Exploring the Impact of Backward and Forward Locomotor Treadmill Training in Chronic Stroke Survivors with Severe Post-Stroke Walking Impairment: A Single-Center Pilot Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Participants
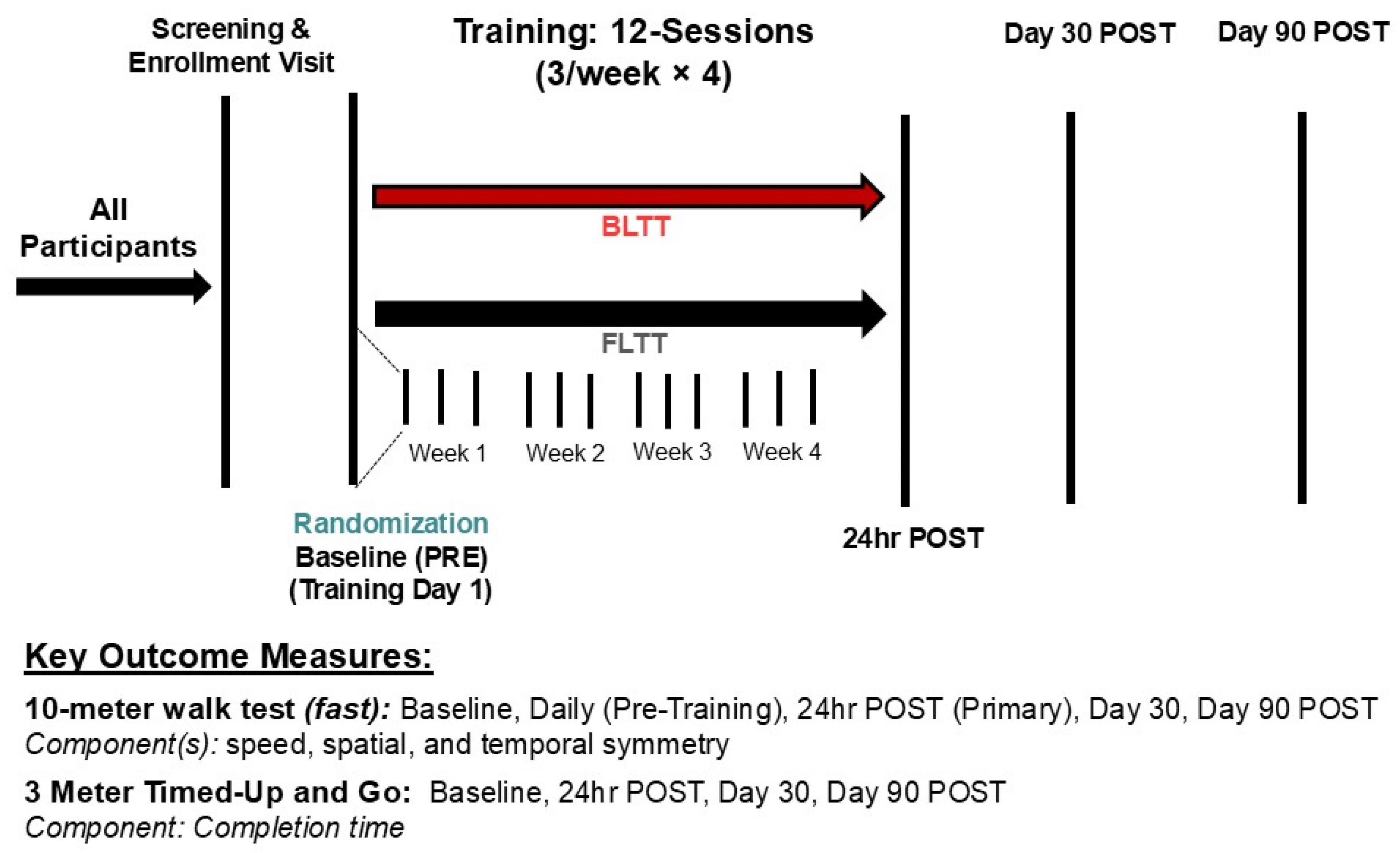
2.2. Training Protocol
2.3. Outcome Measures
2.3.1. Overground Walking Speed (10-Meter Walk Test)
2.3.2. Symmetry During Overground Walking
2.3.3. Dynamic Balance—Instrumented Timed up and Go
2.3.4. Statistics
3. Results
3.1. Safety/Tolerability/Study Fidelity
3.2. Overground Walking Speed at 24 h POST (Primary Outcome)
3.3. Retention of Walking Speed
3.4. Spatial (Step Length) Symmetry Index
3.5. Temporal (% Single Support Time) Symmetry Index
3.6. 3-Meter Timed Up and Go
4. Discussion
4.1. Backward vs. Forward Training on Spatiotemporal Measures
4.2. Backward vs. Forward Training on Dynamic Balance
4.3. Factors Predicting Response
4.4. Key Logistical Considerations
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moore, S.A.; Boyne, P.; Fulk, G.; Verheyden, G.; Fini, N.A. Walk the Talk: Current Evidence for Walking Recovery After Stroke, Future Pathways and a Mission for Research and Clinical Practice. Stroke 2022, 53, 3494–3505. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.; Garrett, M.; Gronley, J.K.; Mulroy, S.J. Classification of walking handicap in the stroke population. Stroke 1995, 26, 982–989. [Google Scholar] [CrossRef]
- Duncan, P.W.; Sullivan, K.J.; Behrman, A.L.; Azen, S.P.; Wu, S.S.; Nadeau, S.E.; Dobkin, B.H.; Rose, D.K.; Tilson, J.K.; Cen, S.; et al. Body-Weight–Supported Treadmill Rehabilitation after Stroke. N. Engl. J. Med. 2011, 364, 2026–2036. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, B.H. Clinical practice. Rehabilitation after stroke. N. Engl. J. Med. 2005, 352, 1677–1684. [Google Scholar] [CrossRef]
- Eng, J.J.; Tang, P. Gait training strategies to optimize walking ability in people with stroke: A synthesis of the evidence. Expert Rev. Neurother. 2007, 7, 1417–1436. [Google Scholar] [CrossRef] [PubMed]
- Stephan, K.M.; Pérennou, D. Mobility After Stroke: Relearning to Walk. In Clinical Pathways in Stroke Rehabilitation: Evidence-based Clinical Practice Recommendations; Platz, T., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Hornby, T.G.; Reisman, D.S.; Ward, I.G.; Scheets, P.L.; Miller, A.; Haddad, D.; Fox, E.J.; Fritz, N.E.; Hawkins, K.; Henderson, C.E.; et al. Clinical Practice Guideline to Improve Locomotor Function Following Chronic Stroke, Incomplete Spinal Cord Injury, and Brain Injury. J. Neurol. Phys. Ther. 2020, 44, 49–100. [Google Scholar] [CrossRef]
- Hosoi, Y.; Kamimoto, T.; Sakai, K.; Yamada, M.; Kawakami, M. Estimation of minimal detectable change in the 10-meter walking test for patients with stroke: A study stratified by gait speed. Front. Neurol. 2023, 14, 1219505. [Google Scholar] [CrossRef]
- Dean, C.M.; Ada, L.; Lindley, R.I. Treadmill training provides greater benefit to the subgroup of community-dwelling people after stroke who walk faster than 0.4m/s: A randomised trial. J. Physiother. 2014, 60, 97–101. [Google Scholar] [CrossRef]
- Bernhardt, J.; Hayward, K.S.; Kwakkel, G.; Ward, N.S.; Wolf, S.L.; Borschmann, K.; Krakauer, J.W.; Boyd, L.A.; Carmichael, S.T.; Corbett, D.; et al. Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable taskforce. Int. J. Stroke 2017, 12, 444–450. [Google Scholar] [CrossRef]
- Boyd, L.A.; Hayward, K.S.; Ward, N.S.; Stinear, C.M.; Rosso, C.; Fisher, R.J.; Carter, A.R.; Leff, A.P.; Copland, D.A.; Carey, L.M.; et al. Biomarkers of stroke recovery: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int. J. Stroke 2017, 12, 480–493. [Google Scholar] [CrossRef]
- Cramer, S.C.; Wolf, S.L.; Adams, H.P.; Chen, D.; Dromerick, A.W.; Dunning, K.; Ellerbe, C.; Grande, A.; Janis, S.; Lansberg, M.G.; et al. Stroke Recovery and Rehabilitation Research: Issues, Opportunities, and the National Institutes of Health StrokeNet. Stroke 2017, 48, 813–819. [Google Scholar] [CrossRef]
- Awosika, O.O.; Chan, D.; Sucharew, H.J.; Boyne, P.; Bhattacharya, A.; Dunning, K.; Kissela, B.M. Backward Locomotor Treadmill Training Differentially Improves Walking Performance across Stroke Walking Impairment Levels. Brain Sci. 2022, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Awosika, O.O.; Matthews, S.; Staggs, E.J.; Boyne, P.; Song, X.; Rizik, B.A.; Sucharew, H.J.; Zhang, C.; Mungcal, G.; Moudgal, R.; et al. Backward locomotor treadmill training combined with transcutaneous spinal direct current stimulation in stroke: A randomized pilot feasibility and safety study. Brain Commun. 2020, 2, fcaa045. [Google Scholar] [CrossRef] [PubMed]
- Kurz, M.J.; Wilson, T.W.; Arpin, D.J. Stride-time variability and sensorimotor cortical activation during walking. Neuroimage 2012, 59, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Kupchenko, Y.; Dreyer-Alster, S.; Broscheid, K.; Kalron, A. Prefrontal hemodynamics during forward and backward walking, with and without a cognitive task, in people with multiple sclerosis. Eur. J. Phys. Rehabil. Med. 2023, 59, 164–173. [Google Scholar] [CrossRef]
- Lin, N.; Liu, C.; Lee, P.; Guo, L.; Sung, J.; Yen, C.; Liaw, L. Backward Walking Induces Significantly Larger Upper-Mu-Rhythm Suppression Effects Than Forward Walking Does. Sensors 2020, 20, 7250. [Google Scholar] [CrossRef]
- Rose, D.K.; DeMark, L.; Fox, E.J.; Clark, D.J.; Wludyka, P. A Backward Walking Training Program to Improve Balance and Mobility in Acute Stroke. J. Neurol. Phys. Ther. 2018, 42, 12–21. [Google Scholar] [CrossRef]
- Balasukumaran, T.; Olivier, B.; Ntsiea, M.V. The effectiveness of backward walking as a treatment for people with gait impairments: A systematic review and meta-analysis. Clin. Rehabil. 2019, 33, 171–182. [Google Scholar] [CrossRef]
- Winter, D.A.; Pluck, N.; Yang, J.F. Backward walking: A simple reversal of forward walking? J. Mot. Behav. 1989, 21, 291–305. [Google Scholar] [CrossRef]
- Donno, L.; Monoli, C.; Frigo, C.A.; Galli, M. Forward and Backward Walking: Multifactorial Characterization of Gait Parameters. Sensors 2023, 23, 4671. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, W.; An, R. Effectiveness of backward walking training on spatial-temporal gait characteristics: A systematic review and meta-analysis. Hum. Mov. Sci. 2018, 60, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.; Ballester, B.R.; Verschure, P.F.M.J. Principles of Neurorehabilitation After Stroke Based on Motor Learning and Brain Plasticity Mechanisms. Front. Syst. Neurosci. 2019, 13, 74. [Google Scholar] [CrossRef]
- Ivey, F.M.; Hafer-Macko, C.E.; Macko, R.F. Task-oriented treadmill exercise training in chronic hemiparetic stroke. J. Rehabil. Res. Dev. 2008, 45, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Wernig, A.; Wernig, S. The Trouble With “Body Weight Support” In Treadmill Training. Arch. Phys. Med. Rehabil. 2010, 91, 1478. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, D.J.; Armstrong, C.W.; Gaul, S. Backward Walking at Three Levels of Treadmill Inclination: An Electromyographic and Kinematic Analysis. J. Orthop. Sports Phys. Ther. 1995, 22, 95–102. [Google Scholar] [CrossRef]
- Kramer, J. Backward walking: A cinematographic and electromyographic pilot study. Physiother. Can. 1981, 33, 77–86. [Google Scholar]
- Schmid, A.; Duncan, P.W.; Studenski, S.; Lai, S.M.; Richards, L.; Perera, S.; Wu, S.S. Improvements in speed-based gait classifications are meaningful. Stroke 2007, 38, 2096–2100. [Google Scholar] [CrossRef]
- Plummer, P.; Behrman, A.L.; Duncan, P.W.; Spigel, P.; Saracino, D.; Martin, J.; Fox, E.; Thigpen, M.; Kautz, S.A. Effects of Stroke Severity and Training Duration on Locomotor Recovery After Stroke: A Pilot Study. Neurorehabilit. Neural Repair 2007, 21, 137–151. [Google Scholar] [CrossRef]
- Li, S. Stroke Recovery Is a Journey: Prediction and Potentials of Motor Recovery after a Stroke from a Practical Perspective. Life 2023, 13, 2061. [Google Scholar] [CrossRef]
- Cheng, D.K.; Dagenais, M.; Alsbury-Nealy, K.; Legasto, J.M.; Scodras, S.; Aravind, G.; Takhar, P.; Nekolaichuk, E.; Salbach, N.M. Distance-limited walk tests post-stroke: A systematic review of measurement properties. NeuroRehabilitation 2021, 48, 413–439. [Google Scholar] [CrossRef]
- Mansfield, A.; Wong, J.S.; McIlroy, W.E.; Biasin, L.; Brunton, K.; Bayley, M.; Inness, E.L. Do measures of reactive balance control predict falls in people with stroke returning to the community? Physiotherapy 2015, 101, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, C.K.; Bowden, M.G.; Neptune, R.R.; Kautz, S.A. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch. Phys. Med. Rehabil. 2007, 88, 43–49. [Google Scholar] [CrossRef]
- Boyne, P.; Doren, S.; Scholl, V.; Staggs, E.; Whitesel, D.; Carl, D.; Shatz, R.; Sawyer, R.; Awosika, O.O.; Reisman, D.S.; et al. Preliminary Outcomes of Combined Treadmill and Overground High-Intensity Interval Training in Ambulatory Chronic Stroke. Front. Neurol. 2022, 13, 812875. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Harris, J.E.; Eng, J.J.; Marigold, D.S.; Tokuno, C.D.; Louis, C.L. Relationship of Balance and Mobility to Fall Incidence in People With Chronic Stroke. Phys. Ther. 2005, 85, 150–158. [Google Scholar] [CrossRef]
- Flansbjer, U.; Holmbäck, A.M.; Downham, D.; Patten, C.; Lexell, J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J. Rehabil. Med. 2005, 37, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yen, J.; Wang, R.; Yen, L.; Lieu, F. Gait outcomes after additional backward walking training in patients with stroke: A randomized controlled trial. Clin. Rehabil. 2005, 19, 264–273. [Google Scholar] [CrossRef]
- Mehrholz, J.; Thomas, S.; Elsner, B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst. Rev. 2017, 8, CD002840. [Google Scholar] [CrossRef]
- Maritz, C.A.; Pigman, J.; Grävare Silbernagel, K.; Crenshaw, J. Effects of Backward Walking Training on Balance, Mobility, and Gait in Community-Dwelling Older Adults. Act. Adapt. Aging 2021, 45, 202–216. [Google Scholar] [CrossRef]
- Soke, F.; Aydin, F.; Karakoc, S.; Gulsen, C.; Yasa, M.E.; Ersoy, N.; Gulsen, E.O.; Yucesan, C. Effects of backward walking training on balance, gait, and functional mobility in people with multiple sclerosis: A randomized controlled study. Mult. Scler. Relat. Disord. 2023, 79, 104961. [Google Scholar] [CrossRef]
- Wen, H.; Wang, M. Backward Walking Training Impacts Positive Effect on Improving Walking Capacity after Stroke: A Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 3370. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; Kautz, S.A.; Neptune, R.R. Step length asymmetry is representative of compensatory mechanisms used in post-stroke hemiparetic walking. Gait Posture 2011, 33, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.L.; Rodgers, M.M.; Macko, R.F.; Forrester, L.W. Effect of treadmill exercise training on spatial and temporal gait parameters in subjects with chronic stroke: A preliminary report. J. Rehabil. Res. Dev. 2008, 45, 221–228. [Google Scholar] [CrossRef]
- Hornby, T.G.; Campbell, D.D.; Kahn, J.H.; Demott, T.; Moore, J.L.; Roth, H.R. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: A randomized controlled study. Stroke 2008, 39, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Silver, K.; Macko, R.F.; Forrester, L.W.; Goldberg, A.P.; Smith, G.V. Effects of Aerobic Treadmill Training on Gait Velocity, Cadence, and Gait Symmetry in Chronic Hemiparetic Stroke: A Preliminary Report. Neurorehabil. Neural Repair 2000, 14, 65–71. [Google Scholar] [CrossRef]
- Reisman, D.S.; McLean, H.; Keller, J.; Danks, K.A.; Bastian, A.J. Repeated split-belt treadmill training improves poststroke step length asymmetry. Neurorehabil. Neural Repair 2013, 27, 460–468. [Google Scholar] [CrossRef]
- Herman, T.; Giladi, N.; Hausdorff, J.M. Properties of the ‘timed up and go’ test: More than meets the eye. Gerontology 2011, 57, 203–210. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; An, R. Effectiveness of backward walking training on balance performance: A systematic review and meta-analysis. Gait Posture 2019, 68, 466–475. [Google Scholar] [CrossRef]
- El-Basatiny, H.M.Y.; Abdel-Aziem, A.A. Effect of backward walking training on postural balance in children with hemiparetic cerebral palsy: A randomized controlled study. Clin. Rehabil. 2015, 29, 457–467. [Google Scholar] [CrossRef]
- Preston, E.; Ada, L.; Stanton, R.; Mahendran, N.; Dean, C.M. Prediction of Independent Walking in People Who Are Nonambulatory Early After Stroke: A Systematic Review. Stroke 2021, 52, 3217–3224. [Google Scholar] [CrossRef]
- Perry, M.K.; Peters, D.M. Neural correlates of walking post-stroke: Neuroimaging insights from the past decade. Exp. Brain Res. 2021, 239, 3439–3446. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.C.; See, J.; Liu, B.; Edwardson, M.; Wang, X.; Radom-Aizik, S.; Haddad, F.; Shahbaba, B.; Wolf, S.L.; Dromerick, A.W.; et al. Genetic Factors, Brain Atrophy, and Response to Rehabilitation Therapy After Stroke. Neurorehabil. Neural Repair 2022, 36, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Awosika, O.O.; Garver, A.; Drury, C.; Sucharew, H.J.; Boyne, P.; Schwab, S.M.; Wasik, E.; Earnest, M.; Dunning, K.; Bhattacharya, A.; et al. Insufficiencies in sensory systems reweighting is associated with walking impairment severity in chronic stroke: An observational cohort study. Front. Neurol. 2023, 14, 1244657. [Google Scholar] [CrossRef] [PubMed]
- Tavares, E.; Coelho, J.; Rogado, P.; Correia, R.; Castro, C.; Fernandes, J.B. Barriers to Gait Training among Stroke Survivors: An Integrative Review. J. Funct. Morphol. Kinesiol. 2022, 7, 85. [Google Scholar] [CrossRef]




| Category | Study Groups | p-Value | |
|---|---|---|---|
| BLTT (n = 7) | FLTT (n = 7) | ||
| Time since Stroke (mo) | 14.0 (7.00–24.0) | 25.0 (10.0–47.0) | 0.32 |
| Age (yrs) | 57.7 ± 7.50 | 53.9 ± 7.41 | 0.47 |
| Mini Mental Status Exam | 29 (28–30) | 30 (29–30) | 0.36 |
| Patient Health Questionnaire-9 | 3 (2.75–6.00) | 3 (1.50–5.00) | 0.83 |
| Sex | 1.00 | ||
| Male | 5 (71.4%) | 6 (85.71%) | |
| Female | 2 (28.6%) | 1 (14.29%) | |
| Assistive Device | 0.10 | ||
| None | 0 (0.00%) | 0 (0.00%) | |
| Cane | 0 (0.00%) | 2 (28.6%) | |
| Quad Cane | 7 (100%) | 4 (57.1%) | |
| Hemiwalker | 0 (0.00%) | 1 (14.3%) | |
| Orthotic Device (AFO) | 1.00 | ||
| Yes | 3 (42.9%) | 4 (57.1%) | |
| No | 4 (57.1%) | 3 (42.9%) | |
| Stroke Type | 1.00 | ||
| Ischemic | 4 (57.1%) | 4 (57.1%) | |
| Hemorrhagic | 3 (42.9%) | 3 (42.9%) | |
| Lateralization | 0.59 | ||
| Right | 3 (42.9%) | 5 (71.4%) | |
| Left | 4 (57.1%) | 2 (28.6%) | |
| Localization | 0.46 | ||
| Hemispheric | 7 (100%) | 5 (71.43%) | |
| Cerebellar | 0 (0%) | 2 (28.57%) | |
| 10 MWT (m/s) | |||
| Self-selected (SS) | 0.20 (0.17–0.30) | 0.26 (0.15–0.30) | 1.00 |
| Fast (FP) | 0.26 (0.20–0.37) | 0.34 (0.18–0.40) | 1.00 |
| Step Length Symmetry | 85.7 (74.6–88.1) | 81.6 (46.1–97.2) | 0.90 |
| % Single Support Time | 83.9 (68.1–85.5) | 84.2 (76.6–92.3) | 0.32 |
| 3-Meter Timed Up and Go | 47.1 (44.9–72.9) | 44.0 (34.6–69.4) | 0.40 |
| Outcome | Group | 24 h POST | p-Value | Day 30 POST | p-Value | Day 90 POST | p-Value |
|---|---|---|---|---|---|---|---|
| Δ 10 MWT (m/s) | BLTT | 0.05 (0.01–0.08) | 0.80 | 0.03 (−0.01–0.14) | >0.99 | 0.04 (−0.00–0.13) | >0.99 |
| FLTT | 0.07 (0.03–0.08) | 0.06 (0.03–0.07) | 0.05 (0.03–0.09) | ||||
| Δ SLS | BLTT | 3.17 (−2.95–4.87) | 0.54 | 3.79 (1.58–5.95) | 0.26 | 1.09 (−6.05–6.93) | 0.18 |
| FLTT | −1.61 (−3.19–3.39) | −0.90 (−1.60–2.24) | −4.54 (−7.14–−1.12) | ||||
| Δ %SST | BLTT | 2.75 (1.17–4.19) | 0.62 | −2.10 (−1.60–2.24) | 0.71 | 0.60 (−0.86–2.34) | 0.10 |
| FLTT | 2.36 (−4.81–4.02) | −1.77 (−5.42–1.49) | −1.85 (−3.69–−0.51) | ||||
| Δ 3M TUG (s) | BLTT | 9.88 (3.04–24.8) | 0.53 | 21.1 (15.3–28.5) | 0.37 | 7.59 (1.91–20.5) | 0.80 |
| FLTT | 6.59 (2.90–9.21) | 10.3 (6.36–17.0) | 5.9 (5.17–12.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naidu, S.; Singh, K.; Murray, T.; Drury, C.; Palermo, E.; Sucharew, H.J.; Xie, C.; Boyne, P.; Dunning, K.; Awosika, O.O. Exploring the Impact of Backward and Forward Locomotor Treadmill Training in Chronic Stroke Survivors with Severe Post-Stroke Walking Impairment: A Single-Center Pilot Randomized Controlled Trial. Brain Sci. 2025, 15, 437. https://doi.org/10.3390/brainsci15050437
Naidu S, Singh K, Murray T, Drury C, Palermo E, Sucharew HJ, Xie C, Boyne P, Dunning K, Awosika OO. Exploring the Impact of Backward and Forward Locomotor Treadmill Training in Chronic Stroke Survivors with Severe Post-Stroke Walking Impairment: A Single-Center Pilot Randomized Controlled Trial. Brain Sciences. 2025; 15(5):437. https://doi.org/10.3390/brainsci15050437
Chicago/Turabian StyleNaidu, Saiprasad, Khwahish Singh, Tamiel Murray, Colin Drury, Erin Palermo, Heidi J. Sucharew, Changchun Xie, Pierce Boyne, Kari Dunning, and Oluwole O. Awosika. 2025. "Exploring the Impact of Backward and Forward Locomotor Treadmill Training in Chronic Stroke Survivors with Severe Post-Stroke Walking Impairment: A Single-Center Pilot Randomized Controlled Trial" Brain Sciences 15, no. 5: 437. https://doi.org/10.3390/brainsci15050437
APA StyleNaidu, S., Singh, K., Murray, T., Drury, C., Palermo, E., Sucharew, H. J., Xie, C., Boyne, P., Dunning, K., & Awosika, O. O. (2025). Exploring the Impact of Backward and Forward Locomotor Treadmill Training in Chronic Stroke Survivors with Severe Post-Stroke Walking Impairment: A Single-Center Pilot Randomized Controlled Trial. Brain Sciences, 15(5), 437. https://doi.org/10.3390/brainsci15050437








