Prognostic Biomarkers in Isocitrate Dehydrogenase Wild-Type Glioblastoma: A Focus on B7-H3
Abstract
1. Introduction
2. Materials and Methods
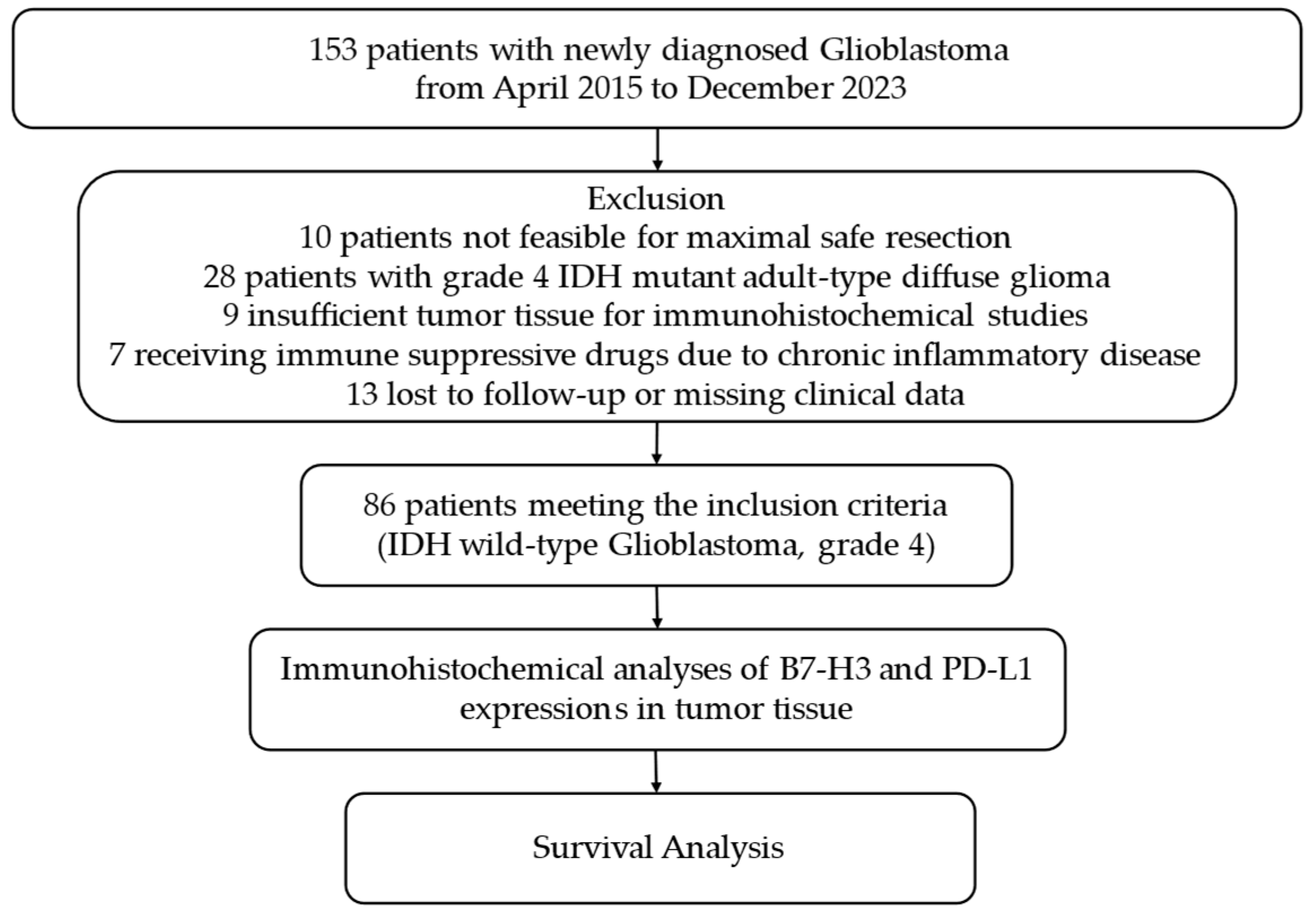
2.1. Randomization of Participants, Data Collection, and Clinical Follow-Up
2.2. Immunohistochemical Evaluation of B7-H3 and PD-L1 Expression in Tumor Tissues
2.3. Statistical Analysis
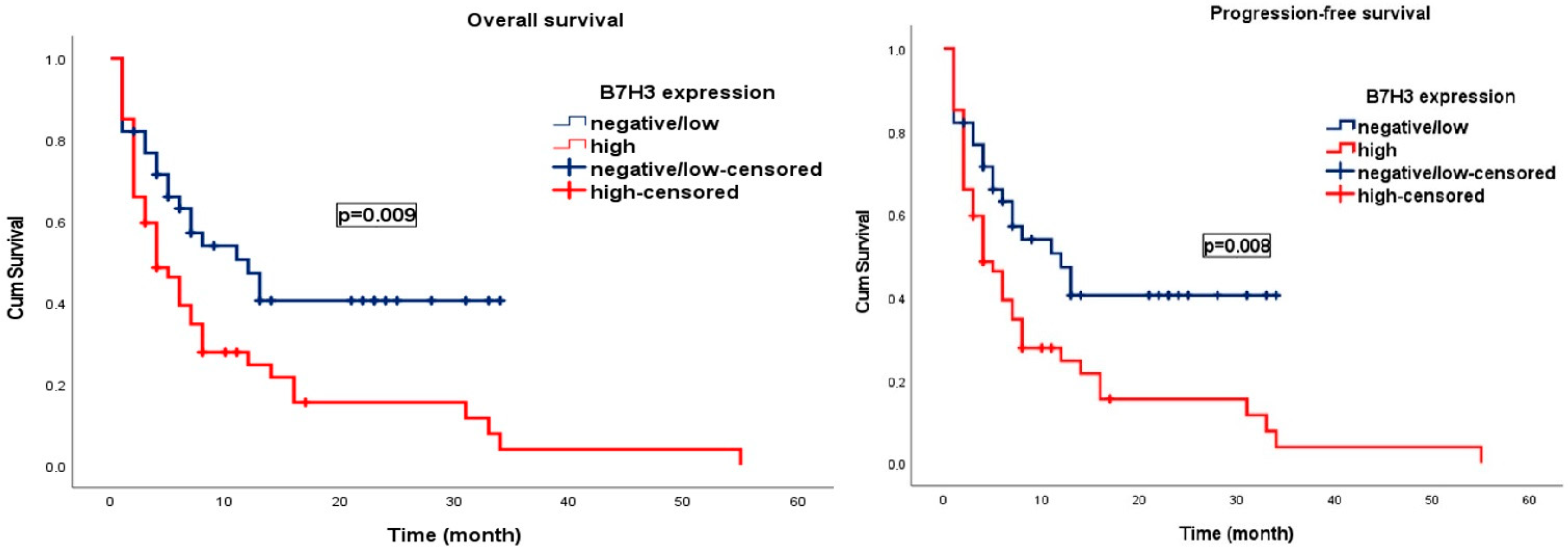
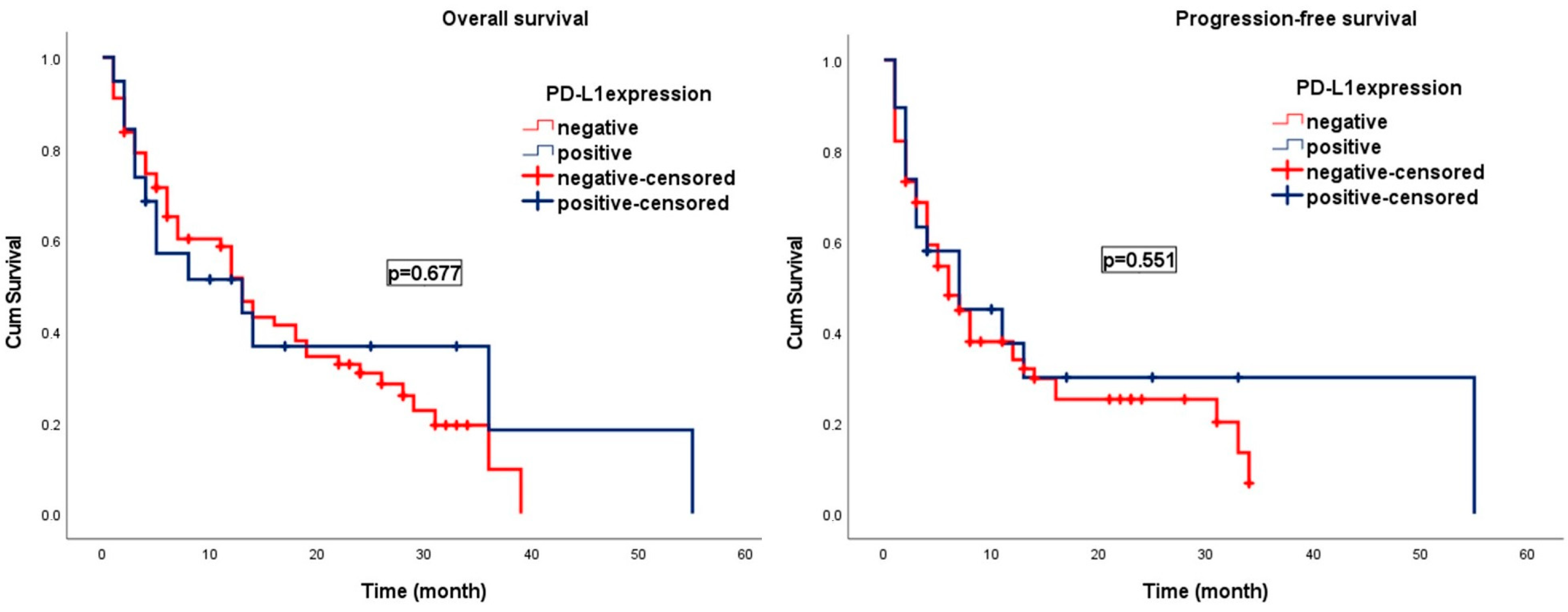
3. Results
Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Alzial, G.; Renoult, O.; Paris, F.; Gratas, C.; Clavreul, A.; Pecqueur, C. Wild-Type Isocitrate Dehydrogenase under the Spotlight in Glioblastoma. Oncogene 2022, 41, 613–621. [Google Scholar] [CrossRef]
- White, K.; Connor, K.; Clerkin, J.; Murphy, B.M.; Salvucci, M.; O’Farrell, A.C.; Rehm, M.; O’Brien, D.; Prehn, J.H.M.; Niclou, S.P.; et al. New Hints towards a Precision Medicine Strategy for IDH Wild-Type Glioblastoma. Ann. Oncol. 2020, 31, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Saaid, A.; Monticelli, M.; Ricci, A.A.; Orlando, G.; Botta, C.; Zeppa, P.; Bianconi, A.; Osella-Abate, S.; Bruno, F.; Pellerino, A.; et al. Prognostic Analysis of the IDH1 G105G (Rs11554137) SNP in IDH-Wildtype Glioblastoma. Genes 2022, 13, 1439. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-T.; Jin, W.-L. B7-H3/CD276: An Emerging Cancer Immunotherapy. Front. Immunol. 2021, 12, 701006. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K. B7-H3 Immunoregulatory Roles in Cancer. Biomed. Pharmacother. 2023, 163, 114890. [Google Scholar] [CrossRef]
- Malapelle, U.; Parente, P.; Pepe, F.; Di Micco, M.C.; Russo, A.; Clemente, C.; Graziano, P.; Rossi, A. B7-H3/CD276 Inhibitors: Is There Room for the Treatment of Metastatic Non-Small Cell Lung Cancer? Int. J. Mol. Sci. 2022, 23, 16077. [Google Scholar] [CrossRef]
- Yu, T.-T.; Zhang, T.; Lu, X.; Wang, R.-Z. B7-H3 Promotes Metastasis, Proliferation, and Epithelial-Mesenchymal Transition in Lung Adenocarcinoma. Onco Targets Ther. 2018, 11, 4693–4700. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, J.; Chen, L.; Qian, Z.; Zhang, Y. A Promising Target for Breast Cancer: B7-H3. BMC Cancer 2024, 24, 182. [Google Scholar] [CrossRef]
- Joshi, V.; Beecher, K.; Lim, M.; Stacey, A.; Feng, Y.; Jat, P.S.; Duijf, P.H.G.; Simpson, P.T.; Lakhani, S.R.; McCart Reed, A.E. B7-H3 Expression in Breast Cancer and Brain Metastasis. Int. J. Mol. Sci. 2024, 25, 3976. [Google Scholar] [CrossRef]
- Guo, C.; Figueiredo, I.; Gurel, B.; Neeb, A.; Seed, G.; Crespo, M.; Carreira, S.; Rekowski, J.; Buroni, L.; Welti, J.; et al. B7-H3 as a Therapeutic Target in Advanced Prostate Cancer. Eur. Urol. 2023, 83, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Xue, H.; Lin, Y.-Y.; Dong, X.; Classen, A.; Wu, R.; Jin, Y.; Lin, D.; Volik, S.; Ong, C.; et al. Influence of ADT on B7-H3 Expression during CRPC Progression from Hormone-Naïve Prostate Cancer. Cancer Gene Ther. 2023, 30, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tian, Z.; Gao, H.; Xiong, F.; Cao, C.; Yu, J.; Shi, W.; Zhan, Q.; Yang, C. Clinical Significance and Correlation of PD-L1, B7-H3, B7-H4, and TILs in Pancreatic Cancer. BMC Cancer 2022, 22, 584. [Google Scholar] [CrossRef]
- Babič, D.; Jovčevska, I.; Zottel, A. B7-H3 in Glioblastoma and beyond: Significance and Therapeutic Strategies. Front. Immunol. 2024, 15, 1495283. [Google Scholar] [CrossRef]
- Zhong, C.; Tao, B.; Chen, Y.; Guo, Z.; Yang, X.; Peng, L.; Xia, X.; Chen, L. B7-H3 Regulates Glioma Growth and Cell Invasion Through a JAK2/STAT3/Slug-Dependent Signaling Pathway. Onco Targets Ther. 2020, 13, 2215–2224. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, S.; Zhang, Y.; Wang, Y.; Zhang, Z.; Yang, M.; Zhu, Y.; Zhang, G.; Guo, G.; Tong, A.; et al. B7-H3 as a Novel CAR-T Therapeutic Target for Glioblastoma. Mol. Ther. Oncolytics 2019, 14, 279–287. [Google Scholar] [CrossRef]
- Mao, Y.; Wei, D.; Fu, F.; Wang, H.; Sun, Z.; Huang, Z.; Wang, Y.; Zhang, G.; Zhang, X.; Jiang, B.; et al. Development of a MMAE-Based Antibody-Drug Conjugate Targeting B7–H3 for Glioblastoma. Eur. J. Med. Chem. 2023, 257, 115489. [Google Scholar] [CrossRef]
- Hao, C.; Chen, G.; Zhao, H.; Li, Y.; Chen, J.; Zhang, H.; Li, S.; Zhao, Y.; Chen, F.; Li, W.; et al. PD-L1 Expression in Glioblastoma, the Clinical and Prognostic Significance: A Systematic Literature Review and Meta-Analysis. Front. Oncol. 2020, 10, 1015. [Google Scholar] [CrossRef] [PubMed]
- Pratt, D.; Dominah, G.; Lobel, G.; Obungu, A.; Lynes, J.; Sanchez, V.; Adamstein, N.; Wang, X.; Edwards, N.A.; Wu, T.; et al. Programmed Death Ligand 1 Is a Negative Prognostic Marker in Recurrent Isocitrate Dehydrogenase-Wildtype Glioblastoma. Neurosurgery 2019, 85, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Kim, T.M.; Frenel, J.; Simonelli, M.; Lopez, J.; Subramaniam, D.S.; Siu, L.L.; Wang, H.; Krishnan, S.; Stein, K.; et al. Treatment with Pembrolizumab in Programmed Death Ligand 1–Positive Recurrent Glioblastoma: Results from the Multicohort Phase 1 KEYNOTE-028 Trial. Cancer 2021, 127, 1620–1629. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bähr, O.; et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma. JAMA Oncol. 2020, 6, 1003–1010. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Z.; Luan, S.; Zheng, M.; Wang, Z.; Chen, Y.; Chen, X.; Tong, A.; Yang, H. Novel Bispecific Antibody-Drug Conjugate Targeting PD-L1 and B7-H3 Enhances Antitumor Efficacy and Promotes Immune-Mediated Antitumor Responses. J. Immunother. Cancer 2024, 12, e009710. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.; Prawira, A.; Antonia, S.; Rahma, O.; Tolcher, A.; Cohen, R.B.; Lou, Y.; Hauke, R.; Vogelzang, N.; Zandberg, D.P.; et al. Dual Checkpoint Targeting of B7-H3 and PD-1 with Enoblituzumab and Pembrolizumab in Advanced Solid Tumors: Interim Results from a Multicenter Phase I/II Trial. J. Immunother. Cancer 2022, 10, e004424. [Google Scholar] [CrossRef]
- Brown, N.F.; Carter, T.; Kitchen, N.; Mulholland, P. Dabrafenib and Trametinib in BRAFV600E Mutated Glioma. CNS Oncol. 2017, 6, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Kaley, T.; Touat, M.; Subbiah, V.; Hollebecque, A.; Rodon, J.; Lockhart, A.C.; Keedy, V.; Bielle, F.; Hofheinz, R.-D.; Joly, F.; et al. BRAF Inhibition in BRAF V600 -Mutant Gliomas: Results From the VE-BASKET Study. J. Clin. Oncol. 2018, 36, 3477–3484. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in Patients with TRK Fusion-Positive Solid Tumours: A Pooled Analysis of Three Phase 1/2 Clinical Trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Franz, D.N.; Belousova, E.; Sparagana, S.; Bebin, E.M.; Frost, M.; Kuperman, R.; Witt, O.; Kohrman, M.H.; Flamini, J.R.; Wu, J.Y.; et al. Efficacy and Safety of Everolimus for Subependymal Giant Cell Astrocytomas Associated with Tuberous Sclerosis Complex (EXIST-1): A Multicentre, Randomised, Placebo-Controlled Phase 3 Trial. Lancet 2013, 381, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Mishchenko, T.A.; Turubanova, V.D.; Gorshkova, E.N.; Krysko, O.; Vedunova, M.V.; Krysko, D.V. Glioma: Bridging the Tumor Microenvironment, Patient Immune Profiles and Novel Personalized Immunotherapy. Front. Immunol. 2024, 14, 1299064. [Google Scholar] [CrossRef] [PubMed]
- Adhikaree, J.; Moreno-Vicente, J.; Kaur, A.P.; Jackson, A.M.; Patel, P.M. Resistance Mechanisms and Barriers to Successful Immunotherapy for Treating Glioblastoma. Cells 2020, 9, 263. [Google Scholar] [CrossRef]
- Brown, M.C.; Ashley, D.M.; Khasraw, M. Low Tumor Mutational Burden and Immunotherapy in Gliomas. Trends Cancer 2022, 8, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Awad, M.; Koyama, T.; Gutierrez, M.; Falchook, G.S.; Piha-Paul, S.A.; Doi, T.; Satoh, T.; Okamoto, N.; Singh, J.; et al. OA05.05 Ifinatamab Deruxtecan (I-DXd; DS-7300) in Patients with Refractory SCLC: A Subgroup Analysis of a Phase 1/2 Study. J. Thorac. Oncol. 2023, 18, S54–S55. [Google Scholar] [CrossRef]
- Digregorio, M.; Coppieters, N.; Lombard, A.; Lumapat, P.N.; Scholtes, F.; Rogister, B. The Expression of B7-H3 Isoforms in Newly Diagnosed Glioblastoma and Recurrence and Their Functional Role. Acta Neuropathol. Commun. 2021, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chang, M.; Wang, Y.; Xing, B.; Ma, W. B7-H3 in Brain Malignancies: Immunology and Immunotherapy. Int. J. Biol. Sci. 2023, 19, 3762–3780. [Google Scholar] [CrossRef]

| Variables, n (%) | B7-H3 Expression | PD-L1 Expression | ||||||
|---|---|---|---|---|---|---|---|---|
| Negative/Low | High | p | Negative | Positive | p | |||
| n, (%) | n, (%) | n, (%) | n, (%) | |||||
| Age | <60 | 43 (50.0) | 19 (48.7) | 24 (51.1) | 0.500 | 33 (49.3) | 10 (52.6) | 0.500 |
| ≥60 | 43 (50.0) | 20 (51.3) | 23 (48.9) | 34 (50.7) | 9 (47.4) | |||
| Sex | Female | 37 (43.0) | 19 (48.7) | 18 (38.3) | 0.226 | 29 (43.3) | 8 (42.1) | 0.570 |
| Male | 49 (57.0) | 20 (51.3) | 29 (61.7) | 38 (56.7) | 11 (57.9) | |||
| Comorbidity | None | 42 (48.8) | 17 (43.6) | 25 (53.2) | 0.252 | 32 (47.8) | 10 (52.6) | 0.454 |
| Present | 44 (51.2) | 22 (56.4) | 22 (46.8) | 35 (52.2) | 9 (47.4) | |||
| ECOG PS | 0–1 | 42 (48.8) | 17 (43.6) | 25 (53.2) | 0.252 | 32 (47.8) | 10 (52.6) | 0.454 |
| ≥2 | 44 (51.2) | 22 (56.4) | 22 (46.8) | 35 (52.2) | 9 (47.4) | |||
| Lateralization | Right | 45 (52.3) | 20 (51.3) | 25 (53.2) | 0.516 | 36 (53.7) | 9 (47.4) | 0.408 |
| Left | 41 (47.79) | 19 (48.7) | 22 (46.8) | 31 (46.3) | 10 (52.6) | |||
| Tumor location | Frontal | 22 (25.6) | 13 (33.3) | 9 (19.1) | 0.157 | 19 (28.4) | 3 (15.8) | 0.143 |
| Parietal | 18 (20.9) | 8 (20.5) | 10 (21.3) | 14 (20.9) | 4 (21.1) | |||
| Occipital | 15 (17.5) | 6 (15.4) | 9 (19.1) | 13 (19.4) | 2 (10.5) | |||
| Temporal | 31 (36.0) | 12 (30.8) | 19 (40.4) | 21 (31.3) | 10 (52.6) | |||
| Adjuvant chemo | No | 38 (44.2) | 20 (51.3) | 18 (38.3) | 0.161 | 30 (44.8) | 8 (42.1) | 0.524 |
| Yes | 48 (55.8) | 19 (48.7) | 29 (61.7) | 37 (55.2) | 11 (57.9) | |||
| Radiotherapy | No | 23 (26.7) | 15 (38.5) | 8 (17.0) | 0.023 | 19 (28.4) | 4 (21.1) | 0.376 |
| Yes | 63 (73.3) | 24 (61.5) | 39 (83.0) | 48 (71.6) | 15 (78.9) | |||
| Selected chemo regimen | Temozolomide | 34 (70.1) | 13 (68.4) | 21 (72.4) | 0.507 | 25 (67.6) | 9 (81.2) | 0.305 |
| Irinotecan plus Bevacizumab | 14 (29.9) | 6 (31.6) | 8 (27.6) | 12 (32.4) | 2 (18.2) | |||
| Tumor focality | Unifocal | 62 (72.1) | 24 (61.5) | 38 (80.9) | 0.040 | 46 (68.7) | 16 (84.2) | 0.148 |
| Multifocal | 24 (27.9) | 15 (38.5) | 9 (19.1) | 21 (31.3) | 3 (15.8) | |||
| Tumor size, mm | <50 | 44 (51.2) | 23 (59.0) | 21 (44.7) | 0.135 | 37 (55.2) | 10 (52.6) | 0.522 |
| ≥50 | 42 (48.8) | 16 (41.0) | 26 (55.3) | 30 (44.8) | 9 (47.4) | |||
| ATRX loss | None | 76 (88.4) | 35 (89.7) | 41 (87.2) | 0.494 | 36 (53.7) | 8 (42.1) | 0.263 |
| Present | 10 (11.6) | 4 (10.3) | 6 (12.8) | 31 (46.3) | 11 (57.9) | |||
| Ki67 | ≤%50 | 59 (68.6) | 26 (66.7) | 33 (70.2) | 0.451 | 57 (85.1) | 19 (100.0) | 0.070 |
| >%50 | 27 (31.4) | 13 (33.3) | 14 (29.8) | 10 (14.9) | 0 (0.0) | |||
| Surgical procedure | Subtotal | 47 (54.7) | 24 (61.5) | 23 (48.9) | 0.171 | 45 (67.2) | 14 (73.7) | 0.405 |
| Gross total | 39 (45.3) | 15 (38.5) | 24 (51.1) | 22 (32.8) | 5 (26.3) | |||
| p53 status | Wild | 41 (47.7) | 18 (46.2) | 23 (48.9) | 0.484 | 33 (49.3) | 8 (42.1) | 0.387 |
| Mutant | 45 (52.3) | 21 (53.8) | 24 (51.1) | 34 (50.7) | 11 (57.9) | |||
| Overall Survival | Progression-Free Survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95.0% CI | p | HR | 95.0% CI | p | ||||
| Lower | Upper | Lower | Upper | ||||||
| Age | 2.56 | 1.50 | 4.37 | 0.001 | Age | 1.86 | 1.11 | 3.10 | 0.018 |
| Sex | 0.99 | 0.59 | 1.65 | 0.972 | Sex | 0.87 | 0.52 | 1.45 | 0.615 |
| Comorbidity | 2.07 | 1.22 | 3.51 | 0.007 | Comorbidity | 1.43 | 0.86 | 2.38 | 0.161 |
| ECOG PS | 1.69 | 1.01 | 2.81 | 0.042 | ECOG PS | 1.48 | 0.89 | 2.46 | 0.127 |
| Tumor location | 0.96 | 0.78 | 1.19 | 0.766 | Tumor location | 0.98 | 0.79 | 1.21 | 0.858 |
| Lateralization | 0.98 | 0.59 | 1.63 | 0.949 | Lateralization | 0.97 | 0.58 | 1.61 | 0.923 |
| Adjuvant chemo | 0.74 | 0.44 | 1.23 | 0.254 | Adjuvant chemo | 0.94 | 0.56 | 1.56 | 0.815 |
| Radiotherapy | 0.97 | 0.54 | 1.75 | 0.929 | Radiotherapy | 1.16 | 0.64 | 2.10 | 0.616 |
| Selected chemo regimen | 0.35 | 0.15 | 0.79 | 0.012 | Selected chemo regimen | 0.44 | 0.19 | 1.01 | 0.054 |
| Tumor focality | 1.26 | 0.71 | 2.23 | 0.422 | Tumor focality | 1.06 | 0.60 | 1.86 | 0.830 |
| Surgical procedure | 0.66 | 0.38 | 1.11 | 0.123 | Surgical procedure | 0.77 | 0.46 | 1.28 | 0.318 |
| Tumor size | 1.09 | 0.66 | 1.82 | 0.718 | Tumor size | 1.04 | 0.63 | 1.73 | 0.858 |
| ATRX loss | 1.53 | 0.75 | 3.13 | 0.241 | ATRX loss | 1.31 | 0.64 | 2.68 | 0.447 |
| Ki67 | 0.95 | 0.54 | 1.66 | 0.874 | Ki67 | 0.91 | 0.52 | 1.58 | 0.744 |
| p53 status | 0.75 | 0.45 | 1.25 | 0.278 | p53 status | 0.72 | 0.43 | 1.20 | 0.215 |
| B7-H3 expression | 1.98 | 1.16 | 3.40 | 0.012 | B7-H3 expression | 1.95 | 1.14 | 3.32 | 0.014 |
| PD-L1 expression | 0.87 | 0.46 | 1.66 | 0.687 | PD-L1 expression | 0.83 | 0.44 | 1.56 | 0.570 |
| Overall Survival | Progression-Free Survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95.0% CI | p | HR | 95.0% CI | p | ||||
| Lower | Upper | Lower | Upper | ||||||
| Age | 1.86 | 0.80 | 4.32 | 0.147 | Age | 2.06 | 1.22 | 3.48 | 0.007 |
| Comorbidity | 1.29 | 0.56 | 2.92 | 0.542 | Comorbidity | - | - | - | - |
| ECOG PS | 1.17 | 0.50 | 2.70 | 0.711 | ECOG PS | - | - | - | - |
| Selected chemo regimen | 0.20 | 0.07 | 0.56 | 0.002 | Selected chemo regimen | - | - | - | - |
| B7-H3 expression | 6.47 | 2.58 | 16.21 | <0.001 | B7-H3 expression | 2.14 | 1.25 | 3.67 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yüceer, R.O.; Kaya, S.; Balcı, S.N.; Eğilmez, H.R.; Yılmaz, M.; Erdıs, E. Prognostic Biomarkers in Isocitrate Dehydrogenase Wild-Type Glioblastoma: A Focus on B7-H3. Brain Sci. 2025, 15, 212. https://doi.org/10.3390/brainsci15020212
Yüceer RO, Kaya S, Balcı SN, Eğilmez HR, Yılmaz M, Erdıs E. Prognostic Biomarkers in Isocitrate Dehydrogenase Wild-Type Glioblastoma: A Focus on B7-H3. Brain Sciences. 2025; 15(2):212. https://doi.org/10.3390/brainsci15020212
Chicago/Turabian StyleYüceer, Ramazan Oğuz, Seyhmus Kaya, Sema Nur Balcı, Hatice Reyhan Eğilmez, Mukaddes Yılmaz, and Eda Erdıs. 2025. "Prognostic Biomarkers in Isocitrate Dehydrogenase Wild-Type Glioblastoma: A Focus on B7-H3" Brain Sciences 15, no. 2: 212. https://doi.org/10.3390/brainsci15020212
APA StyleYüceer, R. O., Kaya, S., Balcı, S. N., Eğilmez, H. R., Yılmaz, M., & Erdıs, E. (2025). Prognostic Biomarkers in Isocitrate Dehydrogenase Wild-Type Glioblastoma: A Focus on B7-H3. Brain Sciences, 15(2), 212. https://doi.org/10.3390/brainsci15020212






