Reward System EEG–fMRI-Pattern Neurofeedback for Major Depressive Disorder with Anhedonia: A Multicenter Pilot Study
Abstract
1. Introduction
2. Methods
2.1. Participants and Recruitment
- Primary diagnosis of MDD with anhedonia, with a SHAPS-C score ≥ 25;
- MDD diagnosis determined via the neuropsychiatric interview (MINI for DSM-5);
- Aged 22 to 50;
- Any gender;
- High school diploma or equivalent;
- Right-handed if undergoing an MRI [27];
- Normal or corrected-to-normal vision and hearing;
- Ability to provide signed, informed consent;
- Ability to adhere to the study schedule;
- Concomitant psychotropic medications allowed if they are at a stable dose for 4 weeks prior to the study.
- History of psychotic disorder or bipolar I;
- Moderate or severe substance use disorder within 3 months of screening;
- Lifetime diagnosis of autism or intellectual disability allowed at the investigators’ discretion;
- Benzodiazepines that cannot be ceased for the duration of the study (with a washout period of at least 2 weeks prior to the first Prism training session) or that cannot be replaced with short-acting benzodiazepines;
- Current diagnosis of PTSD;
- Treatment-resistant depression, defined as episodes that did not have a ≥50% symptom reduction to at least two full trials;
- Any past or current use of DA (dopamine agonist)-acting drugs;
- Recent initiation of any evidence-based MDD psychotherapy was excluded but continuation of established therapy was allowed.
2.2. Study Design, Device Description, Outcome Measures, and Statistical Methods
2.2.1. Study Design
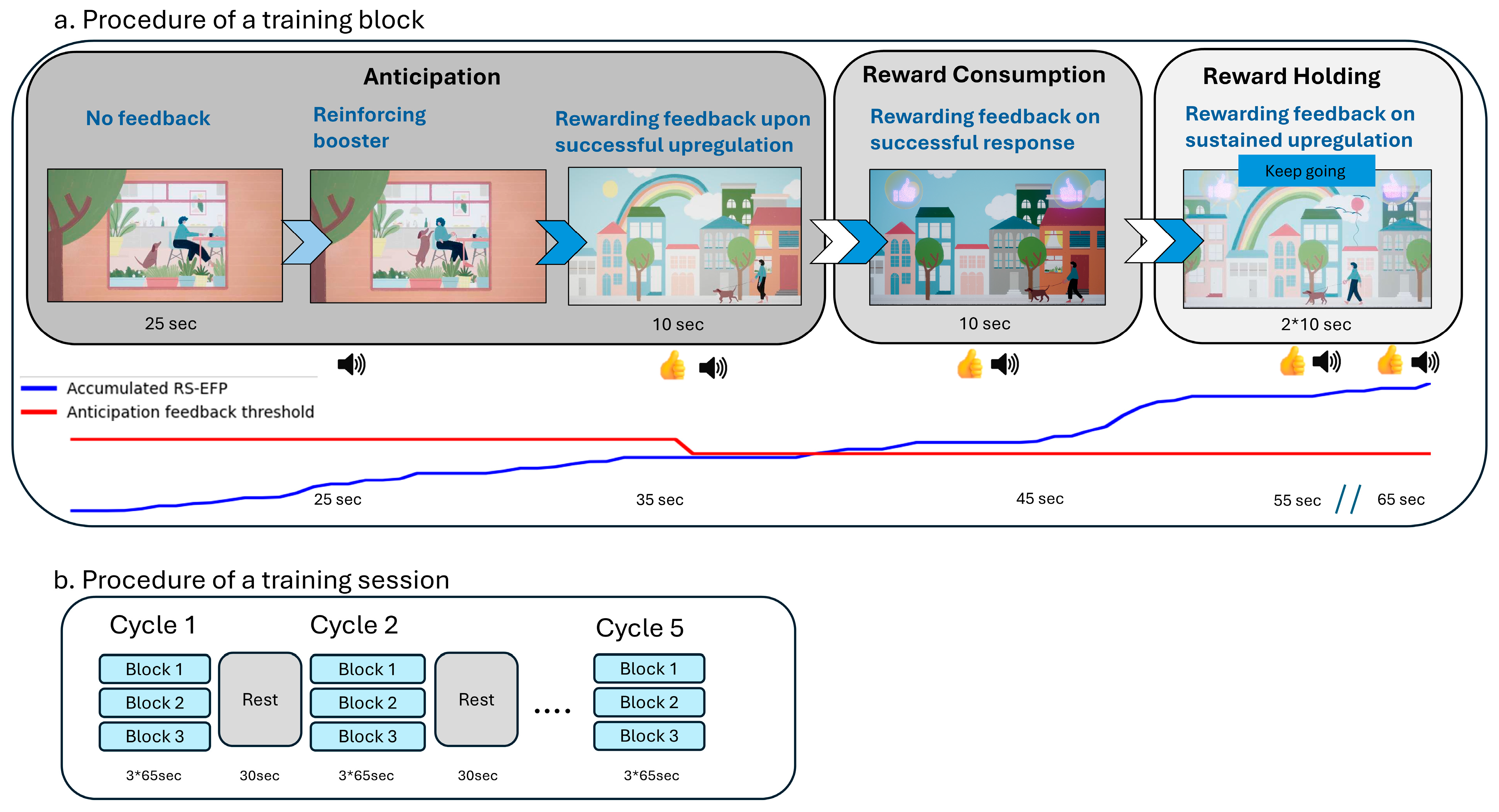
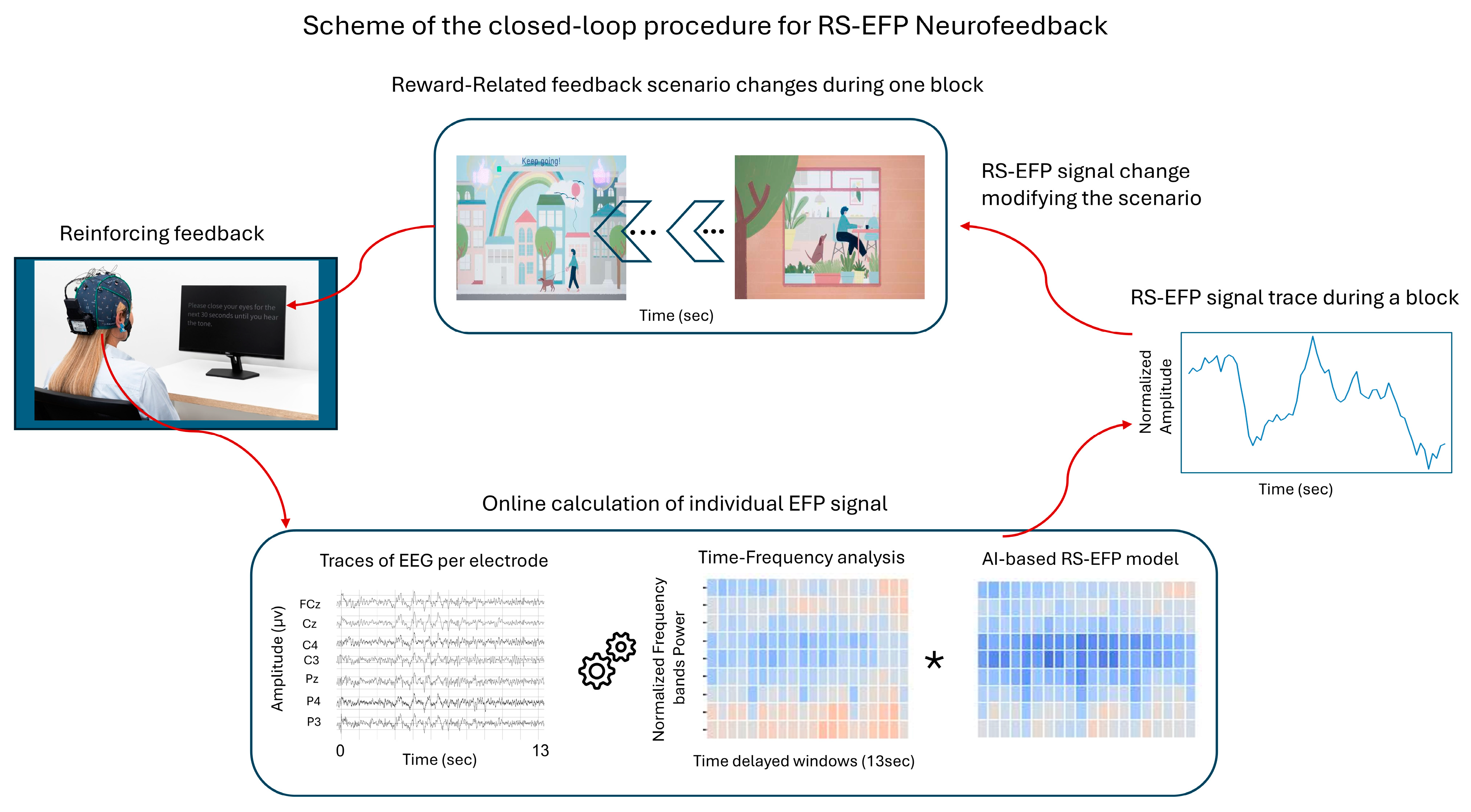
2.2.2. Device Description
2.2.3. Outcome Measures
- Null Hypothesis: mean change from baseline to the post-Prism training visit in the Clinician HDRS-17 (week 6), HDRS ≥ −4;
- Alternative Hypothesis: mean change from baseline to the post-Prism training visit in HDRS-17 (week 6), HDRS < −4.
2.2.4. Statistical Methods
3. Results
3.1. Safety Analysis (AEs)
3.2. Demographic and Baseline Characteristics (FA Set)
3.3. Clinical Outcomes (EF Set)
3.3.1. Primary Efficacy Outcomes
3.3.2. Secondary Efficacy Outcomes
3.3.3. Effect Sizes and Clinical Significance
3.3.4. Patient Satisfaction
4. Discussion
4.1. Addressing the Anhedonia Treatment Gap in MDD
4.2. Comparison with Existing Treatment Approaches
4.3. Potential for Scalable Clinical Implementation/Feasibility
4.4. Methodological Considerations and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Anterior cingulate cortex |
| AE | Adverse event |
| AI | Artificial intelligence |
| ANCOVA | Analysis of covariance |
| CGI-I | Clinical Global Impression-Improvement |
| CI | Confidence interval |
| CRED-nf | Consensus on the Reporting of Experimental Design neurofeedback |
| DA | Dopamine agonist |
| DSM-5 | Diagnostic and statistical manual for mental disorders |
| EEG | Electroencephalogram |
| EF | Efficacy Analysis |
| FA | Full Analysis |
| fMRI | Functional magnetic resonance imaging |
| GAD-7 | General Anxiety Disorder Scale |
| HDRS-17 | Hamilton Depression Rating Scale |
| LSMean | Least squared mean |
| MDD | Major depressive disorder |
| MINI | Mini-International Neuropsychiatric Interview |
| NF | Neurofeedback |
| PAI | Positive activity intervention |
| PASS | Positive affect stimulation sustainment |
| PAT | Positive affect treatment |
| PHQ-9 | Patient Health Questionnaire |
| PP | Per Protocol |
| QIDS-SR-16 | Quick Inventory Depressive Symptomatology |
| RDoC | Research domain criteria |
| RS-EFP-NF | Reward system electronic fingerprint neurofeedback |
| SE | Standard error |
| SHAPS-C | Snaith–Hamilton Pleasure Scale |
| SNRIs | Serotonin and norepinephrine reuptake inhibitors |
| SSRIs | Selective serotonin reuptake inhibitors |
| STAR*D | Sequenced treatment alternatives to relieve depression |
| TMS | Transcranial magnetic stimulation |
| VS | Ventral striatum |
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar]
- Shankman, S.A.; Katz, A.C.; DeLizza, A.A. The Different Facets of Anhedonia and Their Associations with Different Psychopathologies. In Anhedonia: A Comprehensive Handbook Volume I; Springer Nature: Berlin/Heidelberger, Germany, 2014; pp. 3–22. [Google Scholar]
- Cao, B.; Park, C.; Subramaniapillai, M. The Efficacy of Vortioxetine on Anhedonia in Patients with Major Depressive Disorder. Front. Psychiatry 2019, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Yin, Y.; He, C. Disrupted Reward Circuits Is Associated with Cognitive Deficits and Depression Severity in Major Depressive Disorder. J. Psychiatr. Res. 2017, 84, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Spijker, J.; Bijl, R.V.; de Graaf, R.; Nolen, W.A. Determinants of Poor 1-Year Outcome of DSM-III-R Major Depression in the General Population: Results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Acta Psychiatr. Scand. 2001, 103, 122–130. [Google Scholar] [CrossRef]
- Vrieze, E.; Demyttenaere, K.; Bruffaerts, R. Dimensions in Major Depressive Disorder and Their Relevance for Treatment Outcome. J. Affect. Disord. 2014, 155, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.; Gunning, F.; Liston, C. Causes and Consequences of Diagnostic Heterogeneity in Depression: Paths to Discovering Novel Biological Depression Subtypes. Biol. Psychiatry 2020, 88, 83–94. [Google Scholar] [CrossRef]
- Uher, R.; Perlis, R.H.; Henigsberg, N. Depression Symptom Dimensions as Predictors of Antidepressant Treatment Outcome: Replicable Evidence for Interest-Activity Symptoms. Psychol. Med. 2011, 42, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Treadway, M.T.; Zald, D.H. Reconsidering Anhedonia in Depression: Lessons from Translational Neuroscience. Neurosci. Biobehav. Rev. 2011, 35, 537–555. [Google Scholar] [CrossRef]
- Serretti, A. Anhedonia and Depressive Disorders. Clin. Psychopharmacol. Neurosci. 2023, 21, 401–409. [Google Scholar] [CrossRef]
- Argyropoulos, S.V.; Nutt, D.J. Anhedonia Revisited: Is There a Role for Dopamine-Targeting Drugs for Depression? J. Psychopharmacol. 2013, 27, 869–877. [Google Scholar] [CrossRef]
- Price, J.; Cole, V.; Goodwin, G.M. Emotional Side-Effects of Selective Serotonin Reuptake Inhibitors: Qualitative Study. Br. J. Psychiatry 2009, 195, 211–217. [Google Scholar] [CrossRef]
- Höflich, A.; Michenthaler, P.; Kasper, S. Circuit Mechanisms of Reward, Anhedonia, and Depression. Int. J. Neuropsychopharmacol. 2018, 22, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, D.A. Toward a Better Understanding of the Mechanisms and Pathophysiology of Anhedonia: Are We Ready for Translation? Am. J. Psychiatry 2022, 179, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, M.; Russo, S.J. Anhedonia and the Brain Reward Circuitry in Depression. Curr. Behav. Neurosci. Rep. 2015, 2, 146–153. [Google Scholar] [CrossRef]
- Pizzagalli, D.A. Depression, Stress, and Anhedonia: Toward a Synthesis and Integrated Model. Annu. Rev. Clin. Psychol. 2014, 10, 393–423. [Google Scholar] [CrossRef]
- Borsini, A.; St John Wallis, A.; Zunszain, P. Characterizing Anhedonia: A Systematic Review of Neuroimaging across the Subtypes of Reward Processing Deficits in Depression. Cogn. Affect. Behav. Neurosci. 2020, 20, 816–841. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.; Roiser, J.P. Neuroscience of Apathy and Anhedonia: A Transdiagnostic Approach. Nat. Rev. Neurosci. 2018, 19, 470–484. [Google Scholar] [CrossRef]
- Treadway, M.T.; Zald, D.H. Parsing Anhedonia: Translational Models of Reward-Processing Deficits in Psychopathology. Curr. Dir. Psychol. Sci. 2013, 22, 244–249. [Google Scholar] [CrossRef]
- Der-Avakian, A.; Markou, A. The Neurobiology of Anhedonia and Other Reward-Related Deficits. Trends Neurosci. 2012, 35, 68–77. [Google Scholar] [CrossRef]
- Rolls, E.T.; Grabenhorst, F. The Orbitofrontal Cortex and beyond: From Affect to Decision-Making. Prog. Neurobiol. 2008, 86, 216–244. [Google Scholar] [CrossRef]
- Winer, E.S.; Jordan, D.G.; Collins, A.C. Conceptualizing Anhedonias and Implications for Depression Treatments. Psychol. Res. Behav. Manag. 2019, 12, 325–335. [Google Scholar] [CrossRef]
- Craske, M.G.; Meuret, A.E.; Ritz, T. Treatment for Anhedonia: A Neuroscience Driven Approach. Depress. Anxiety 2016, 33, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Keynan, J.N.; Meir-Hasson, Y.; Gilam, G.; Cohen, A.; Jackont, G.; Kinreich, S.; Ikar, L.; Or-Borichev, A.; Etkin, A.; Gyurak, A.; et al. Limbic Activity Modulation Guided by FMRI-Inspired EEG Improves Implicit Emotion Regulation Short Title: FMRI Inspired EEG-NF for Improved Emotion Regulation. Biol. Psychiatry 2016, 80, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Singer, N.; Poker, G.; Dunsky-Moran, N.; Nemni, S.; Reznik Balter, S.; Doron, M.; Baker, T.; Dagher, A.; Zatorre, R.J.; Hendler, T. Development and Validation of an FMRI-Informed EEG Model of Reward-Related Ventral Striatum Activation. Neuroimage 2023, 276, 120183. [Google Scholar] [CrossRef] [PubMed]
- Lubianiker, N.; Goldway, N.; Fruchtman-Steinbok, T. Process-Based Framework for Precise Neuromodulation. Nat. Hum. Behav. 2019, 3, 436–445. [Google Scholar] [CrossRef]
- Chapman, L.J.; Chapman, J.P. The measurement of handedness. Brain Cogn. 1987, 6, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, F.; Cincotti, F.; Carducci, F. Spatial Enhancement of EEG Data by Surface Laplacian Estimation: The Use of Magnetic Resonance Imaging-Based Head Models. Clin. Neurophysiol. 2001, 112, 724–727. [Google Scholar] [CrossRef]
- Browne, R.H. On the Use of a Pilot Sample for Sample Size Determination. Stat. Med. 1995, 14, 1933–1940. [Google Scholar] [CrossRef]
- Cohen, A.; Keynan, J.N.; Jackont, G. Multi-Modal Virtual Scenario Enhances Neurofeedback Learning. Front. Robot. AI 2016, 3, 52. [Google Scholar] [CrossRef]
- Harmer, C.J.; Duman, R.S.; Cowen, P.J. How Do Antidepressants Work? New Perspectives for Refining Future Treatment Approaches. Lancet Psychiatry 2017, 4, 409–418. [Google Scholar] [CrossRef]
- Serretti, A.; Calati, R.; Goracci, A. Antidepressants in Healthy Subjects: What Are the Psychotropic/Psychological Effects? Eur. Neuropsychopharmacol. 2010, 20, 433–453. [Google Scholar] [CrossRef]
- Mendelewicz, J. Towards Achieving Remission in the Treatment of Depression. Dialog. Clin. Neurosci. 2008, 10, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Young, K.D.; Siegle, G.J.; Zotev, V.; Phillips, R.; Misaki, M.; Yuan, H.; Drevets, W.C.; Bodurka, J. Randomized Clinical Trial of Real-Time FMRI Amygdala Neurofeedback for Major Depressive Disorder: Effects on Symptoms and Autobiographical Memory Recall. Am. J. Psychiatry 2017, 174, 748–755. [Google Scholar] [CrossRef]
- Patil, A.U.; Lin, C.; Lee, S.H. Review of EEG-Based Neurofeedback as a Therapeutic Intervention to Treat Depression. Psychiatry Res. Neuroimaging 2023, 329, 111591. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Alvarez, J.; Grassi, M.; Colombo, D. Efficacy of Bio- and Neurofeedback for Depression: A Meta-Analysis. Psychol. Med. 2022, 52, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.; Cuthbert, B.; Garvey, M.; Heinssen, R.; Pine, D.; Quinn, K.; Sanislow, C.; Wang, P. Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on Mental Disorders. Am. J. Psychiatry 2010, 167, 748–751. [Google Scholar] [CrossRef]
- Fine, N.B.; Helpman, L.; Bardin Armon, D.; Gurevitch, G.; Sheppes, G.; Seligman, Z.; Hendler, T.; Bloch, M. Amygdala-Related Electroencephalogram Neurofeedback as Add-on Therapy for Treatment-Resistant Childhood Sexual Abuse Posttraumatic Stress Disorder: Feasibility Study. Psychiatry Clin. Neurosci. 2024, 78, 19–28. [Google Scholar] [CrossRef]
| Number of Subjects | |
|---|---|
| Screened | 49 |
| Screen Failures | 5 |
| FA analysis set | 44 |
| Completed 1 session | 1 |
| Completed 3 sessions | 2 |
| Completed 4 sessions | 1 |
| Completed 6 sessions | 1 |
| Completed 7 sessions | 2 |
| Did not start NF sessions | 3 |
| EF analysis set | 34 |
| Major Protocol Violation | 0 |
| PP analysis set | 34 |
| FA Set | |||
|---|---|---|---|
| Age (years) | N | 44 | |
| Mean (SD) | 39.9 (11.03) | ||
| Median [Range] | 39.1 [21.5; 64.6] | ||
| Gender | Male | % (n/N) | 25.0% (11/44) |
| Female | % (n/N) | 75.0% (33/44) | |
| Ethnicity | Not of Hispanic or Latino origin | % (n/N) | 100% (44/44) |
| Race | Caucasian | % (n/N) | 93.2% (41/44) |
| Other | % (n/N) | 6.8% (3/44) | |
| Years of Education | Did not finish high school | % (n/N) | 2.3% (1/44) |
| High school diploma or equivalent | % (n/N) | 40.9% (18/44) | |
| Some college, no degree | % (n/N) | 15.9% (7/44) | |
| Associate degree (for example: AA or AS) | % (n/N) | 4.5% (2/44) | |
| Bachelor’s degree (for example: BA or BS) | % (n/N) | 31.8% (14/44) | |
| Master’s degree (For example: MA or MS) | % (n/N) | 4.5% (2/44) | |
| Marital Status | Married | % (n/N) | 40.9% (18/44) |
| Divorced | % (n/N) | 29.5% (13/44) | |
| Single | % (n/N) | 29.5% (13/44) | |
| Laterality | Right | % (n/N) | 95.5% (42/44) |
| Ambidextrous | % (n/N) | 4.5% (2/44) | |
| Duration of Current Episode | Mean (SD) in months | 72 (88.8) | |
| Concomitant Meds | SSRIs/SNRIs | % (n/N) | 66% (29/44) |
| Cannabis | % (n/N) | 16% (7/44) | |
| Benzodiazepines | % (n/N) | 30% (13/44) | |
| Other | % (n/N) | 36.4% (16/44) | |
| Comorbidities | Fibromyalgia | % (n/N) | 20.5% (9/44) |
| Insomnia | % (n/N) | 6.8% (3/44) | |
| PTSD | % (n/N) | 4.5% (2/44) | |
| Migraine | % (n/N) | 4.5% (2/44) | |
| Irritable bowel syndrome (IBS) | % (n/N) | 4.5% (2/44) |
| Primary and Secondary Endpoints | Baseline Value |
|---|---|
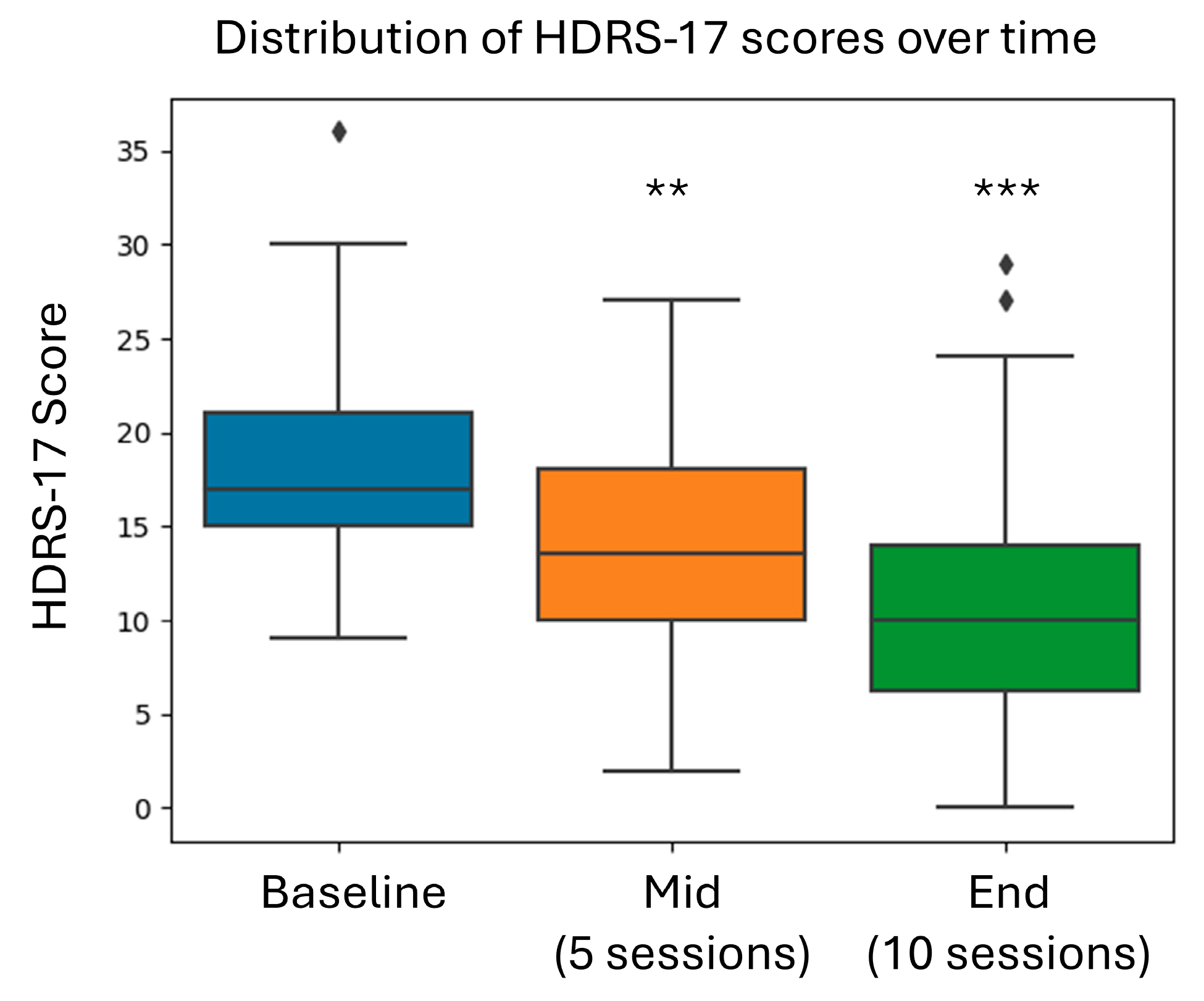
| HDRS-17 | 19.1 ± 6.26 |
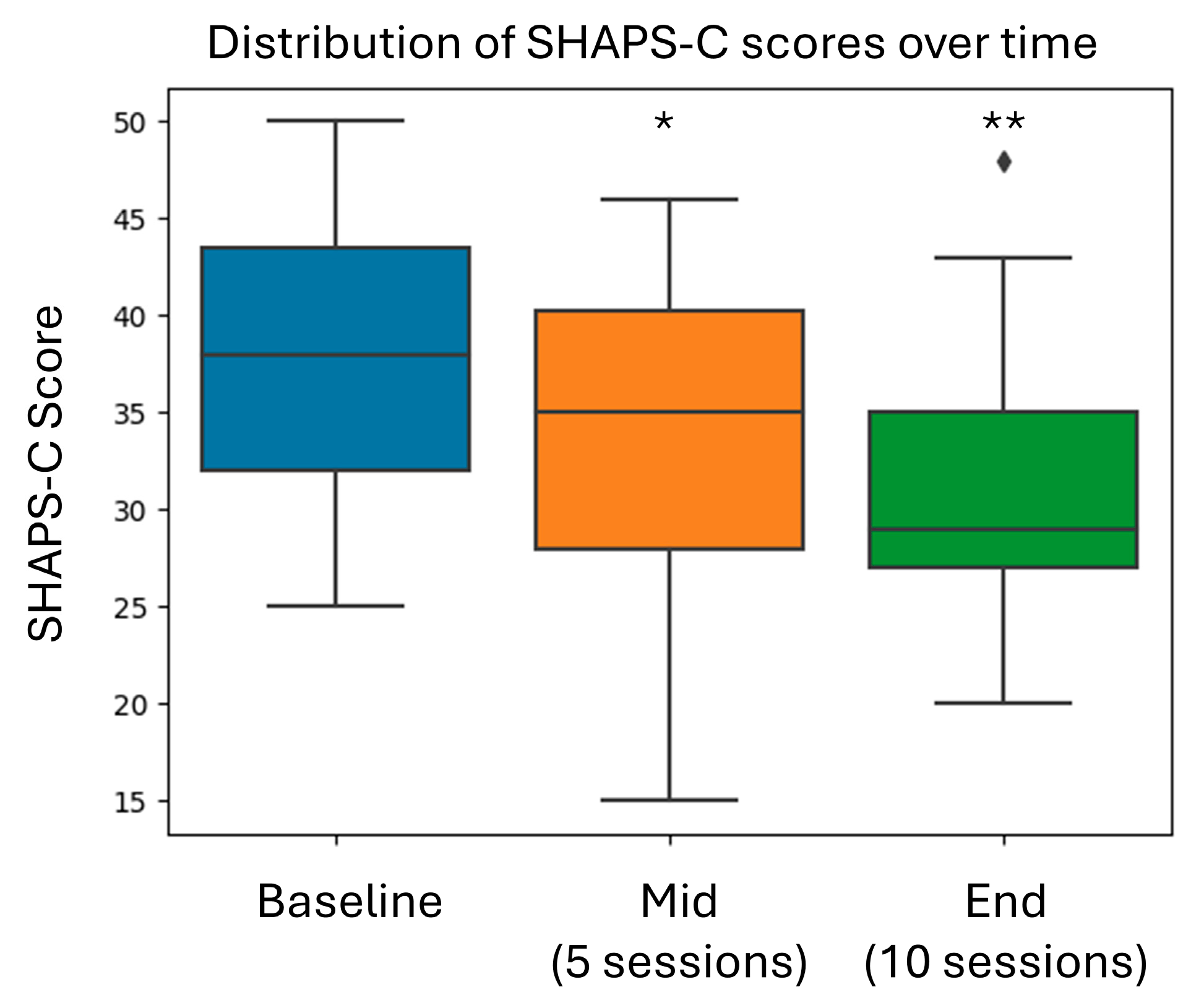
| SHAPS-C | 38.8 ± 6.85 |
| QIDS-SR-16 | 15.5 ± 5.03 |
| GAD-7 | 11.4 ± 5.74 |
| PHQ-9 | 12.4 ± 4.7 |
| Instrument | Baseline to 6-Week Assessment | |
|---|---|---|
| LS Means (95% CI) | p-Value | |
| HDRS-17 | −8.0 (−10.5 to −5.41) | <0.0001 |
| SHAPS-C | −6.3 (−8.51 to −4.14) | <0.0001 |
| CGI-I | 2.5 (2.22 to 0.72) | <0.0001 |
| QIDS-SR-16 | −4.3 (−5.97 to −2.62) | <0.0001 |
| GAD-7 | −3.3 (−4.47 to −2.12) | <0.0001 |
| PHQ-9 | −4.7 (−7.94 to −1.40) | <0.01 |
| Instrument | Effect Size | p-Value |
|---|---|---|
| HDRS-17 | 1.22 [95% CI: 0.7 to 1.74] | <0.00001 |
| SHAPS-C | 0.92 [95% CI: 0.42 to 1.42] | 0.0003 |
| QIDS-SR-16 | 0.81 [95% CI: 0.31 to 1.3] | 0.001 |
| GAD-7 | 0.54 [95% CI: 0.06 to 1.03] | 0.03 |
| PHQ-9 | 0.75 [95% CI: 0.03 to 1.47] | 0.04 |
| Mean (SD) | ||
|---|---|---|
| EF | To what extant were you satisfied with the NF training in this trial? | 3.6 (1.05) |
| In your opinion, how effective was the NF training? | 3.6 (1.13) | |
| Would you recommend the use of the PRISM system (used in this trial) to your friends/family members? | 3.7 (1.24) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amital, D.; Gross, R.; Goldental, N.; Fruchter, E.; Yaron-Wachtel, H.; Tendler, A.; Stern, Y.; Deutsch, L.; Voigt, J.D.; Hendler, T.; et al. Reward System EEG–fMRI-Pattern Neurofeedback for Major Depressive Disorder with Anhedonia: A Multicenter Pilot Study. Brain Sci. 2025, 15, 476. https://doi.org/10.3390/brainsci15050476
Amital D, Gross R, Goldental N, Fruchter E, Yaron-Wachtel H, Tendler A, Stern Y, Deutsch L, Voigt JD, Hendler T, et al. Reward System EEG–fMRI-Pattern Neurofeedback for Major Depressive Disorder with Anhedonia: A Multicenter Pilot Study. Brain Sciences. 2025; 15(5):476. https://doi.org/10.3390/brainsci15050476
Chicago/Turabian StyleAmital, Daniela, Raz Gross, Nadav Goldental, Eyal Fruchter, Haya Yaron-Wachtel, Aron Tendler, Yaki Stern, Lisa Deutsch, Jeffrey D. Voigt, Talma Hendler, and et al. 2025. "Reward System EEG–fMRI-Pattern Neurofeedback for Major Depressive Disorder with Anhedonia: A Multicenter Pilot Study" Brain Sciences 15, no. 5: 476. https://doi.org/10.3390/brainsci15050476
APA StyleAmital, D., Gross, R., Goldental, N., Fruchter, E., Yaron-Wachtel, H., Tendler, A., Stern, Y., Deutsch, L., Voigt, J. D., Hendler, T., Harmelech, T., Singer, N., & Sharon, H. (2025). Reward System EEG–fMRI-Pattern Neurofeedback for Major Depressive Disorder with Anhedonia: A Multicenter Pilot Study. Brain Sciences, 15(5), 476. https://doi.org/10.3390/brainsci15050476








