A Methodological Evaluation of Four Different Paired Associative Stimulation Paradigms in Healthy Controls
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Transcranial Magnetic Stimulation:
2.4. Electromyography
2.5. Peripheral Nerve Stimulation
2.6. Resting Motor Thresholds and PAS Stimulation Intensity
2.7. PAS Testing
2.8. Attention
2.9. Statistics
2.10. Graphics
3. Results
3.1. Subject Demographics
3.2. No Significant Difference in RMT Between Test Sessions
3.3. PAS-10 with 180 or 225 Paired Pulses Did Not Result in Decreased but Increased Corticospinal Excitation
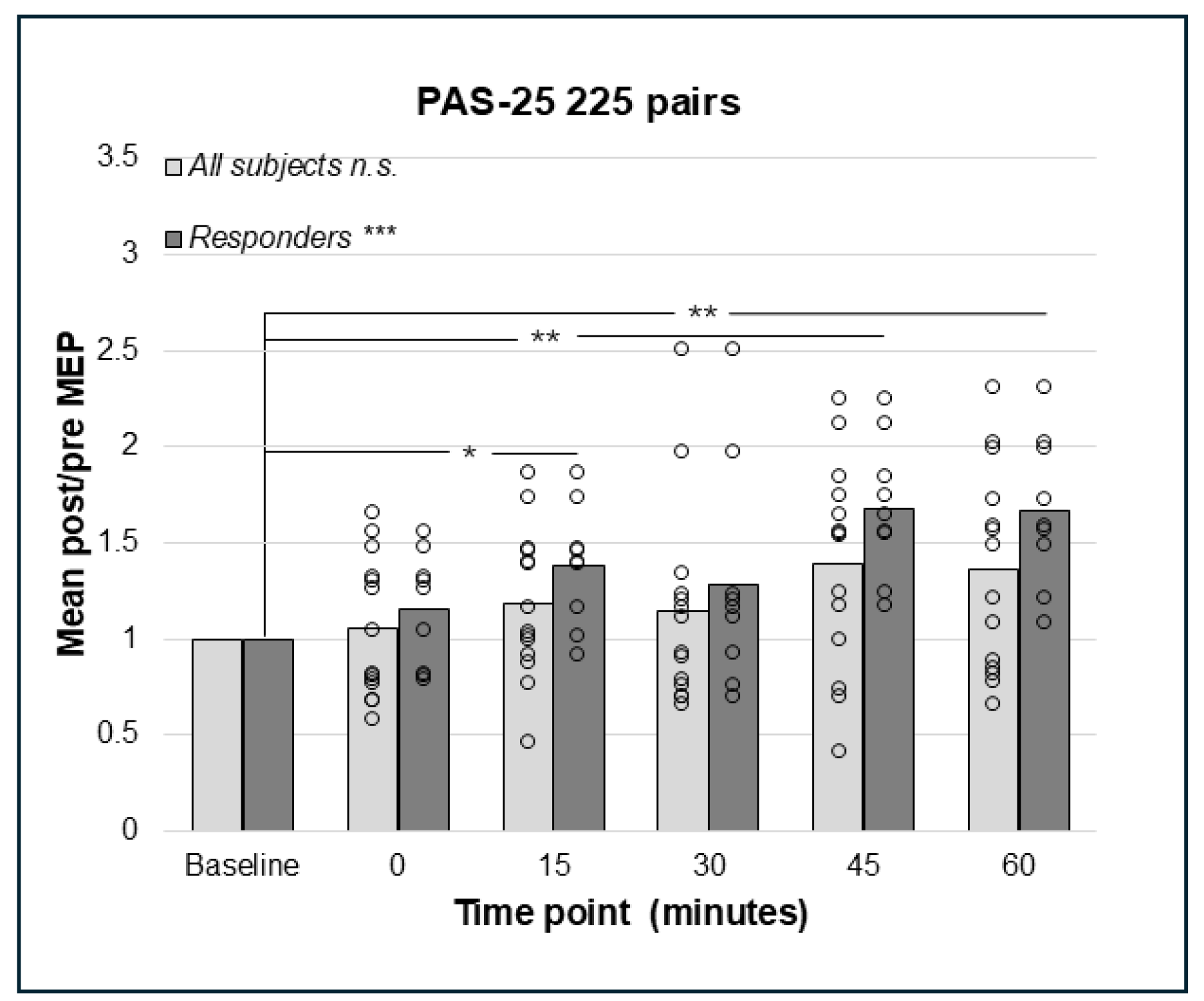
3.4. PAS-25 with 180 and 225 Paired Pulses Resulted in a Significant Increase in the Corticospinal Excitation
3.5. Attentional Measure
4. Discussion
4.1. PAS-25 with 225 Pairings
4.2. PAS-10 Increased Corticospinal Excitability
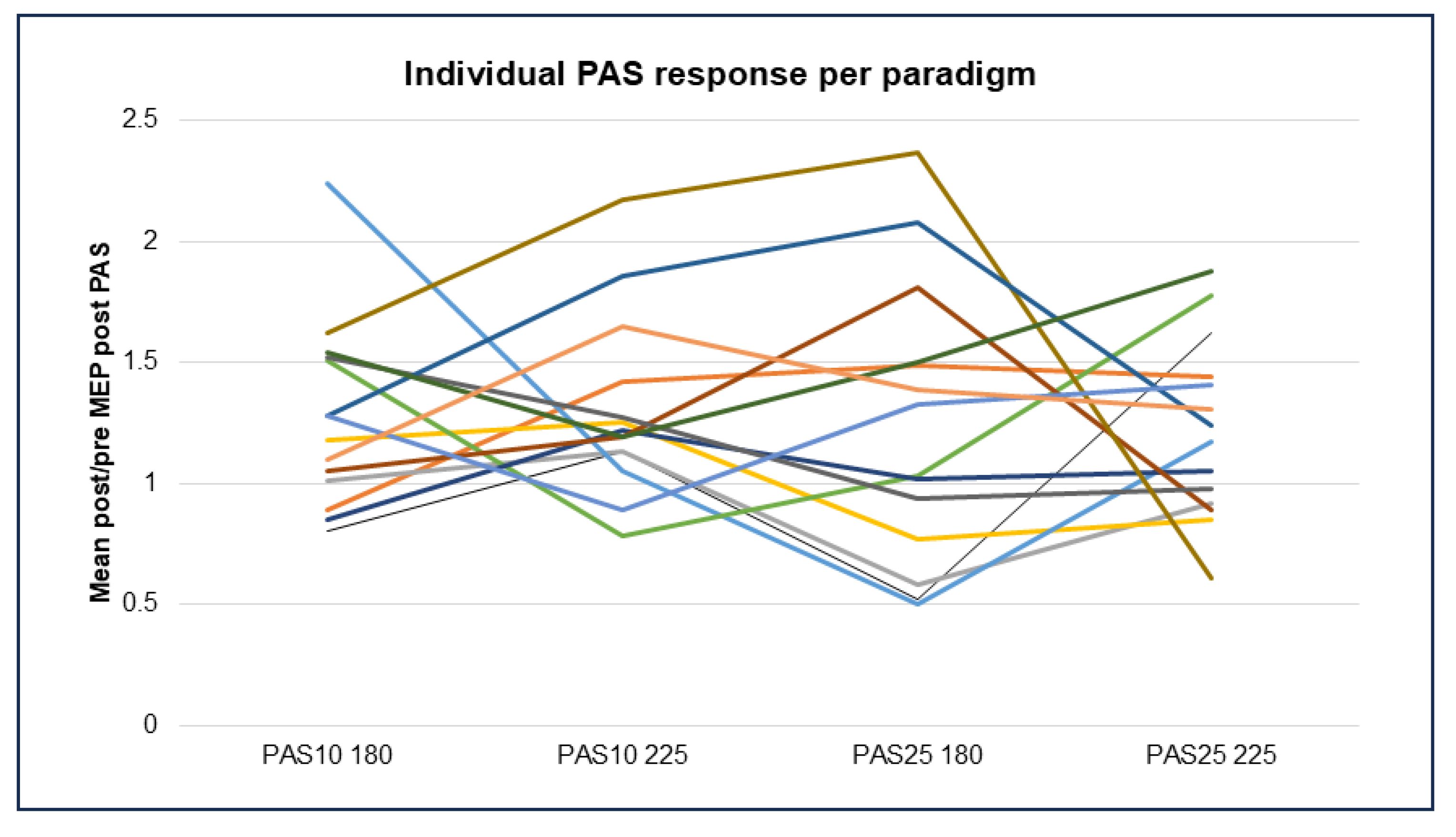
4.3. Patterns of Response in the Different PAS Paradigms
5. Limitations
6. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EMG | Electromyographic |
| M1 | Primary motor cortex |
| LTP | Long-term potentiation |
| LTD | Long-term depression |
| rTMS | Repetitive transcranial magnetic stimulation |
| PAS | Paired associative stimulation |
| PnS | Peripheral nerve stimulation |
| RMT | Resting motor threshold |
| STDP | Spike-timing-dependent plasticity |
References
- Kolb, B.; Harker, A.; Gibb, R. Principles of plasticity in the developing brain. Dev. Med. Child Neurol. 2017, 59, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Froudist-Walsh, S.; López-Barroso, D.; Torres-Prioris, M.J.; Croxson, P.L.; Berthier, M.L. Plasticity in the Working Memory System: Life Span Changes and Response to Injury. Neuroscientist 2018, 24, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, C.; Duman, R.S. Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology 2008, 33, 88–109. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A.; González-Burgos, G. Neuroplasticity of Neocortical Circuits in Schizophrenia. Neuropsychopharmacology 2008, 33, 141–165. [Google Scholar] [CrossRef]
- Levy, M.J.F.; Boulle, F.; Steinbusch, H.W.; van den Hove, D.; Kenis, G.; Lanfumey, L. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology 2018, 235, 2195–2220. [Google Scholar] [CrossRef]
- Liston, C.; Chen, A.C.; Zebley, B.D.; Drysdale, A.T.; Gordon, R.; Leuchter, B.; Voss, H.U.; Casey, B.; Etkin, A.; Dubin, M.J. Default Mode Network Mechanisms of Transcranial Magnetic Stimulation in Depression. Biol. Psychiatry 2014, 76, 517–526. [Google Scholar] [CrossRef]
- Persson, J.; Struckmann, W.; Gingnell, M.; Fällmar, D.; Bodén, R. Intermittent theta burst stimulation over the dorsomedial prefrontal cortex modulates resting-state connectivity in depressive patients: A sham-controlled study. Behav. Brain Res. 2020, 394, 112834. [Google Scholar] [CrossRef]
- Sehatpour, P.; Kantrowitz, J.T. Finding the Right Dose: NMDA Receptor-Modulating Treatments for Cognitive and Plasticity Deficits in Schizophrenia and the Role of Pharmacodynamic Target Engagement. Biol. Psychiatry 2025, 97, 128–138. [Google Scholar] [CrossRef]
- Frantseva, M.V.; Fitzgerald, P.B.; Chen, R.; Möller, B.; Daigle, M.; Daskalakis, Z.J. Evidence for Impaired Long-Term Potentiation in Schizophrenia and Its Relationship to Motor Skill Leaning. Cereb. Cortex 2008, 18, 990–996. [Google Scholar] [CrossRef]
- Appelbaum, L.G.; Shenasa, M.A.; Stolz, L.; Daskalakis, Z. Synaptic plasticity and mental health: Methods, challenges and opportunities. Neuropsychopharmacology 2023, 48, 113–120. [Google Scholar] [CrossRef]
- Stefan, K.; Kunesch, E.; Cohen, L.G.; Benecke, R.; Classen, J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain J. Neurol. 2000, 123 Pt 3, 572–584. [Google Scholar] [CrossRef]
- Ziemann, U.; Iliać, T.V.; Pauli, C.; Meintzschel, F.; Ruge, D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J. Neurosci. 2004, 24, 1666–1672. [Google Scholar] [CrossRef]
- Suppa, A.; Quartarone, A.; Siebner, H.; Chen, R.; Di Lazzaro, V.; Del Giudice, P.; Paulus, W.; Rothwell, J.C.; Ziemann, U.; Classen, J. The associative brain at work: Evidence from paired associative stimulation studies in humans. Clin. Neurophysiol. 2017, 128, 2140–2164. [Google Scholar] [CrossRef] [PubMed]
- Stefan, K.; Kunesch, E.; Benecke, R.; Cohen, L.G.; Classen, J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J. Physiol. 2002, 543, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Wolters, A.; Sandbrink, F.; Schlottmann, A.; Kunesch, E.; Stefan, K.; Cohen, L.G.; Benecke, R.; Classen, J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J. Neurophysiol. 2003, 89, 2339–2345. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.N.; Orekhov, Y.; Ziemann, U. Suppression of LTP-like plasticity in human motor cortex by the GABAB receptor agonist baclofen. Exp. Brain Res. 2007, 180, 181–186. [Google Scholar] [CrossRef]
- Monte-Silva, K.; Kuo, M.-F.; Thirugnanasambandam, N.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Dose-Dependent Inverted U-Shaped Effect of Dopamine (D2-Like) Receptor Activation on Focal and Nonfocal Plasticity in Humans. J. Neurosci. 2009, 29, 6124–6131. [Google Scholar] [CrossRef]
- Grundey, J.; Thirugnanasambandam, N.; Kaminsky, K.; Drees, A.; Skwirba, A.C.; Lang, N.; Paulus, W.; Nitsche, M.A. Neuroplasticity in Cigarette Smokers Is Altered under Withdrawal and Partially Restituted by Nicotine Exposition. J. Neurosci. 2012, 32, 4156–4162. [Google Scholar] [CrossRef]
- Thirugnanasambandam, N.; Grundey, J.; Adam, K.; Drees, A.; Skwirba, A.C.; Lang, N.; Paulus, W.; Nitsche, M.A. Nicotinergic Impact on Focal and Non-Focal Neuroplasticity Induced by Non-Invasive Brain Stimulation in Non-Smoking Humans. Neuropsychopharmacology 2011, 36, 879–886. [Google Scholar] [CrossRef]
- Stefan, K.; Wycislo, M.; Classen, J. Modulation of Associative Human Motor Cortical Plasticity by Attention. J. Neurophysiol. 2004, 92, 66–72. [Google Scholar] [CrossRef]
- Wischnewski, M.; Schutter, D.J. Efficacy and time course of paired associative stimulation in cortical plasticity: Implications for neuropsychiatry. Clin. Neurophysiol. 2016, 127, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Rajji, T.K.; Liu, S.-K.; Frantseva, M.V.; Mulsant, B.H.; Thoma, J.; Chen, R.; Fitzgerald, P.B.; Daskalakis, Z.J. Exploring the effect of inducing long-term potentiation in the human motor cortex on motor learning. Brain Stimul. 2011, 4, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-K.; Bliem, B.; Jung, P.; Arai, N.; Tsai, C.-H.; Ziemann, U. Modulation of preparatory volitional motor cortical activity by paired associative transcranial magnetic stimulation. Hum. Brain Mapp. 2009, 30, 3645–3656. [Google Scholar] [CrossRef]
- Alder, G.; Signal, N.; Olsen, S.; Taylor, D. A Systematic Review of Paired Associative Stimulation (PAS) to Modulate Lower Limb Corticomotor Excitability: Implications for Stimulation Parameter Selection and Experimental Design. Front. Neurosci. 2019, 13, 895. [Google Scholar] [CrossRef]
- Weise, D.; Schramm, A.; Stefan, K.; Wolters, A.; Reiners, K.; Naumann, M.; Classen, J. The two sides of associative plasticity in writer’s cramp. Brain 2006, 129, 2709–2721. [Google Scholar] [CrossRef]
- Morgante, F.; Espay, A.J.; Gunraj, C.; Lang, A.E.; Chen, R. Motor cortex plasticity in Parkinson’s disease and levodopa-induced dyskinesias. Brain 2006, 129, 1059–1069. [Google Scholar] [CrossRef]
- Lücke, C.; Heidegger, T.; Röhner, M.; Toennes, S.W.; Krivanekova, L.; Müller-Dahlhaus, F.; Ziemann, U. Deleterious Effects of a Low Amount of Ethanol on LTP-Like Plasticity in Human Cortex. Neuropsychopharmacology 2014, 39, 1508–1518. [Google Scholar] [CrossRef]
- Jung, P.; Ziemann, U. Homeostatic and Nonhomeostatic Modulation of Learning in Human Motor Cortex. J. Neurosci. 2009, 29, 5597–5604. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Weise, D.; Schramm, A.; Beck, M.; Reiners, K.; Classen, J. Loss of topographic specificity of LTD-like plasticity is a trait marker in focal dystonia. Neurobiol. Dis. 2011, 42, 171–176. [Google Scholar] [CrossRef]
- Kang, J.-S.; Terranova, C.; Hilker, R.; Quartarone, A.; Ziemann, U. Deficient homeostatic regulation of practice-dependent plasticity in writer’s cramp. Cereb. Cortex 2011, 21, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Wada, M.; Nakajima, S.; Takano, M.; Taniguchi, K.; Honda, S.; Mimura, M.; Noda, Y. Neuroplasticity of the left dorsolateral prefrontal cortex in patients with treatment-resistant depression as indexed with paired associative stimulation: A TMS–EEG study. Cereb. Cortex 2024, 34, bhad515. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.F.M.; Orekhov, Y.; Liu, Y.; Ziemann, U. Homeostatic plasticity in human motor cortex demonstrated by two consecutive sessions of paired associative stimulation. Eur. J. Neurosci. 2007, 25, 3461–3468. [Google Scholar] [CrossRef]
- Ngezahayo, A.; Schachner, M.; Artola, A. Synaptic Activity Modulates the Induction of Bidirectional Synaptic Changes in Adult Mouse Hippocampus. J. Neurosci. 2000, 20, 2451–2458. [Google Scholar] [CrossRef]
- López-Alonso, V.; Cheeran, B.; Río-Rodríguez, D.; Fernández-Del-Olmo, M. Inter-individual Variability in Response to Non-invasive Brain Stimulation Paradigms. Brain Stimul. 2014, 7, 372–380. [Google Scholar] [CrossRef]
- Elahi, B.; Hutchison, W.D.; Daskalakis, Z.J.; Gunraj, C.; Chen, R. Dose-response curve of associative plasticity in human motor cortex and interactions with motor practice. J. Neurophysiol. 2014, 111, 594–601. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Profice, P.; Saturno, E.; Pilato, F.; Insola, A.; Mazzone, P.; Tonali, P.; Rothwell, J. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr. Clin. Neurophysiol. Mot. Control. 1998, 109, 397–401. [Google Scholar] [CrossRef]
- Cash, R.; Jegatheeswaran, G.; Ni, Z.; Chen, R. Modulation of the Direction and Magnitude of Hebbian Plasticity in Human Motor Cortex by Stimulus Intensity and Concurrent Inhibition. Brain Stimul. 2017, 10, 83–90. [Google Scholar] [CrossRef]
- Minkova, L.; Peter, J.; Abdulkadir, A.; Schumacher, L.V.; Kaller, C.P.; Nissen, C.; Klöppel, S.; Lahr, J. Determinants of Inter-Individual Variability in Corticomotor Excitability Induced by Paired Associative Stimulation. Front. Neurosci. 2019, 13, 841. [Google Scholar] [CrossRef]
- Sale, M.V.; Ridding, M.C.; Nordstrom, M.A. Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp. Brain Res. 2007, 181, 615–626. [Google Scholar] [CrossRef]
- Fratello, F.; Veniero, D.; Curcio, G.; Ferrara, M.; Marzano, C.; Moroni, F.; Pellicciari, M.; Bertini, M.; Rossini, P.; Degennaro, L. Modulation of corticospinal excitability by paired associative stimulation: Reproducibility of effects and intraindividual reliability. Clin. Neurophysiol. 2006, 117, 2667–2674. [Google Scholar] [CrossRef] [PubMed]
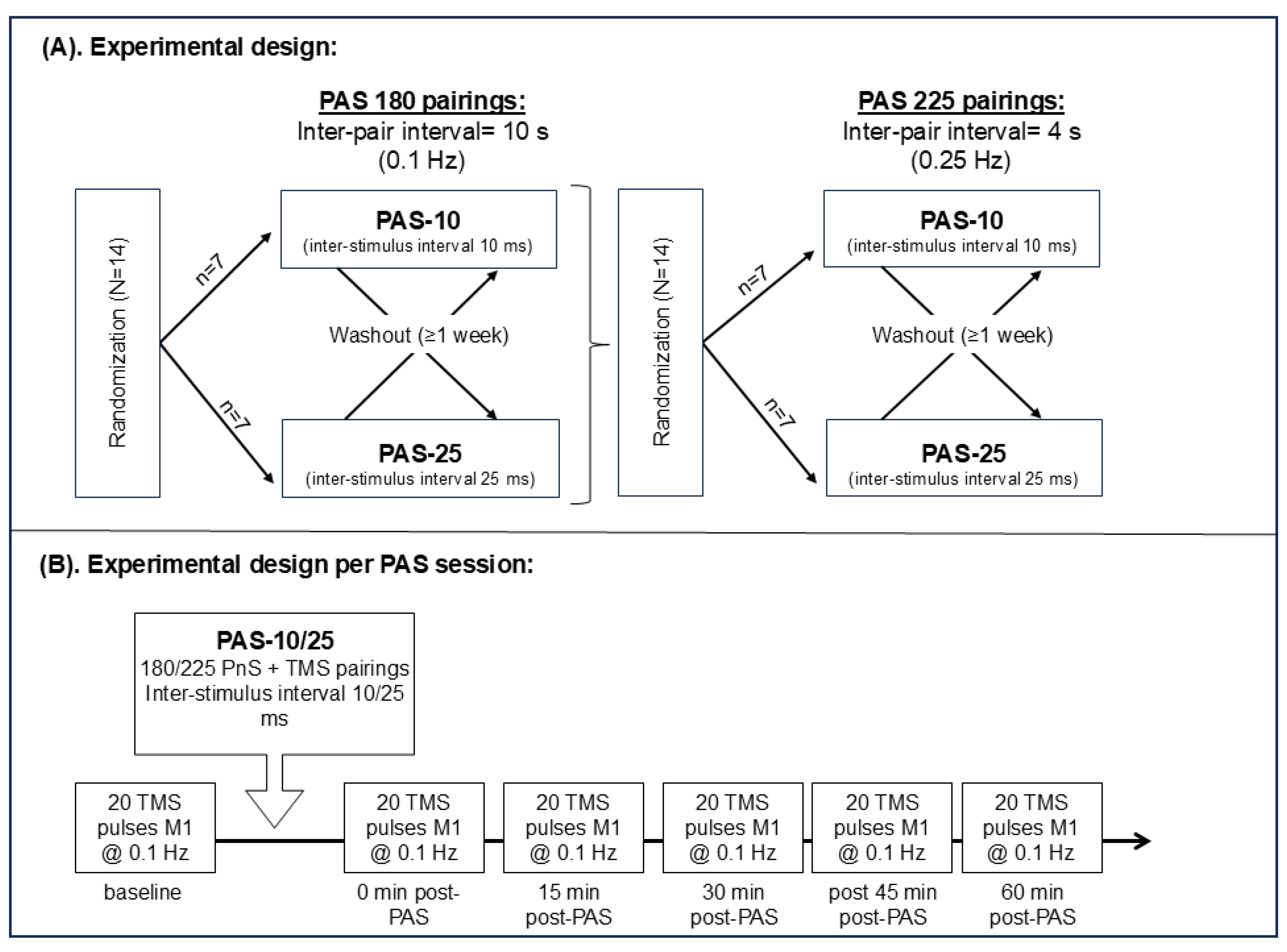
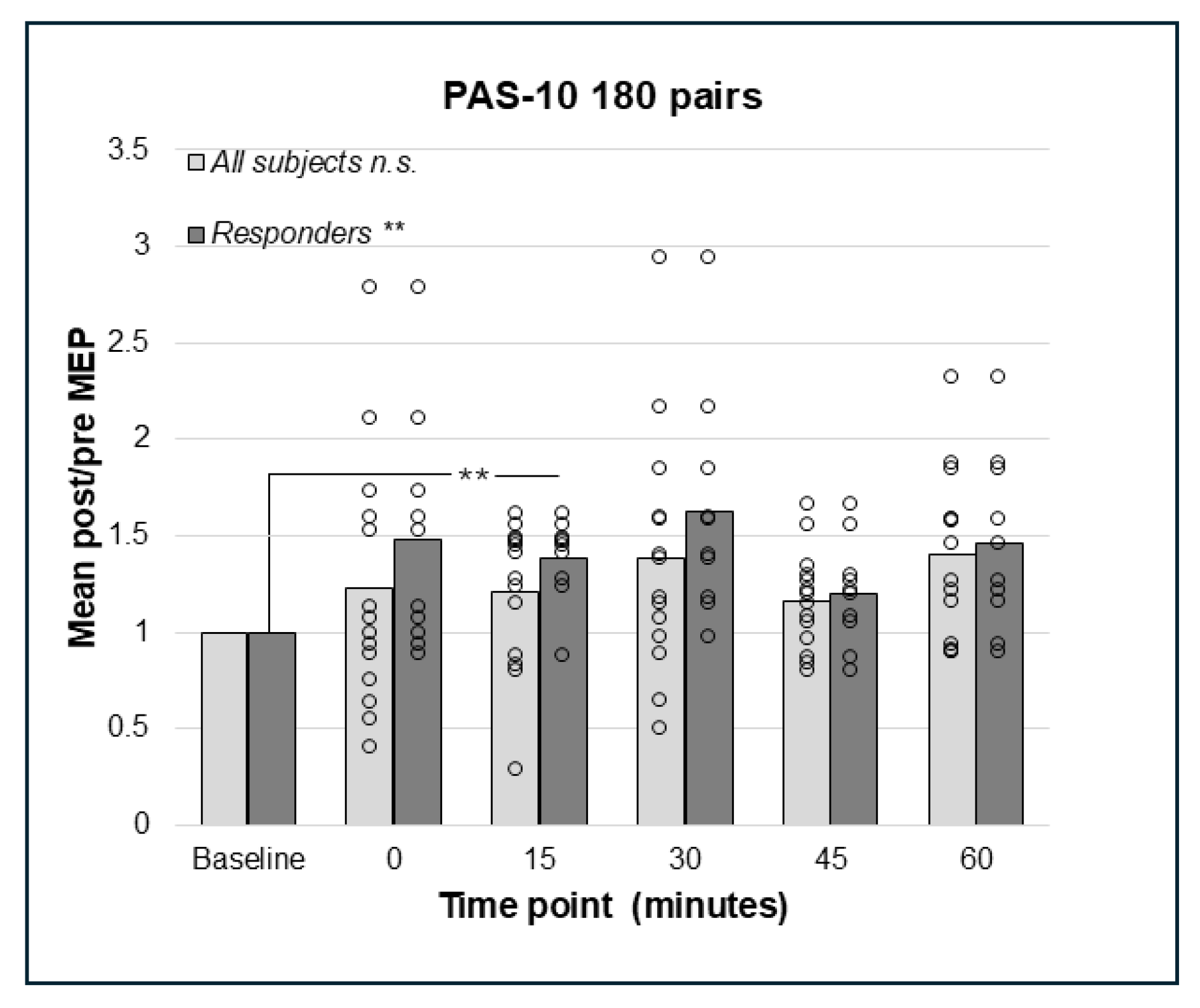
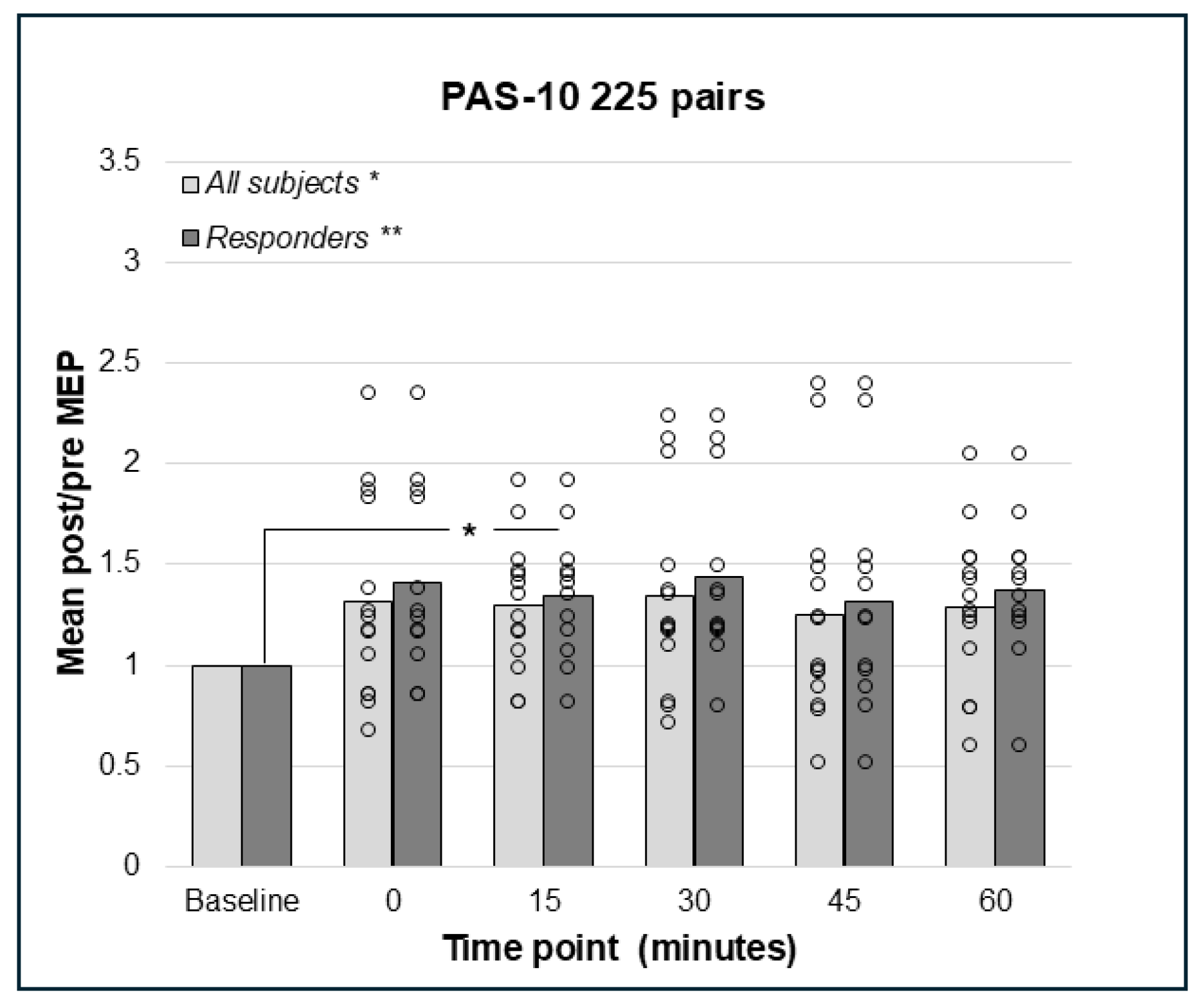
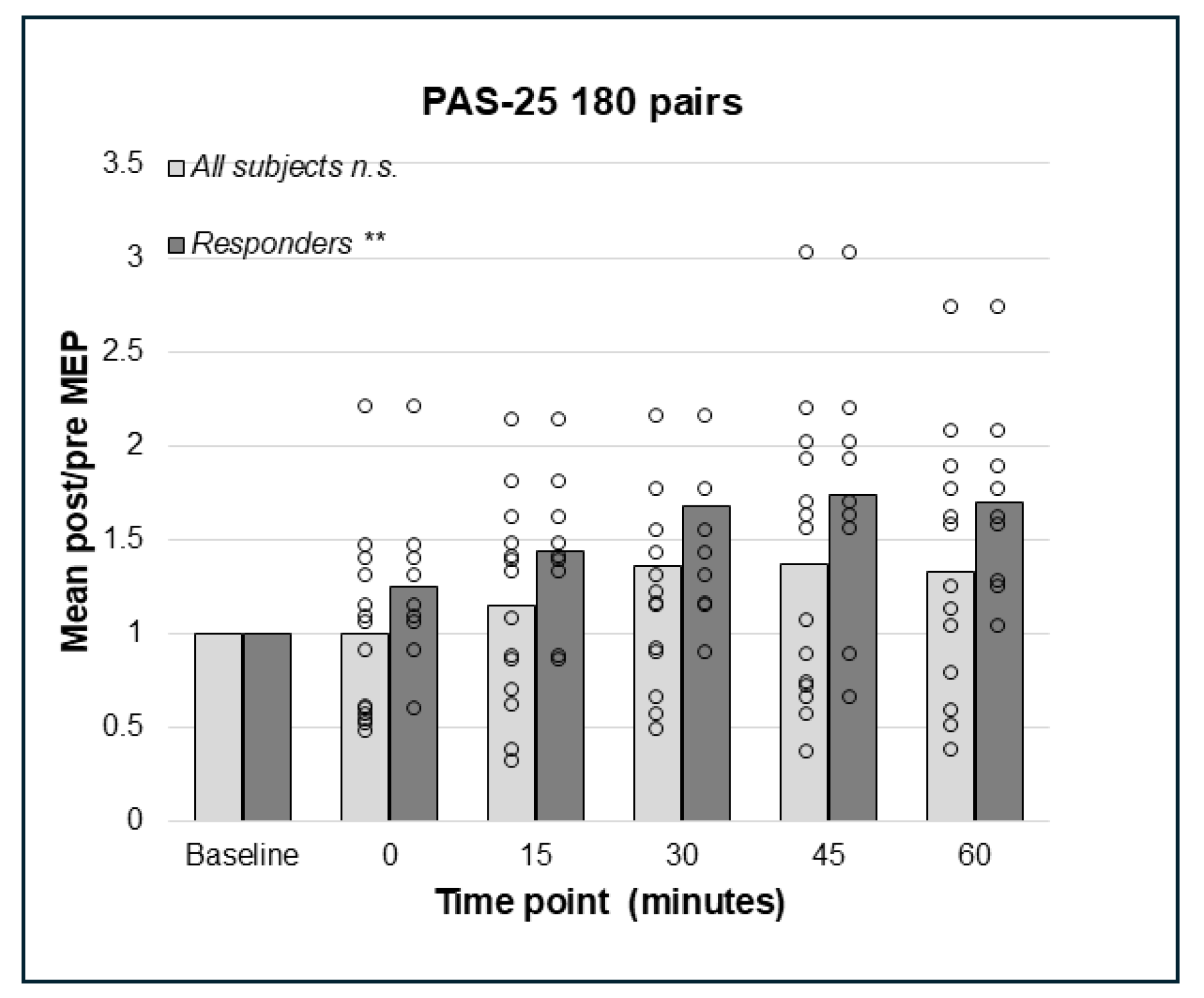
| Phase 1 | Phase 2 | |||
|---|---|---|---|---|
| PAS paradigm | PAS-10 (180) | PAS-25 (180) | PAS-10 (225) | PAS-25 (225) |
| Inter-stimulus interval (PnS to TMS) | 10 ms | 25 ms | 10 ms | 25 ms |
| Number of PnS + TMS pairs | 180 | 180 | 225 | 225 |
| Inter-pair interval | 10 s (0.1 Hz) | 10 s (0.1 Hz) | 4 s (0.25 Hz) | 4 s (0.25 Hz) |
| Group | Full Participation Group | Mean Post/Pre > 1.0 | Mean Post/Pre > 1.0 | Mean Post/Pre > 1.0 | Mean Post/Pre > 1.0 |
|---|---|---|---|---|---|
| Paradigm | All | PAS-10 180 pairs | PAS-10 225 pairs | PAS-25 180 pairs | PAS-25 225 pairs |
| Participants (#) | 14 | 10 | 12 | 9 | 9 |
| Females/males | 6/8 | 2/8 | 6/6 | 4/5 | 4/5 |
| Mean (±s.d.) age years baseline | 32.29 (7.72) | 34.90 (6.37) | 31.58 (8.10) | 33.78 (7.16) | 33.00 (8.03) |
| Mean (±s.d.) height (cm) baseline | 172 (12.70) | 174.00 (12.95) | 172.92 (11.60) | 171.56 (15.57) | 170.89 (14.49) |
| Mean (±s.d.) AUDITc baseline | 4 (1.3) | n.a. | n.a. | n.a. | n.a. |
| Mean (±s.d.) RMT % | 39.79 (6.07) | 39.80 (5.79) | 40.25 (7.21) | 39.89 (7.54) | 39.67 (6.71) |
| Mean (±s.d.) stimulus intensity % | n.a. | 50.40 (8:41) | 48.25 (10.36) | 52.00 (12.51) | 47.67 (10.38) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodzic, K.; Thordstein, M.; Strandberg, J.; Jerlhag, E.; Wass, C.E. A Methodological Evaluation of Four Different Paired Associative Stimulation Paradigms in Healthy Controls. Brain Sci. 2025, 15, 461. https://doi.org/10.3390/brainsci15050461
Hodzic K, Thordstein M, Strandberg J, Jerlhag E, Wass CE. A Methodological Evaluation of Four Different Paired Associative Stimulation Paradigms in Healthy Controls. Brain Sciences. 2025; 15(5):461. https://doi.org/10.3390/brainsci15050461
Chicago/Turabian StyleHodzic, Kenan, Magnus Thordstein, Joakim Strandberg, Elisabet Jerlhag, and Caroline E. Wass. 2025. "A Methodological Evaluation of Four Different Paired Associative Stimulation Paradigms in Healthy Controls" Brain Sciences 15, no. 5: 461. https://doi.org/10.3390/brainsci15050461
APA StyleHodzic, K., Thordstein, M., Strandberg, J., Jerlhag, E., & Wass, C. E. (2025). A Methodological Evaluation of Four Different Paired Associative Stimulation Paradigms in Healthy Controls. Brain Sciences, 15(5), 461. https://doi.org/10.3390/brainsci15050461







