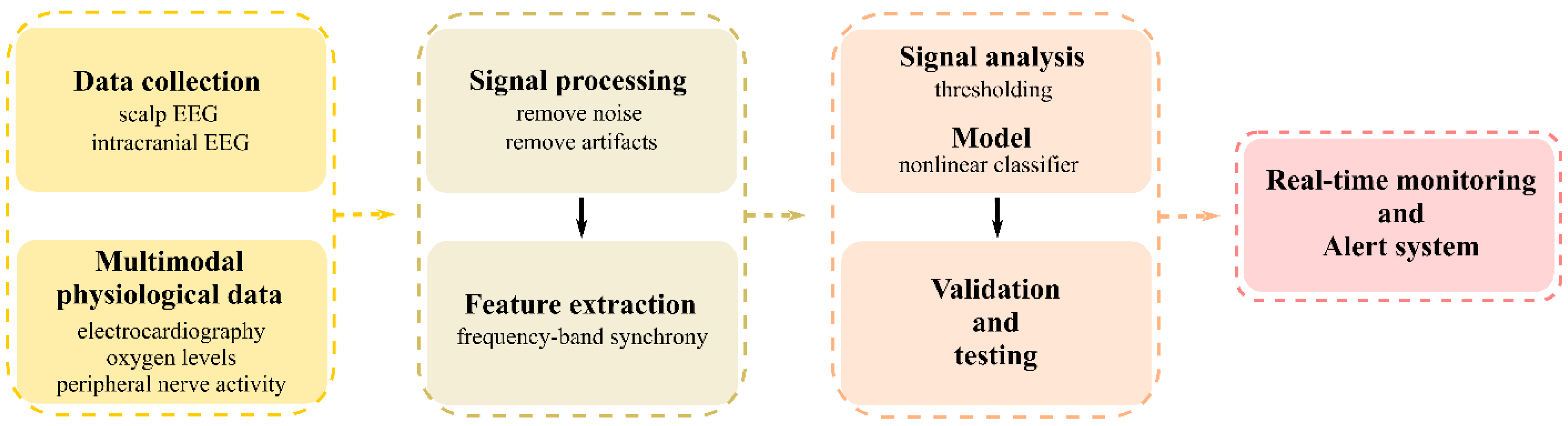
4. Application of Assistive AI in Clinical Care for LMICs
5. The Socio-Economic Impact of Assistive AI for Epilepsy in LMICs
6. Future Directions and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fisher, R.S.; van Emde Boas, W.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J., Jr. Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef]
- Milligan, T.A. Epilepsy: A Clinical Overview. Am. J. Med. 2021, 134, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Sander, J.W. The global burden of epilepsy report: Implications for low- and middle-income countries. Epilepsy Behav. 2020, 105, 106949. [Google Scholar] [CrossRef]
- Perez, D.L.; LaFrance, W.C., Jr. Nonepileptic seizures: An updated review. CNS Spectr. 2016, 21, 239–246. [Google Scholar] [CrossRef]
- Stafstrom, C.E.; Carmant, L. Seizures and epilepsy: An overview for neuroscientists. Cold Spring Harb. Perspect. Med. 2015, 5, a022426. [Google Scholar] [CrossRef]
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; Peltola, J.; Roulet Perez, E.; et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef]
- Fisher, R.S.; Cross, J.H.; D’Souza, C.; French, J.A.; Haut, S.R.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia 2017, 58, 531–542. [Google Scholar] [CrossRef]
- National Guideline Centre (UK). Evidence Review: Diagnosis of Epilepsies; National Guideline Centre: London, UK, 2022. [Google Scholar]
- Tatum, W.O.; Rubboli, G.; Kaplan, P.W.; Mirsatari, S.M.; Radhakrishnan, K.; Gloss, D.; Caboclo, L.O.; Drislane, F.W.; Koutroumanidis, M.; Schomer, D.L.; et al. Clinical utility of EEG in diagnosing and monitoring epilepsy in adults. Clin. Neurophysiol. 2018, 129, 1056–1082. [Google Scholar] [CrossRef]
- Koutroumanidis, M.; Arzimanoglou, A.; Caraballo, R.; Goyal, S.; Kaminska, A.; Laoprasert, P.; Oguni, H.; Rubboli, G.; Tatum, W.; Thomas, P.; et al. The role of EEG in the diagnosis and classification of the epilepsy syndromes: A tool for clinical practice by the ILAE Neurophysiology Task Force (Part 2). Epileptic Disord. 2017, 19, 385–437. [Google Scholar] [CrossRef] [PubMed]
- Jehi, L. The Epileptogenic Zone: Concept and Definition. Epilepsy Curr. 2018, 18, 12–16. [Google Scholar] [CrossRef]
- Benbadis, S.R.; Beniczky, S.; Bertram, E.; MacIver, S.; Moshé, S.L. The role of EEG in patients with suspected epilepsy. Epileptic Disord. 2020, 22, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Beghi, E. The Epidemiology of Epilepsy. Neuroepidemiology 2020, 54, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.N.; Filippi, D.; Hauser, W.A. The descriptive epidemiology of epilepsy—A review. Epilepsy Res. 2009, 85, 31–45. [Google Scholar] [CrossRef]
- Beghi, E.; Giussani, G.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; Abraha, H.N.; Adib, M.G.; Agrawal, S.; Alandab, F.; Awasthi, A.; et al. Global, regional, and national burden of epilepsy, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 357–375. [Google Scholar] [CrossRef]
- Fiest, K.M.; Sauro, K.M.; Wiebe, S.; Patten, S.B.; Kwon, C.S.; Dykeman, J.; Pringsheim, T.; Lorenzetti, D.L.; Jette, N. Prevalence and incidence of epilepsy: A systematic review and meta-analysis of international studies. Neurology 2017, 88, 296–303. [Google Scholar] [CrossRef]
- Begley, C.; Wagner, R.G.; Abraham, A.; Beghi, E.; Newton, C.; Kwon, C.S.; Labiner, D.; Winkler, A. The global cost of epilepsy: A systematic review and extrapolation. Epilepsia 2022, 63, 892–903. [Google Scholar] [CrossRef]
- Wilmshurst, J.M.; Birbeck, G.L.; Newton, C.R. Epilepsy is ubiquitous, but more devastating in the poorer regions of the world… or is it? Epilepsia 2014, 55, 1322–1325. [Google Scholar] [CrossRef]
- Newton, C.R.; Garcia, H.H. Epilepsy in poor regions of the world. Lancet 2012, 380, 1193–1201. [Google Scholar] [CrossRef]
- Thomas, S.V.; Sarma, P.S.; Alexander, M.; Pandit, L.; Shekhar, L.; Trivedi, C.; Vengamma, B. Economic burden of epilepsy in India. Epilepsia 2001, 42, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Shan, Y.; Du, Q.; Ding, Y.; Shen, C.H.; Wang, S.; Ding, M.P.; Xu, Y.F. Gender and Socioeconomic Disparities in Global Burden of Epilepsy: An Analysis of Time Trends From 1990 to 2017. Front. Neurol. 2021, 12, 643450. [Google Scholar] [CrossRef]
- Lagunju, I.A.; Imam, Z.O.; Adedokun, B.O. Cost of epilepsy in children attending a tertiary centre in Nigeria. Int. Health 2011, 3, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Mogal, Z.; Aziz, H. Epilepsy treatment gap and stigma reduction in Pakistan: A tested public awareness model. Epilepsy Behav. 2020, 102, 106637. [Google Scholar] [CrossRef]
- Adjei, P.; Nkromah, K.; Akpalu, A.; Laryea, R.; Osei Poku, F.; Ohene, S.; Puplampu, P.; Twumasi Aboagye, E. A cross-sectional comparative study of perceived stigma between patients with epilepsy and patients living with HIV/AIDS in Accra, Ghana. Epilepsy Behav. 2018, 89, 1–7. [Google Scholar] [CrossRef]
- Wolf, P. Has stigma changed? The image of epilepsy in literature. An essay. Epilepsy Behav. 2022, 137, 108921. [Google Scholar] [CrossRef]
- Hohmann, L.; Berger, J.; Kastell, S.U.; Holtkamp, M. Perceived epilepsy-related stigma is linked to the socioeconomic status of the residence. Front. Public. Health 2022, 10, 952585. [Google Scholar] [CrossRef] [PubMed]
- Karakas, N.; Saritas, S.C.; Aktura, S.C.; Karabulutlu, E.Y.; Oruc, F.G. Investigation of factors associated with stigma and social support in patients with epilepsy in Turkey: A cross-sectional study. Epilepsy Behav. 2022, 128, 108572. [Google Scholar] [CrossRef]
- Placencia, M.; Farmer, P.J.; Jumbo, L.; Sander, J.W.A.S.; Shorvon, S.D. Levels of Stigmatization of Patients with Previously Untreated Epilepsy in Northern Ecuador. Neuroepidemiology 1995, 14, 147–154. [Google Scholar] [CrossRef]
- Fiest, K.M.; Birbeck, G.L.; Jacoby, A.; Jette, N. Stigma in Epilepsy. Curr. Neurol. Neurosci. Rep. 2014, 14, 444. [Google Scholar] [CrossRef]
- Lim, K.S.; Tan, C.T. Epilepsy stigma in Asia: The meaning and impact of stigma. Neurol. Asia 2014, 19, 1–10. [Google Scholar]
- Baker, G.A. Comments on De Boer JE et al. The global burden and stigma of epilepsy. Epilepsy & Behavior 2008;12:540–546. Epilepsy Behav. 2014, 40, 20–21. [Google Scholar] [CrossRef]
- de Boer, H.M.; Mula, M.; Sander, J.W. The global burden and stigma of epilepsy. Epilepsy Behav. 2008, 12, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Sahar, N.-U. Assessment of psychological distress in epilepsy: Perspective from pakistan. Epilepsy Res. Treat. 2012, 2012, 171725. [Google Scholar] [CrossRef]
- Mula, M.; Kaufman, K.R. Double stigma in mental health: Epilepsy and mental illness. BJPsych. Open 2020, 6, e72. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.S.; Wagner, R.G.; Carpio, A.; Jette, N.; Newton, C.R.; Thurman, D.J. The worldwide epilepsy treatment gap: A systematic review and recommendations for revised definitions—A report from the ILAE Epidemiology Commission. Epilepsia 2022, 63, 551–564. [Google Scholar] [CrossRef]
- Aziz, H.; Akhtar, S.W.; Hasan, K.Z. Epilepsy in Pakistan: Stigma and Psychosocial Problems. A Population-Based Epidemiologic Study. Epilepsia 1997, 38, 1069–1073. [Google Scholar] [CrossRef]
- Cameron, A.; Bansal, A.; Dua, T.; Hill, S.R.; Moshe, S.L.; Mantel-Teeuwisse, A.K.; Saxena, S. Mapping the availability, price, and affordability of antiepileptic drugs in 46 countries. Epilepsia 2012, 53, 962–969. [Google Scholar] [CrossRef]
- Bakhsh, A. Value of neuroimaging in epilepsy: An experience from Pakistan. J. Neurosci. Rural. Pract. 2013, 4, S35–S39. [Google Scholar] [CrossRef]
- Khan, T.A.; Hussain, S.; Ikram, A.; Mahmood, S.; Riaz, H.; Jamil, A.; Amin, A.; Haider, Y.G.; Sandhu, M.; Mushtaq, A.; et al. Prevalence and treatment of neurological and psychiatric disorders among tertiary hospitals in Pakistan; findings and implications. Hosp. Pract. 2020, 48, 145–160. [Google Scholar] [CrossRef]
- Asghar, M.A.; Rehman, A.A.; Raza, M.L.; Shafiq, Y.; Asghar, M.A. Analysis of treatment adherence and cost among patients with epilepsy: A four-year retrospective cohort study in Pakistan. BMC Health Serv. Res. 2021, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Neligan, A.; Sander, J.W. The treatment gap in epilepsy: A global perspective. Epileptology 2013, 1, 28–30. [Google Scholar] [CrossRef]
- Brodie, M.J.; Barry, S.J.E.; Bamagous, G.A.; Norrie, J.D.; Kwan, P. Patterns of treatment response in newly diagnosed epilepsy. Neurology 2012, 78, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Watila, M.M.; Xiao, F.L.; Keezer, M.R.; Miserocchi, A.; Winkler, A.S.; McEvoy, A.W.; Sander, J.W. Epilepsy surgery in low- and middle-income countries: A scoping review. Epilepsy Behav. 2019, 92, 311–326. [Google Scholar] [CrossRef]
- Dua, T.; de Boer, H.M.; Prilipko, L.L.; Saxena, S. Epilepsy care in the world: Results of an ILAE/IBE/WHO global campaign against epilepsy survey. Epilepsia 2006, 47, 1225–1231. [Google Scholar] [CrossRef]
- Dua, T.; De Boer, H.M.; Prilipko, L.L. Atlas: Epilepsy care in the world. Epilepsia 2005, 46, 28. [Google Scholar]
- Rajbhandari, K.C. Epilepsy in Nepal. Can. J. Neurol. Sci. 2004, 31, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.; Ernesto, C.; Torres, S.; Salazar-Cabrera, R.; Lopez, D.M.; Vargas-Canas, R. Intelligent Telehealth System To Support Epilepsy Diagnosis. J. Multidiscip. Health 2020, 13, 433–445. [Google Scholar] [CrossRef]
- Furbass, F.; Ossenblok, P.; Hartmann, M.; Perko, H.; Skupch, A.M.; Lindinger, G.; Elezi, L.; Pataraia, E.; Colon, A.J.; Baumgartner, C.; et al. Prospective multi-center study of an automatic online seizure detection system for epilepsy monitoring units. Clin. Neurophysiol. 2015, 126, 1124–1131. [Google Scholar] [CrossRef]
- Patterson, V. Managing Epilepsy by Telemedicine in Resource-Poor Settings. Front. Public. Health 2019, 7, 321. [Google Scholar] [CrossRef]
- Patterson, V.; Samant, S.; Singh, M.B.; Jain, P.; Agavane, V.; Jain, Y. Diagnosis of epileptic seizures by community health workers using a mobile app: A comparison with physicians and a neurologist. Seizure-Eur. J. Epilep 2018, 55, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Patterson, V.; Samant, S.; Jain, Y.; Singh, M.B. Computer-naive health workers can use a tablet-based epilepsy diagnosis app. Epilepsy Behav. 2017, 70, 274–275. [Google Scholar] [CrossRef]
- Giuliano, L.; Cicero, C.E.; Trimarchi, G.; Todaro, V.; Colli, C.; Gomez, E.B.C.; Bartoloni, A.; Sofia, V.; Patterson, V.; Zappia, M.; et al. Usefulness of a smartphone application for the diagnosis of epilepsy: Validation study in high-income and rural low-income countries. Epilepsy Behav. 2021, 115, 107680. [Google Scholar] [CrossRef]
- Rajbhandari, H.; Joshi, S.; Malakar, S.; Pander, P.; Jain, P.; Uppadaya, K.; Singh, M.; Patterson, V. Epilepsy field workers, a smartphone application and telephone telemedicine: Safe and effective epilepsy care in rural Nepal. Seizure-Eur. J. Epilep 2019, 64, 54–58. [Google Scholar] [CrossRef]
- Patterson, V. The development of a smartphone application to help manage epilepsy in resource-limited settings. Seizure-Eur. J. Epilep 2020, 79, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Beniczky, S.; Asadi-Pooya, A.A.; Perucca, E.; Rubboli, G.; Tartara, E.; Larsen, P.M.; Ebrahimi, S.; Farzinmehr, S.; Rampp, S.; Sperling, M.R. A web-based algorithm to rapidly classify seizures for the purpose of drug selection. Epilepsia 2021, 62, 2474–2484. [Google Scholar] [CrossRef] [PubMed]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An introduction to Statistical Learning: With Applications in R; Springer: New York, NY, USA, 2013. [Google Scholar]
- Bishop, C.M.; Bishop, H. Deep Learning: Foundations and Concepts; Springer International Publishing: Cham, Switzerland, 2023. [Google Scholar]
- Van Houdt, G.; Mosquera, C.; Nápoles, G. A review on the long short-term memory model. Artif. Intell. Rev. 2020, 53, 5929–5955. [Google Scholar] [CrossRef]
- Alammar, J.; Grootendorst, M. Hands-On Large Language Models; O’Reilly Media: Sebastopol, CA, USA, 2024. [Google Scholar]
- Lucas, A.; Revell, A.; Davis, K.A. Artificial intelligence in epilepsy–applications and pathways to the clinic. Nat. Rev. Neurol. 2024, 20, 319–336. [Google Scholar] [CrossRef]
- Nair, P.P.; Aghoram, R.; Khilari, M.L. Applications of Artificial Intelligence in Epilepsy. Int. J. Adv. Med. Health Res. 2021, 8, 41–48. [Google Scholar] [CrossRef]
- Ein Shoka, A.A.; Dessouky, M.M.; El-Sayed, A.; Hemdan, E.E. EEG seizure detection: Concepts, techniques, challenges, and future trends. Multimed. Tools Appl. 2023, 82, 42021–42051. [Google Scholar] [CrossRef]
- An, S.; Kang, C.; Lee, H.W. Artificial Intelligence and Computational Approaches for Epilepsy. J. Epilepsy Res. 2020, 10, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Shoeibi, A.; Khodatars, M.; Ghassemi, N.; Jafari, M.; Moridian, P.; Alizadehsani, R.; Panahiazar, M.; Khozeimeh, F.; Zare, A.; Hosseini-Nejad, H.; et al. Epileptic Seizures Detection Using Deep Learning Techniques: A Review. Int. J. Environ. Res. Public. Health 2021, 18, 5780. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, L.; Lehnertz, K.; Richardson, M.P.; Schelter, B.; Zaveri, H.P. Seizure prediction—Ready for a new era. Nat. Rev. Neurol. 2018, 14, 618–630. [Google Scholar] [CrossRef]
- Kiral-Kornek, I.; Roy, S.; Nurse, E.; Mashford, B.; Karoly, P.; Carroll, T.; Payne, D.; Saha, S.; Baldassano, S.; O’Brien, T.; et al. Epileptic Seizure Prediction Using Big Data and Deep Learning: Toward a Mobile System. EBioMedicine 2018, 27, 103–111. [Google Scholar] [CrossRef]
- Schelter, B.; Timmer, J.; Schulze-Bonhage, A. Seizure Prediction in Epilepsy: From Basic Mechanisms to Clinical Applications; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Carney, P.R.; Myers, S.; Geyer, J.D. Seizure prediction: Methods. Epilepsy Behav. 2011, 22 (Suppl. 1), S94–S101. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.R.; Hagiwara, Y.; Adeli, H. Automated seizure prediction. Epilepsy Behav. 2018, 88, 251–261. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, L.; Zhang, Z.; Chen, Z.; Zhou, Y. Early prediction of epileptic seizures using a long-term recurrent convolutional network. J. Neurosci. Methods 2019, 327, 108395. [Google Scholar] [CrossRef]
- Wei, X.; Cao, X.; Zhou, Y.; Zhen, Z. Epileptic seizure prediction from multivariate sequential signals using Multidimensional convolution network. J. Neurol. Disord. 2022, 10, 517. [Google Scholar]
- Cousyn, L.; Navarro, V.; Chavez, M. Preictal state detection using prodromal symptoms: A machine learning approach. Epilepsia 2021, 62, e42–e47. [Google Scholar] [CrossRef]
- Tran, L.V.; Tran, H.M.; Le, T.M.; Huynh, T.T.M.; Tran, H.T.; Dao, S.V.T. Application of Machine Learning in Epileptic Seizure Detection. Diagnostics 2022, 12, 2879. [Google Scholar] [CrossRef]
- Ghaempour, M.; Hassanli, K.; Abiri, E. An approach to detect and predict epileptic seizures with high accuracy using convolutional neural networks and single-lead-ECG signal. Biomed. Phys. Eng. Express 2024, 10, 025041. [Google Scholar] [CrossRef]
- Srinivasan, S.; Dayalane, S.; Mathivanan, S.K.; Rajadurai, H.; Jayagopal, P.; Dalu, G.T. Detection and classification of adult epilepsy using hybrid deep learning approach. Sci. Rep. 2023, 13, 17574. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, L.; Zhang, G.Q.; Lhatoo, S.D. Can Big Data guide prognosis and clinical decisions in epilepsy? Epilepsia 2021, 62 (Suppl. 2), S106–S115. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, E.M.; Yao, R.; Sirven, J.I.; Crepeau, A.Z.; Noe, K.H.; Drazkowski, J.F.; Hoerth, M.T.; Salinas, E.; Csernak, L.; Mehta, N. Usage of EpiFinder clinical decision support in the assessment of epilepsy. Epilepsy Behav. 2018, 82, 140–143. [Google Scholar] [CrossRef]
- Chan, A.M.; Sun, F.T.; Boto, E.H.; Wingeier, B.M. Automated seizure onset detection for accurate onset time determination in intracranial EEG. Clin. Neurophysiol. 2008, 119, 2687–2696. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, K.; Azizi, E.; Haddadnia, J. Optimized Seizure Detection Algorithm: A Fast Approach for Onset of Epileptic in EEG Signals Using GT Discriminant Analysis and K-NN Classifier. J. Biomed. Phys. Eng. 2016, 6, 81–94. [Google Scholar]
- Wang, L.; Xue, W.; Li, Y.; Luo, M.; Huang, J.; Cui, W.; Huang, C. Automatic Epileptic Seizure Detection in EEG Signals Using Multi-Domain Feature Extraction and Nonlinear Analysis. Entropy 2017, 19, 222. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.; Yang, S.; Kim, H.; Choi, Y.S.; Kim, H.J.; Lee, H.W.; Lee, B.-U. Early Seizure Detection by Applying Frequency-Based Algorithm Derived from the Principal Component Analysis. Front. Neurosci. 2017, 11, 52. [Google Scholar] [CrossRef]
- Emami, A.; Kunii, N.; Matsuo, T.; Shinozaki, T.; Kawai, K.; Takahashi, H. Seizure detection by convolutional neural network-based analysis of scalp electroencephalography plot images. NeuroImage Clin. 2019, 22, 101684. [Google Scholar] [CrossRef]
- Zhou, M.; Tian, C.; Cao, R.; Wang, B.; Niu, Y.; Hu, T.; Guo, H.; Xiang, J. Epileptic Seizure Detection Based on EEG Signals and CNN. Front. Neuroinform. 2018, 12, 95. [Google Scholar] [CrossRef]
- Hussein, R.; Palangi, H.; Ward, R.K.; Wang, Z.J. Optimized deep neural network architecture for robust detection of epileptic seizures using EEG signals. Clin. Neurophysiol. 2019, 130, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-J.; Cho, K.-O. Dual deep neural network-based classifiers to detect experimental seizures. Korean J. Physiol. Pharmacol. 2019, 23, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Guttag, A.S.J. Application of machine learning to epileptic seizure detection. In Proceedings of the 27th International Conference on International Conference on Machine Learning, Haifa, Israel, 21–24 June 2010; pp. 975–982. [Google Scholar]
- Subasi, A.; Kevric, J.; Canbaz, M.A. Epileptic seizure detection using hybrid machine learning methods. Neural Comput. Appl. 2019, 31, 317–325. [Google Scholar] [CrossRef]
- Cho, K.-O.; Jang, H.-J. Comparison of different input modalities and network structures for deep learning-based seizure detection. Sci. Rep. 2020, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Chaovalitwongse, W.A.; Prokopyev, O.A.; Pardalos, P.M. Electroencephalogram (EEG) time series classification: Applications in epilepsy. Ann. Oper. Res. 2006, 148, 227–250. [Google Scholar] [CrossRef]
- Hogan, R.; Mathieson, S.R.; Luca, A.; Ventura, S.; Griffin, S.; Boylan, G.B.; O’Toole, J.M. Scaling convolutional neural networks achieves expert level seizure detection in neonatal EEG. npj Digit. Med. 2025, 8, 17. [Google Scholar] [CrossRef]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, Ł.; Polosukhin, I. Attention is all you need. In Proceedings of the 31st International Conference on Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; pp. 6000–6010. [Google Scholar]
- Huang, Z.; Yang, Y.; Ma, Y.; Dong, Q.; Su, J.; Shi, H.; Zhang, S.; Hu, L. EEG detection and recognition model for epilepsy based on dual attention mechanism. Sci. Rep. 2025, 15, 9404. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Wang, Y.; Liu, X.; Qiu, X. A survey of transformers. AI Open 2022, 3, 111–132. [Google Scholar] [CrossRef]
- Siddiqui, M.K.; Islam, M.Z.; Kabir, M.A. A novel quick seizure detection and localization through brain data mining on ECoG dataset. Neural Comput. Appl. 2019, 31, 5595–5608. [Google Scholar] [CrossRef]
- Donos, C.; Dümpelmann, M.; Schulze-Bonhage, A. Early Seizure Detection Algorithm Based on Intracranial EEG and Random Forest Classification. Int. J. Neural Syst. 2015, 25, 1550023. [Google Scholar] [CrossRef]
- Alickovic, E.; Kevric, J.; Subasi, A. Performance evaluation of empirical mode decomposition, discrete wavelet transform, and wavelet packed decomposition for automated epileptic seizure detection and prediction. Biomed. Signal Process. Control 2018, 39, 94–102. [Google Scholar] [CrossRef]
- Wang, X.; Gong, G.; Li, N.; Qiu, S. Detection Analysis of Epileptic EEG Using a Novel Random Forest Model Combined with Grid Search Optimization. Front. Hum. Neurosci. 2019, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Manzouri, F.; Heller, S.; Dümpelmann, M.; Woias, P.; Schulze-Bonhage, A. A Comparison of Machine Learning Classifiers for Energy-Efficient Implementation of Seizure Detection. Front. Syst. Neurosci. 2018, 12, 43. [Google Scholar] [CrossRef]
- Mursalin, M.; Islam, S.; Noman, K. Epileptic seizure classification using statistical sampling and a novel feature selection algorithm. arXiv 2019, arXiv:1902.09962. [Google Scholar]
- Logesparan, L.; Casson, A.J.; Rodriguez-Villegas, E. Optimal features for online seizure detection. Med. Biol. Eng. Comput. 2012, 50, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.K.; Islam, M.Z.; Kabir, M.A. Analyzing Performance of Classification Techniques in Detecting Epileptic Seizure. In Proceedings of the Advanced Data Mining and Applications (ADMA 2017), Singapore, 5–6 November 2017; pp. 386–398. [Google Scholar]
- Selvakumari, R.S.; Mahalakshmi, M.; Prashalee, P. Patient-Specific Seizure Detection Method using Hybrid Classifier with Optimized Electrodes. J. Med. Syst. 2019, 43, 121. [Google Scholar] [CrossRef]
- Zabihi, M.; Kiranyaz, S.; Ince, T.; Gabbouj, M. Patient-specific epileptic seizure detection in long-term EEG recording in paediatric patients with intractable seizures. In Proceedings of the IET Intelligent Signal Processing Conference 2013 (ISP 2013), London, UK, 2–3 December 2013; pp. 1–7. [Google Scholar]
- Fergus, P.; Hussain, A.; Hignett, D.; Al-Jumeily, D.; Abdel-Aziz, K.; Hamdan, H. A machine learning system for automated whole-brain seizure detection. Appl. Comput. Inform. 2016, 12, 70–89. [Google Scholar] [CrossRef]
- Logesparan, L.; Rodriguez-Villegas, E.; Casson, A.J. The impact of signal normalization on seizure detection using line length features. Med. Biol. Eng. Comput. 2015, 53, 929–942. [Google Scholar] [CrossRef]
- Olsen, D.E.; Cristion, J.A.; Spaur, C.W. Automatic detection of seizures using electroencephalographic signals. Johns Hopkins APL Tech. Dig. 1991, 12, 2. [Google Scholar]
- Koolen, N.; Jansen, K.; Vervisch, J.; Matic, V.; De Vos, M.; Naulaers, G.; Van Huffel, S. Line length as a robust method to detect high-activity events: Automated burst detection in premature EEG recordings. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2014, 125, 1985–1994. [Google Scholar] [CrossRef]
- Guo, L.; Rivero, D.; Dorado, J.; Rabuñal, J.R.; Pazos, A. Automatic epileptic seizure detection in EEGs based on line length feature and artificial neural networks. J. Neurosci. Methods 2010, 191, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Mosquera, C.; Trigueros, A.M.; Franco, J.I.; Navia-Vázquez, A. New feature extraction approach for epileptic EEG signal detection using time-frequency distributions. Med. Biol. Eng. Comput. 2010, 48, 321–330. [Google Scholar] [CrossRef]
- Pal, P.R.; Rajanikant, P. Classification of EEG signals for epileptic seizure evaluation. In Proceedings of the 2010 IEEE Students Technology Symposium (TechSym), Kharagpur, India, 3–4 April 2010; pp. 72–76. [Google Scholar]
- Chen, S.; Zhang, X.; Chen, L.; Yang, Z. Automatic Diagnosis of Epileptic Seizure in Electroencephalography Signals Using Nonlinear Dynamics Features. IEEE Access 2019, 7, 61046–61056. [Google Scholar] [CrossRef]
- Sharma, R.R.; Varshney, P.; Pachori, R.B.; Vishvakarma, S.K. Automated System for Epileptic EEG Detection Using Iterative Filtering. IEEE Sens. Lett. 2018, 2, 1–4. [Google Scholar] [CrossRef]
- Ocak, H. Automatic detection of epileptic seizures in EEG using discrete wavelet transform and approximate entropy. Expert Syst. Appl. 2009, 36, 2027–2036. [Google Scholar] [CrossRef]
- Song, Y.; Liò, P. A new approach for epileptic seizure detection: Sample entropy based feature extraction and extreme learning machine. J. Biomed. Sci. Eng. 2010, 3, 556–567. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Wang, J.; Zheng, X. Comparison of classification methods on EEG signals based on wavelet packet decomposition. Neural Comput. Appl. 2015, 26, 1217–1225. [Google Scholar] [CrossRef]
- Acharya, U.R.; Molinari, F.; Sree, S.V.; Chattopadhyay, S.; Ng, K.-H.; Suri, J.S. Automated diagnosis of epileptic EEG using entropies. Biomed. Signal Process. Control 2012, 7, 401–408. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhou, W.; Zhang, L.; Zhang, F.; Xu, F.; Leng, Y.; Wei, D.; Chen, M. Epileptic seizure detection based on imbalanced classification and wavelet packet transform. Seizure 2017, 50, 99–108. [Google Scholar] [CrossRef]
- Birjandtalab, J.; Baran Pouyan, M.; Cogan, D.; Nourani, M.; Harvey, J. Automated seizure detection using limited-channel EEG and non-linear dimension reduction. Comput. Biol. Med. 2017, 82, 49–58. [Google Scholar] [CrossRef]
- Birjandtalab, J.; Jarmale, V.N.; Nourani, M.; Harvey, J. Imbalance Learning Using Neural Networks for Seizure Detection. In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS), Cleveland, OH, USA, 17–19 October 2018; pp. 1–4. [Google Scholar]
- Tzallas, A.T.; Tsipouras, M.G.; Fotiadis, D.I. Automatic seizure detection based on time-frequency analysis and artificial neural networks. Comput. Intell. Neurosci. 2007, 2007, 80510. [Google Scholar] [CrossRef]
- Raghu, S.; Sriraam, N. Classification of focal and non-focal EEG signals using neighborhood component analysis and machine learning algorithms. Expert Syst. Appl. 2018, 113, 18–32. [Google Scholar] [CrossRef]
- Parvez, M.Z.; Paul, M. Epileptic seizure detection by analyzing EEG signals using different transformation techniques. Neurocomputing 2014, 145, 190–200. [Google Scholar] [CrossRef]
- Amin, H.U.; Malik, A.S.; Ahmad, R.F.; Badruddin, N.; Kamel, N.; Hussain, M.; Chooi, W.-T. Feature extraction and classification for EEG signals using wavelet transform and machine learning techniques. Australas. Phys. Eng. Sci. Med. 2015, 38, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Wardrope, A.; Jamnadas-Khoda, J.; Broadhurst, M.; Grunewald, R.A.; Heaton, T.J.; Howell, S.J.; Koepp, M.; Parry, S.W.; Sisodiya, S.; Walker, M.C.; et al. Machine learning as a diagnostic decision aid for patients with transient loss of consciousness. Neurol-Clin. Pract. 2020, 10, 96–105. [Google Scholar] [CrossRef]
- Pevy, N.; Christensen, H.; Walker, T.; Reuber, M. Feasibility of using an automated analysis of formulation effort in patients’ spoken seizure descriptions in the differential diagnosis of epileptic and nonepileptic seizures. Seizure-Eur. J. Epilep 2021, 91, 141–145. [Google Scholar] [CrossRef]
- Raghu, S.; Sriraam, N.; Temel, Y.; Rao, S.V.; Kubben, P.L. EEG based multi-class seizure type classification using convolutional neural network and transfer learning. Neural Netw. 2020, 124, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Dunnmon, J.A.; Saab, K.; Zhang, X.; Huang, Q.; Dubost, F.; Rubin, D.; Lee-Messer, C. Self-Supervised Graph Neural Networks for Improved Electroencephalographic Seizure Analysis. In Proceedings of the International Conference on Learning Representations, Virtual Event, Austria, 3–7 May 2021. [Google Scholar]
- Dwi Saputro, I.R.; Maryati, N.D.; Solihati, S.R.; Wijayanto, I.; Hadiyoso, S.; Patmasari, R. Seizure Type Classification on EEG Signal using Support Vector Machine. J. Phys. Conf. Ser. 2019, 1201, 012065. [Google Scholar] [CrossRef]
- Springer, M.; Khalaf, A.; Vincent, P.; Ryu, J.H.; Abukhadra, Y.; Beniczky, S.; Glauser, T.; Krestel, H.; Blumenfeld, H. A machine-learning approach for predicting impaired consciousness in absence epilepsy. Ann. Clin. Transl. Neurol. 2022, 9, 1538–1550. [Google Scholar] [CrossRef]
- Johansson, D.; Ohlsson, F.; Krýsl, D.; Rydenhag, B.; Czarnecki, M.; Gustafsson, N.; Wipenmyr, J.; McKelvey, T.; Malmgren, K. Tonic-clonic seizure detection using accelerometry-based wearable sensors: A prospective, video-EEG controlled study. Seizure 2019, 65, 48–54. [Google Scholar] [CrossRef]
- Kołodziej, M.; Majkowski, A.; Rysz, A. Implementation of Machine Learning and Deep Learning Techniques for the Detection of Epileptic Seizures Using Intracranial Electroencephalography. Appl. Sci. 2023, 13, 8747. [Google Scholar] [CrossRef]
- Benbadis, S.R.; Lin, K. Errors in EEG interpretation and misdiagnosis of epilepsy—Which EEG patterns are overread? Eur. Neurol. 2008, 59, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.M. EEG in the diagnosis, classification, and management of patients with epilepsy. J. Neurol. Neurosurg. Psychiatry 2005, 76, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, E.; Bachir, D.H.A.; Sakadi, F.; Williams, J.; Vogel, A.C.; Schaekermann, M.; Tassiou, N.; Bah, A.K.; Khatri, V.; Hotan, G.C.; et al. Tablet-based electroencephalography diagnostics for patients with epilepsy in the West African Republic of Guinea. Eur. J. Neurol. 2020, 27, 1570–1577. [Google Scholar] [CrossRef]
- McKenzie, E.D.; Lim, A.S.P.; Leung, E.C.W.; Cole, A.J.; Lam, A.D.; Eloyan, A.; Nirola, D.K.; Tshering, L.; Thibert, R.; Garcia, R.Z.; et al. Validation of a smartphone-based EEG among people with epilepsy: A prospective study. Sci. Rep. 2017, 7, 45567. [Google Scholar] [CrossRef]
- Stopczynski, A.; Stahlhut, C.; Larsen, J.E.; Petersen, M.K.; Hansen, L.K. The Smartphone Brain Scanner: A Portable Real-Time Neuroimaging System. PLoS ONE 2014, 9, e86733, Erratum in PLoS ONE 2014, 9, e96652. [Google Scholar] [CrossRef]
- Fisher, R.S.; Scharfman, H.E.; Decurtis, M. How Can We Identify Ictal and Interictal Abnormal Activity? In Issues in Clinical Epileptology: A View from the Bench; Springer: Dordrecht, The Netherlands, 2014; Volume 813, pp. 3–23. [Google Scholar] [CrossRef]
- Le Van Quyen, M. Anticipating epileptic seizures: From mathematics to clinical applications. Comptes Rendus Biol. 2005, 328, 187–198. [Google Scholar] [CrossRef]
- Buettner, R.; Frick, J.; Rieg, T. High-performance detection of epilepsy in seizure-free EEG recordings: A novel machine learning approach using very specific epileptic EEG sub-bands. In Proceedings of the International Conference on Interaction Sciences, Busan, Republic of Korea, 16–18 August 2019. [Google Scholar]
- Rieg, T.; Frick, J.; Buettner, R. Machine Learning-Based Diagnosis of Epilepsy in Clinical Routine: Lessons Learned from a Retrospective Pilot Study. Inf. Syst. Neurosci. 2020, 43, 276–283. [Google Scholar] [CrossRef]
- van Diessen, E.; Diederen, S.J.H.; Braun, K.P.J.; Jansen, F.E.; Stam, C.J. Functional and structural brain networks in epilepsy: What have we learned? Epilepsia 2013, 54, 1855–1865. [Google Scholar] [CrossRef]
- Wagh, N.; Varatharajah, Y. EEG-GCNN: Augmenting Electroencephalogram-based Neurological Disease Diagnosis using a Domain-guided Graph Convolutional Neural Network. Proc. Mach. Learn. Res. 2020, 136, 367–378. [Google Scholar]
- Cao, J.; Grajcar, K.; Shan, X.C.; Zhao, Y.F.; Zou, J.R.; Chen, L.Y.; Li, Z.Q.; Grunewald, R.; Zis, P.; De Marco, M.; et al. Using interictal seizure-free EEG data to recognise patients with epilepsy based on machine learning of brain functional connectivity. Biomed. Signal Process. Control. 2021, 67, 102554. [Google Scholar] [CrossRef]
- Li, Q.; Gao, J.B.; Zhang, Z.W.; Huang, Q.; Wu, Y.; Xu, B. Distinguishing Epileptiform Discharges from Normal Electroencephalograms Using Adaptive Fractal and Network Analysis: A Clinical Perspective. Front. Physiol. 2020, 11, 828. [Google Scholar] [CrossRef]
- Chawla, S.; Kurani, S.; Wren, S.M.; Stewart, B.; Burnham, G.; Kushner, A.; McIntyre, T. Electricity and generator availability in LMIC hospitals: Improving access to safe surgery. J. Surg. Res. 2018, 223, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Wallis, L.; Blessing, P.; Dalwai, M.; Shin, S.D. Integrating mHealth at point of care in low- and middle-income settings: The system perspective. Glob. Health Action 2017, 10, 1327686. [Google Scholar] [CrossRef] [PubMed]
- Witter, S.; Thomas, S.; Topp, S.M.; Barasa, E.; Chopra, M.; Cobos, D.; Blanchet, K.; Teddy, G.; Atun, R.; Ager, A. Health system resilience: A critical review and reconceptualisation. Lancet Glob. Health 2023, 11, e1454–e1458. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.; Harutyunyan, A.; Sargsyan, K. Digitization of Clinical Pathways in Low- and Middle-Income Countries. In Digitalization of Medicine in Low- and Middle-Income Countries: Paradigm Changes in Healthcare and Biomedical Research; Kozlakidis, Z., Muradyan, A., Sargsyan, K., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 141–148. [Google Scholar]
- Kruk, M.E.; Gage, A.D.; Arsenault, C.; Jordan, K.; Leslie, H.H.; Roder-DeWan, S.; Adeyi, O.; Barker, P.; Daelmans, B.; Doubova, S.V.; et al. High-quality health systems in the Sustainable Development Goals era: Time for a revolution. Lancet Glob. Health 2018, 6, e1196–e1252. [Google Scholar] [CrossRef]
- Ciecierski-Holmes, T.; Singh, R.; Axt, M.; Brenner, S.; Barteit, S. Artificial intelligence for strengthening healthcare systems in low- and middle-income countries: A systematic scoping review. npj Digit. Med. 2022, 5, 162. [Google Scholar] [CrossRef]
- Beghi, E. Addressing the burden of epilepsy: Many unmet needs. Pharmacol. Res. 2016, 107, 79–84. [Google Scholar] [CrossRef]
- Friedman, E.A. Computer-assisted medical diagnosis for rural Sub-Saharan Africa. IEEE Technol. Soc. Mag. 2009, 28, 18–27. [Google Scholar] [CrossRef]
- Clarke, S.; Karoly, P.J.; Nurse, E.; Seneviratne, U.; Taylor, J.; Knight-Sadler, R.; Kerr, R.; Moore, B.; Hennessy, P.; Mendis, D.; et al. Computer-assisted EEG diagnostic review for idiopathic generalized epilepsy. Epilepsy Behav. 2021, 121, 106556. [Google Scholar] [CrossRef]
- Seidman, G.; Atun, R. Does task shifting yield cost savings and improve efficiency for health systems? A systematic review of evidence from low-income and middle-income countries. Hum. Resour. Health 2017, 15, 29. [Google Scholar] [CrossRef]
- Matheny, M.E.; Whicher, D.; Israni, S.T. Artificial Intelligence in Health Care a Report from the National Academy of Medicine. JAMA-J. Am. Med. Assoc. 2020, 323, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Liu, C.; Friedman, D. Artificial intelligence/machine learning for epilepsy and seizure diagnosis. Epilepsy Behav. 2024, 155, 109736. [Google Scholar] [CrossRef] [PubMed]
- Alkhaldi, M.; Abu Joudeh, L.; Ahmed, Y.B.; Husari, K.S. Artificial intelligence and telemedicine in epilepsy and EEG: A narrative review. Seizure 2024, 121, 204–210. [Google Scholar] [CrossRef]
- Singh, G.; Singh, M.B.; Ding, D.; Maulik, P.; Sander, J.W. Implementing WHO’s Intersectoral Global Action Plan for epilepsy and other neurological disorders in Southeast Asia: A proposal. Lancet Reg. Health Southeast. Asia 2023, 10, 100135. [Google Scholar] [CrossRef] [PubMed]
- Lekadir, K.; Frangi, A.F.; Porras, A.R.; Glocker, B.; Cintas, C.; Langlotz, C.P.; Weicken, E.; Asselbergs, F.W.; Prior, F.; Collins, G.S.; et al. FUTURE-AI: International consensus guideline for trustworthy and deployable artificial intelligence in healthcare. BMJ 2025, 388, e081554. [Google Scholar] [CrossRef]
- Milne-Ives, M.; Brownson-Smith, R.; Ananthakrishnan, A.; Wang, Y.; Cong, C.; Winston, G.P.; Meinert, E. The use of AI in epilepsy and its applications for people with intellectual disabilities: Commentary. Acta Epileptol. 2025, 7, 13. [Google Scholar] [CrossRef]

| Metric | High-Income Countries | Low- and Middle-Income Countries |
|---|---|---|
| Annual new epilepsy cases per 100,000 population | 49 | 139 |
| Lifetime prevalence of epilepsy per 1000 population | 5.18 | 8.75 |
| Median point prevalence of epilepsy per 1000 population | 5.49 | 6.68 |
| Annual epilepsy-related deaths | Less than 20% of 125,000 | More than 80% of 125,000 |
| Features | Brain State | Technique | Performance | Citations |
|---|---|---|---|---|
| Time-domain features | ||||
| Mean | Pre-ictal/ictal | Decision forest | Average accuracy: 98.5–99.7% | [96] |
| Pre-ictal/ictal | Random forest | Sensitivity: 93.8% | [97] | |
| Pre-ictal/ictal/interictal | Random forest | Accuracy: 94.3% | [98] | |
| Ictal | Random forest | Area under the ROC curve: 0.99 | [99] | |
| Ictal/interictal | Random forest | Area under the ROC curve: 0.90 | [100] | |
| Ictal/interictal | Random forest | Average accuracy: 98.6% | [101] | |
| Ictal | Decision forest | Area under the ROC curve: 0.64 | [102] | |
| Ictal | Support vector machine | Accuracy: 99.4% | [103] | |
| K-nearest neighbors | Accuracy: 99.4% | |||
| Root mean square | Ictal/interictal | Support vector machine | Accuracy: 95.6% | [104] |
| Ictal/interictal | Support vector machine | Accuracy: 99.1% | [105] | |
| Ictal/interictal | K-nearest neighbors | Area under the ROC curve: 0.91 | [106] | |
| Variance | Ictal/interictal | Support vector machine | Accuracy: 95.6% | [104] |
| Pre-ictal, ictal | Random forest | Sensitivity: 93.8% | [97] | |
| Ictal/interictal | Support vector machine | Accuracy: 99.1% | [105] | |
| Ictal/interictal | K-nearest neighbors | Area under the ROC curve: 0.91 | [106] | |
| Ictal/interictal | Random forest | Area under the ROC curve: 0.90 | [100] | |
| Maxima and minima | Ictal/interictal | Support vector machine | Accuracy: 99.1% | [105] |
| Ictal/interictal | Random forest | Average accuracy: 98.6% | [101] | |
| Ictal | Support vector machine | Accuracy: 99.4% | [103] | |
| K-nearest neighbors | Accuracy: 99.4% | |||
| Pre-ictal/ictal | Decision forest | Average accuracy: 98.5–99.7% | [96] | |
| Ictal | Decision forest | Area under the ROC curve: 0.67 | [102] | |
| Mode and median | Ictal/interictal | Random forest | Average accuracy: 98.6% | [101] |
| Skewness | Pre-ictal/ictal | Decision forest | Average accuracy: 98.5–99.7% | [96] |
| Ictal | Support vector machine | Accuracy: 99.4% | [103] | |
| K-nearest neighbors | Accuracy: 99.4% | |||
| Ictal/interictal | Random forest | Average accuracy: 98.60% | [101] | |
| Pre-ictal/ictal/interictal | Random forest | Accuracy: 94.3% | [98] | |
| Pre-ictal/ictal | Random forest | Sensitivity: 93.8% | [97] | |
| Ictal/interictal | Support vector machine | Accuracy: 99.1% | [105] | |
| Ictal/interictal | K-nearest neighbors | Area under the ROC curve: 0.91 | [106] | |
| Ictal/interictal | Random forest | Area under the ROC curve: 0.90 | [100] | |
| Kurtosis | Pre-ictal/ictal | Decision forest | Average accuracy: 98.5–99.7% | [96] |
| Ictal | Support vector machine | Accuracy: 99.4% | [103] | |
| K-nearest neighbors | Accuracy: 99.4% | |||
| Ictal/interictal | Random forest | Average accuracy: 98.6% | [101] | |
| Pre-ictal/ictal/interictal | Random forest | Accuracy: 94.3% | [98] | |
| Ictal/interictal | Support vector machine | Accuracy: 99.1% | [105] | |
| Ictal/interictal | K-nearest neighbors | Area under the ROC curve: 0.91 | [106] | |
| Ictal/interictal | Random forest | Area under the ROC curve: 0.90 | [100] | |
| Line length | Pre-ictal/ictal | Decision forest Support vector machine K-nearest neighbors Random forest Random forest Random forest Support vector machine Decision forest Neural network Burst detection algorithm Multi-layer perceptron neural network | Average accuracy: 98.5–99.7% Accuracy: 99.4% Accuracy: 99.4% Area under the ROC curve: 0.90 Accuracy: 94.3% Sensitivity: 93.8% Area under the ROC curve: 0.88 Area under the ROC curve: 0.77 -Accuracy: 84.2% Accuracy: 99.6% | [96] [103] [100] [98] [97] [107] [102] [108] [109] [110] |
| Ictal | ||||
| Ictal/interictal | ||||
| Pre-ictal/ictal/interictal | ||||
| Pre-ictal/ictal | ||||
| Ictal/interictal | ||||
| Ictal | ||||
| Ictal | ||||
| Ictal | ||||
| Ictal | ||||
| Ictal | ||||
| Energy | Ictal | Decision forest | Area under the ROC curve: 0.74 | [102] |
| Ictal/interictal | Support vector machine | Accuracy: 99.4% | [103] | |
| K-nearest neighbors | Accuracy: 99.4% | |||
| Ictal | Independent component analysis | Area under the ROC curve: 0.92 | [111] | |
| Ictal | Support vector machine | Accuracy: 95.6% | [104] | |
| Ictal | Automated classification algorithm | Accuracy: 99.4% | [112] | |
| Ictal/interictal | Support vector machine | Accuracy: 99.1% | [105] | |
| Pre-ictal/ictal | Decision forest | Average accuracy: 98.5–99.7% | [96] | |
| Power | Ictal | Decision forest | Area under the ROC curve: 0.74 Area under the ROC curve: 0.99 | [102] [99] |
| Ictal | Random forest | |||
| Shannon entropy | Ictal/interictal | Support vector machine | Accuracy: 99.5% | [113] |
| Ictal/interictal | Random forest | Average accuracy: 98.6% | [101] | |
| Ictal | Support vector machine | Accuracy: 99.4% | [103] | |
| K-nearest neighbors | Accuracy: 99.4% | |||
| Pre-ictal, ictal | Decision forest | Average accuracy: 98.5–99.7% | [96] | |
| Sample and approximate entropies | Ictal | K-nearest neighbor | Accuracy: 98.0% | [114] |
| Ictal | Discrete wavelet transformation | Accuracy: 98.0% | [115] | |
| Ictal/interictal | Extreme learning machine | Accuracy: 95.6% | [116] | |
| Ictal/interictal | Extreme learning machine | Accuracy: 99.6% | [117] | |
| Support vector machine | Accuracy: 100% | |||
| Pre-ictal | Fuzzy Sugeno Classifier | Accuracy: 98.1% | [118] | |
| Ictal/interictal | Support vector machine | Accuracy: 99.1% | [105] | |
| Ictal/interictal | K-nearest neighbors | Area under the ROC curve: 0.91 | [106] | |
| Fuzzy entropy | Ictal/interictal | Support vector machine | Accuracy: 99.5% | [113] |
| Hurst exponent | Ictal/interictal | Random forest | Average accuracy: 98.6% | [101] |
| Standard deviation | Pre-ictal, ictal | Decision forest | Average accuracy: 98.5–99.7% | [96] |
| Ictal | Random forest | Area under the ROC curve: 0.99 | [99] | |
| Pre-ictal/ictal/interictal | Random forest | Accuracy: 94.3% | [98] | |
| Ictal/interictal | Support vector machine | Accuracy: 99.1% | [105] | |
| Ictal/interictal | Random forest | Average accuracy: 98.6% | [101] | |
| Ictal | Support vector machine | Accuracy: 99.4% | [103] | |
| K-nearest neighbors | Accuracy: 99.4% | |||
| Autocorrelation | Pre-ictal, ictal | Random forest | Sensitivity: 93.8% | [97] |
| Ictal/interictal | Random forest | Area under the ROC curve: 0.90 | [100] | |
| Mean absolute deviation | Ictal/interictal | Random forest | Area under the ROC curve: 0.90 | [100] |
| Amplitude | Ictal/interictal | Extreme learning machine | Sensitivity: 97.7% | [119] |
| Pattern match regularity statistic | Ictal/interictal | Extreme learning machine | Sensitivity: 97.7% | [119] |
| Frequency-domain features | ||||
| Spectral power | Pre-ictal/ictal | Random forest | Sensitivity: 93.8% | [97] |
| Ictal | Random forest | Sensitivity: 80.8% | [120] | |
| Ictal | Artificial neural network | F-measure: 0.82 | [121] | |
| Ictal/interictal | Support vector machine | Accuracy: 99.1% | [105] | |
| Ictal/interictal | Artificial neural network | Accuracy: 97.7–100% | [122] | |
| Spectral entropy | Ictal/interictal | Support vector machine | Accuracy: 99.1% | [105] |
| Peak frequency | Ictal/interictal | K-nearest neighbors | Area under the ROC curve: 0.91 | [106] |
| Median frequency | Ictal/interictal | Support vector machine | Accuracy: 99.1% | [105] |
| Ictal/interictal | K-nearest neighbors | Area under the ROC curve: 0.91 | [106] | |
| Power spectral density | Ictal | Random forest | Sensitivity: 80.8% | [120] |
| Ictal/interictal | Extreme learning machine | Sensitivity: 97.7% | [119] | |
| Average power and power ratio | Ictal/interictal | Random forest | Area under the ROC curve: 0.90 | [100] |
| Mean frequency | Ictal/interictal | Support vector machine | Accuracy: 96.1% | [123] |
| Total spectral power | Ictal | Random forest | Sensitivity: 80.8% | [120] |
| Ictal | Artificial neural network | F-measure: 0.82 | [121] | |
| Root mean square bandwidth | Ictal/interictal | Support vector machine | Accuracy: 96.1% | [123] |
| Discrete cosine transform | Ictal/interictal | Support vector machine | Accuracy: 84.1% | [124] |
| Wavelet transformation features | ||||
| DWT features | ||||
| Bounded variation | Ictal | Decision forest | Area under the ROC curve: 0.53 | [102] |
| Coefficients | Ictal | Decision forest | Area under the ROC curve: 0.66 | [102] |
| Interictal | K-nearest neighbors | Accuracy: 98.0% | [125] | |
| Ictal/interictal | Support vector machine | Accuracy: 84.1% | [124] | |
| Energy | Ictal | Decision forest | Area under the ROC curve: 0.71 | [102] |
| Interictal | K-nearest neighbors | Accuracy: 98.0% | [125] | |
| Relative power | Interictal | K-nearest neighbors | Accuracy: 98.0% | [125] |
| Ictal | Decision forest | Area under the ROC curve: 0.81 | [102] | |
| Entropy | Ictal | Decision forest | Area under the ROC curve: 0.71 | [102] |
| Relative bounded variation | Ictal | Decision forest | Area under the ROC curve: 0.54 | [102] |
| Relative scale energy | Ictal | Decision forest | Area under the ROC curve: 0.61 | [102] |
| CWT features | ||||
| Energy standard deviation | Ictal | Decision forest | Area under the ROC curve: 0.70 | [102] |
| Coefficient z-score | Ictal | Decision forest | Area under the ROC curve: 0.69 | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koirala, N.; Adhikari, S.R.; Adhikari, M.; Yadav, T.; Anwar, A.R.; Ciolac, D.; Shrestha, B.; Adhikari, I.; Khanal, B.; Muthuraman, M. Assistive Artificial Intelligence in Epilepsy and Its Impact on Epilepsy Care in Low- and Middle-Income Countries. Brain Sci. 2025, 15, 481. https://doi.org/10.3390/brainsci15050481
Koirala N, Adhikari SR, Adhikari M, Yadav T, Anwar AR, Ciolac D, Shrestha B, Adhikari I, Khanal B, Muthuraman M. Assistive Artificial Intelligence in Epilepsy and Its Impact on Epilepsy Care in Low- and Middle-Income Countries. Brain Sciences. 2025; 15(5):481. https://doi.org/10.3390/brainsci15050481
Chicago/Turabian StyleKoirala, Nabin, Shishir Raj Adhikari, Mukesh Adhikari, Taruna Yadav, Abdul Rauf Anwar, Dumitru Ciolac, Bibhusan Shrestha, Ishan Adhikari, Bishesh Khanal, and Muthuraman Muthuraman. 2025. "Assistive Artificial Intelligence in Epilepsy and Its Impact on Epilepsy Care in Low- and Middle-Income Countries" Brain Sciences 15, no. 5: 481. https://doi.org/10.3390/brainsci15050481
APA StyleKoirala, N., Adhikari, S. R., Adhikari, M., Yadav, T., Anwar, A. R., Ciolac, D., Shrestha, B., Adhikari, I., Khanal, B., & Muthuraman, M. (2025). Assistive Artificial Intelligence in Epilepsy and Its Impact on Epilepsy Care in Low- and Middle-Income Countries. Brain Sciences, 15(5), 481. https://doi.org/10.3390/brainsci15050481








