AI-Based Retinal Image Analysis for the Detection of Choroidal Neovascular Age-Related Macular Degeneration (AMD) and Its Association with Brain Health
Abstract
1. Introduction
2. Method
2.1. Dataset
2.1.1. Primary Dataset
2.1.2. Validation Dataset
2.2. AMD Classification
2.3. Image Pre-Processing
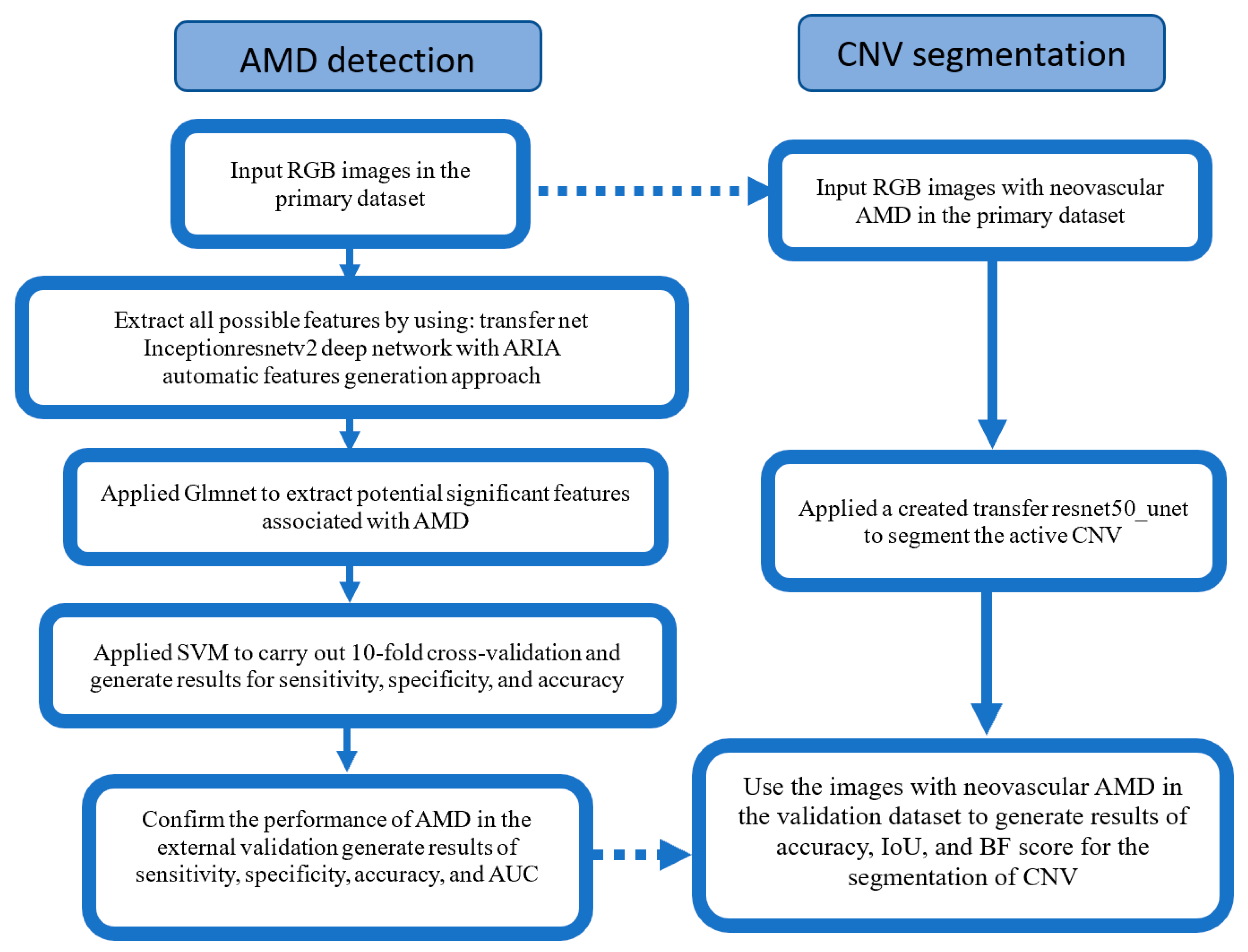
2.4. Development of the Automatic Retinal Analysis Method for AMD
2.5. Statistical Analysis
3. Results
3.1. Primary Dataset and Validation Dataset
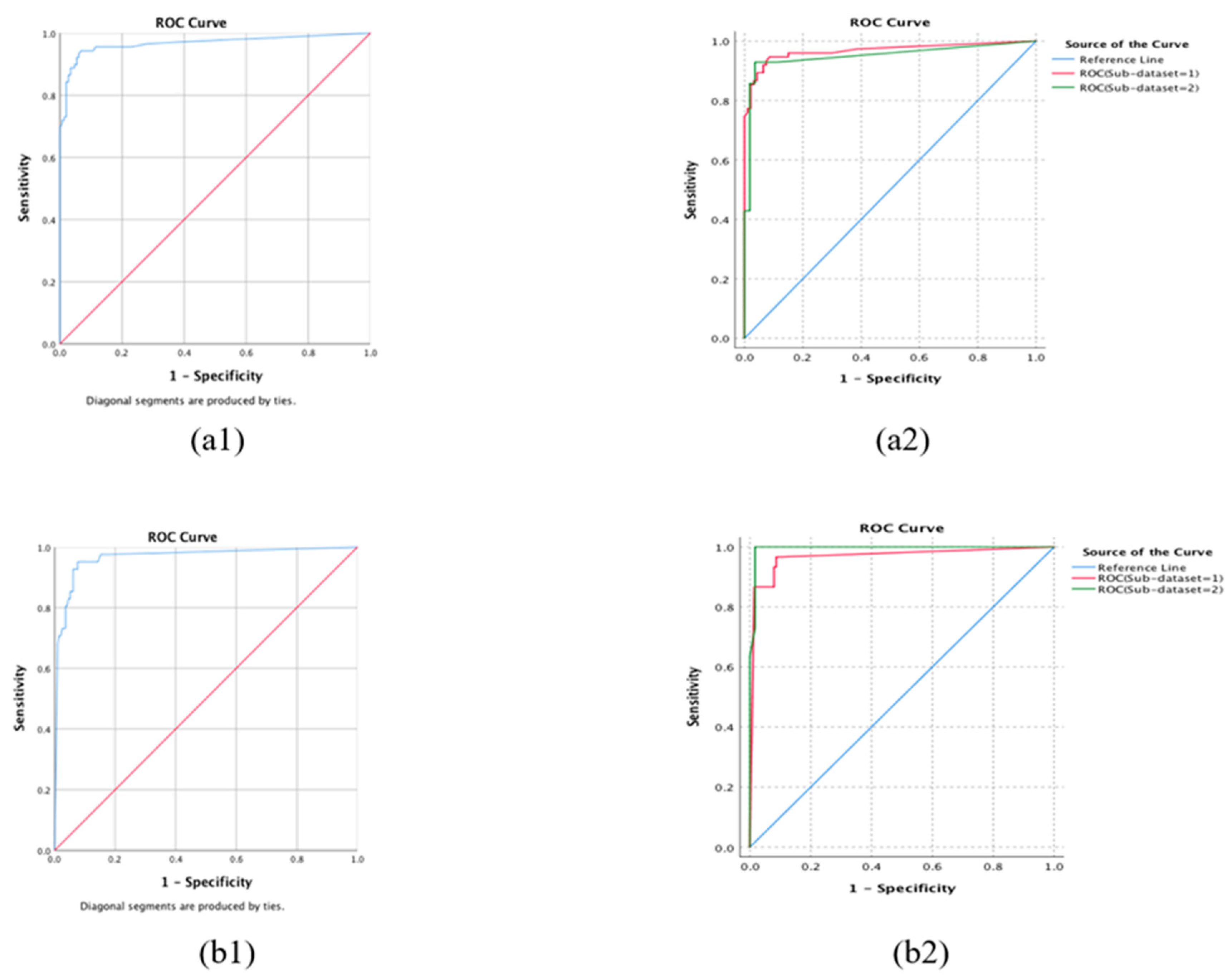
3.2. Internal 10-Fold Cross-Validation
3.3. External Validation
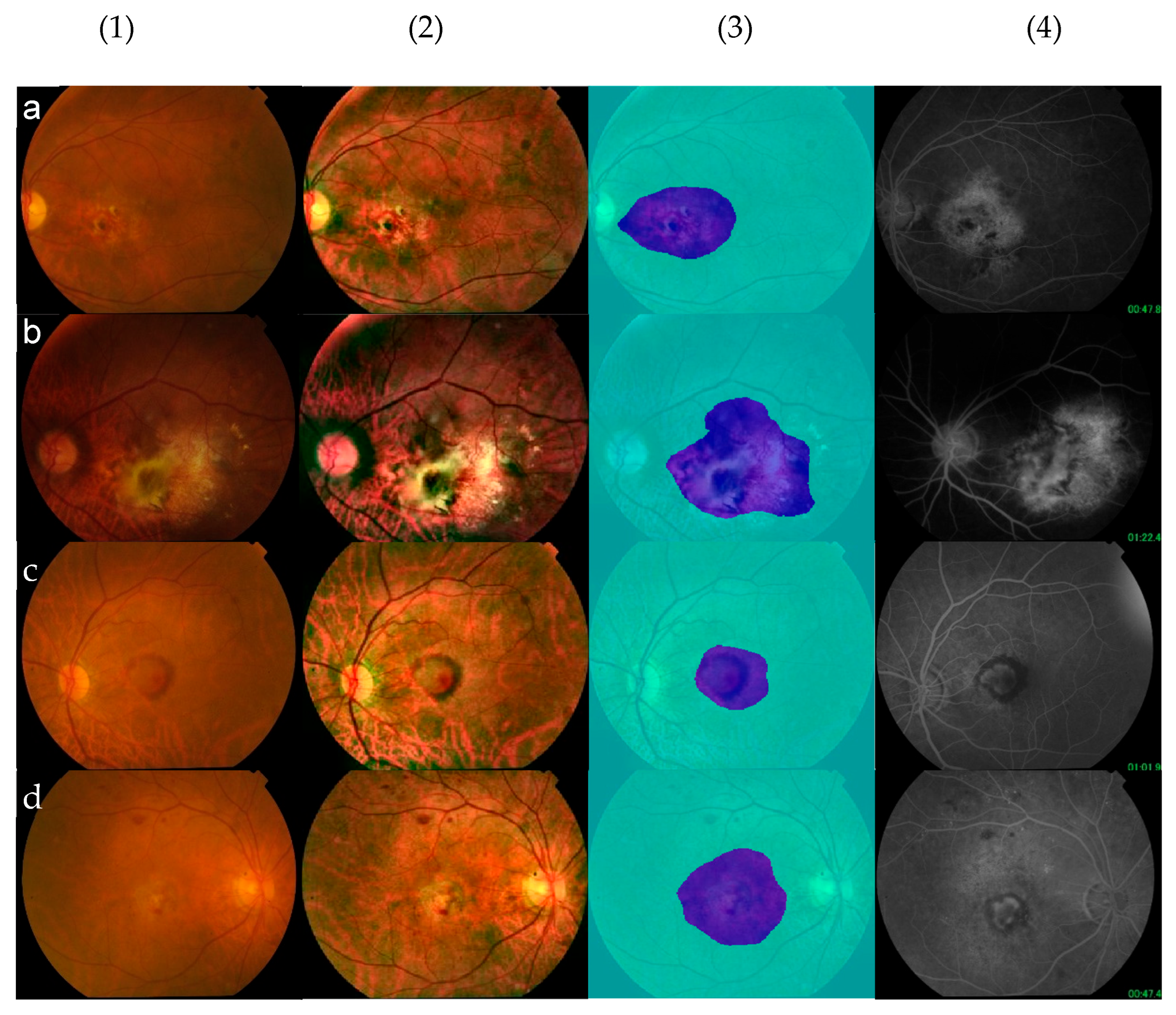
3.4. Segmentation—A Visual Presentation of an Explainable AI System
3.5. Association with WMH as a Risk Factor for Dementia and Depression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Zhu, Z.; Huang, Y.; Zhang, X.; Wang, W.; Shi, D.; Jiang, Y.; Yang, X.; He, M. Associations of ophthalmic and systemic conditions with incident dementia in the UK Biobank. Br. J. Ophthalmol. 2023, 107, 275–282. [Google Scholar] [CrossRef]
- Dawson, S.R.; Mallen, C.D.; Gouldstone, M.B.; Yarham, R.; Mansell, G. The prevalence of anxiety and depression in people with age-related macular degeneration: A systematic review of observational study data. BMC Ophthalmol. 2014, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Ferris FL3rd Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R.; Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group; SanGiovanni, J.P.; Chew, E.Y.; Clemons, T.E.; Ferris, F.L., 3rd; Gensler, G.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; Sperduto, R.D. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch. Ophthalmol. 2007, 125, 1225–1232. [Google Scholar]
- Peng, Y.; Dharssi, S.; Chen, Q.; Keenan, T.D.; Agrón, E.; Wong, W.T.; Chew, E.Y.; Lu, Z. DeepSeeNet: A Deep Learning Model for Automated Classification of Patient-based Age-related Macular Degeneration Severity from Color Fundus Photographs. Ophthalmology 2019, 126, 565–575. [Google Scholar] [CrossRef]
- Bhuiyan, A.; Wong, T.Y.; Ting, D.S.W.; Govindaiah, A.; Souied, E.H.; Smith, R.T. Artificial Intelligence to Stratify Severity of Age-Related Macular Degeneration (AMD) and Predict Risk of Progression to Late AMD. Transl. Vis. Sci. Technol. 2020, 9, 25. [Google Scholar] [CrossRef]
- González-Gonzalo, C.; Sánchez-Gutiérrez, V.; Hernández-Martínez, P.; Contreras, I.; Lechanteur, Y.T.; Domanian, A.; van Ginneken, B.; Sánchez, C.I. Evaluation of a deep learning system for the joint automated detection of diabetic retinopathy and age-related macular degeneration. Acta Ophthalmol. 2020, 98, 368–377. [Google Scholar] [CrossRef]
- Burlina, P.M.; Joshi, N.; Pekala, M.; Pacheco, K.D.; Freund, D.E.; Bressler, N.M. Automated Grading of Age-Related Macular Degeneration from Color Fundus Images Using Deep Convolutional Neural Networks. JAMA Ophthalmol. 2017, 135, 1170–1176. [Google Scholar] [CrossRef]
- Burlina, P.; Pacheco, K.D.; Joshi, N.; Freund, D.E.; Bressler, N.M. Comparing humans and deep learning performance for grading AMD: A study in using universal deep features and transfer learning for automated AMD analysis. Comput. Biol. Med. 2017, 82, 80–86. [Google Scholar] [CrossRef]
- Ferris, F.L., 3rd; Fine, S.L.; Hyman, L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch. Ophthalmol. 1984, 102, 1640–1642. [Google Scholar] [CrossRef]
- Flaxel, C.J.; Adelman, R.A.; Bailey, S.T.; Fawzi, A.; Lim, J.I.; Vemulakonda, G.A.; Ying, G.S. Age-Related Macular Degeneration Preferred Practice Pattern®. Ophthalmology 2020, 127, P1–P65. [Google Scholar] [CrossRef]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Grey, R.H.; Bird, A.C.; Chisholm, I.H. Senile disciform macular degeneration: Features indicating suitability for photocoagulation. Br. J. Ophthalmol. 1979, 63, 85–89. [Google Scholar] [CrossRef]
- Borrelli, E.; Grosso, D.; Vella, G.; Sacconi, R.; Battista, M.; Querques, L.; Zucchiatti, I.; Prascina, F.; Bandello, F.; Querques, G. Short-term outcomes of patients with neovascular exudative AMD: The effect of COVID-19 pandemic. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2621–2628. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Guidelines. In Age-Related Macular Degeneration: Diagnosis and Management; National Institute for Health and Care Excellence (NICE) Copyright © NICE: London, UK, 2018; Available online: https://www.nice.org.uk/guidance/ng82 (accessed on 17 February 2023).
- Sickenberg, M. Early detection, diagnosis and management of choroidal neovascularisation in age-related macular degeneration: The role of ophthalmologists. Ophthalmologica 2001, 215, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Macular Photocoagulation Study Group. Visual outcome after laser photocoagulation for subfoveal choroidal neovascularisation secondary to age-related macular degeneration: The influence of initial lesion size and initial visual acuity. Arch. Ophthalmol. 1994, 112, 480–488. [Google Scholar] [CrossRef]
- Guyer, D.R.; Fine, S.L.; Maguire, M.G.; Hawkins, B.S.; Owens, S.L.; Murphy, R.P. Subfoveal choroidal neovascular membranes in age-related macular degeneration. Visual prognosis in eyes with relatively good initial visual acuity. Arch. Ophthalmol. 1986, 104, 702–705. [Google Scholar] [CrossRef]
- Doris, N.; Hart, P.M.; Chakravarthy, U.; McCleland, J.; Stevenson, M.; Hudson, C.; Jackson, J. Relation between macular morphology and visual function in patients with choroidal neovascularisation of age related macular degeneration. Br. J. Ophthalmol. 2001, 85, 184–188. [Google Scholar] [CrossRef]
- Ying, G.S.; Huang, J.; Maguire, M.G.; Jaffe, G.J.; Grunwald, J.E.; Toth, C.; Daniel, E.; Klein, M.; Pieramici, D.; Wells, J.; et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology 2013, 120, 122–129. [Google Scholar] [CrossRef]
- Do, D.V. Detection of new-onset choroidal neovascularisation. Curr. Opin. Ophthalmol. 2013, 24, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A. The Diagnosis and Treatment of Age-Related Macular Degeneration. Dtsch. Arztebl. Int. 2020, 117, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Yannuzzi, L.A.; Rohrer, K.T.; Tindel, L.J.; Sobel, R.S.; Costanza, M.A.; Shields, W.; Zang, E. Fluorescein angiography complication survey. Ophthalmology 1986, 93, 611–617. [Google Scholar] [CrossRef]
- Kwiterovich, K.A.; Maguire, M.G.; Murphy, R.P.; Schachat, A.P.; Bressler, N.M.; Bressler, S.B.; Fine, S.L. Frequency of adverse systemic reactions after fluorescein angiography. Results of a prospective study. Ophthalmology 1991, 98, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F. Fundus Imaging of AMD. In Age-Related Macular Degeneration; Holz, F.G., Pauleikhoff, D., Spaide, R.F., Bird, A.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 141–161. Available online: https://link.springer.com/chapter/10.1007/978-3-642-22107-1_9 (accessed on 5 June 2025).
- Gualino, V.; Tadayoni, R.; Cohen, S.Y.; Erginay, A.; Fajnkuchen, F.; Haouchine, B.; Krivosic, V.; Quentel, G.; Vicaut, E.; Gaudric, A. Optical Coherence Tomography, Fluorescein Angiography, and Diagnosis of Choroidal Neovascularization in Age-Related Macular Degeneration. Retina 2019, 39, 1664–1671. [Google Scholar] [CrossRef]
- Zee, C.Y.; Lee, J.W.; Li, Q. Method and Device for Retinal Image Analysis. U.S. Patent 8787638 B2, 22 July 2014. [Google Scholar]
- Falk, T.; Mai, D.; Bensch, R.; Çiçek, Ö.; Abdulkadir, A.; Marrakchi, Y.; Böhm, A.; Deubner, J.; Jäckel, Z.; Seiwald, K.; et al. U-Net: Deep learning for cell counting, detection, and morphometry. Nat. Methods 2019, 16, 67–70. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]
- Fan, R.-E.; Chen, P.-H.; Lin, C.-J. Working set selection using second order information for training support vector machines. J. Mach. Learn. Res. 2005, 6, 1889–1918. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer: Berlin/Heidelberg, Germany, 2009; Volume 2. [Google Scholar]
- Chow, S.-C.; Wang, H.; Shao, J. Sample Size Calculations in Clinical Research; Chapman and Hall/CRC: New York, NY, USA, 2003. [Google Scholar]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Csurka, G.; Larlus, D. What Is a Good Evaluation Measure for Semantic Segmentation? Bmvc. Bristol. 2013. Available online: https://www.bmva-archive.org.uk/bmvc/2013/Papers/paper0032/paper0032.pdf (accessed on 17 February 2023).
- Lau, A.; Mok, V.; Lee, J.; Fan, Y.; Zeng, J.; Lam, B.; Wong, A.; Kwok, C.; Lai, M.; Zee, B. Retinal image analytics detects white matter hyperintensities in healthy adults. Ann. Clin. Transl. Neurol. 2019, 6, 98–105. [Google Scholar] [CrossRef]
- Zee, B.; Wong, Y.; Lee, J.; Fan, Y.; Zeng, J.; Lam, B.; Wong, A.; Shi, L.; Lee, A.; Kwok, C.; et al. Machine-learning method for localization of cerebral white matter hyperintensities in healthy adults based on retinal images. Brain Commun. 2021, 3, fcab124. [Google Scholar] [CrossRef]
- Hung, H.; Lai, M.; Lee, J.; Chow, J.; Lau, A.; Qu, Y.; Lee, E.; Chan, K.L.; Tang, N.; Li, X.; et al. AI-based Automatic Retinal Image Analysis (ARIA) for Detecting Depression Risk in Adults. In Proceedings of the 49th International Exhibition of Inventions Geneva, Geneva, Switzerland, 16–20 April 2024. [Google Scholar]
- Tsai, C.L.; Yang, Y.L.; Chen, S.J.; Lin, K.S.; Chan, C.H.; Lin, W.Y. Automatic characterization of classic choroidal neovascularization by using AdaBoost for supervised learning. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2767–2774. [Google Scholar] [CrossRef]
- Tsai, Y.Y.; Lin, W.Y.; Chen, S.J.; Ruamviboonsuk, P.; King, C.H.; Tsai, C.L. Diagnosis of Polypoidal Choroidal Vasculopathy from Fluorescein Angiography Using Deep Learning. Transl. Vis. Sci. Technol. 2022, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hormel, T.T.; Gao, L.; Zang, P.; Guo, Y.; Wang, X.; Bailey, S.T.; Jia, Y. Automated diagnosis and segmentation of choroidal neovascularization in OCT angiography using deep learning. Biomed. Opt. Express 2020, 11, 927–944. [Google Scholar] [CrossRef]
- Told, R.; Reiter, G.S.; Mittermüller, T.J.; Schranz, M.; Reumueller, A.; Schlanitz, F.G.; Weigert, G.; Pollreisz, A.; Sacu, S.; Schmidt-Erfurth, U. Profiling neovascular age-related macular degeneration choroidal neovascularization lesion response to anti-vascular endothelial growth factor therapy using SSOCTA. Acta Ophthalmol. 2021, 99, e240–e246. [Google Scholar] [CrossRef]
- Agurto, C.; Barriga, E.S.; Murray, V.; Nemeth, S.; Crammer, R.; Bauman, W.; Zamora, G.; Pattichis, M.S.; Soliz, P. Automatic detection of diabetic retinopathy and age-related macular degeneration in digital fundus images. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5862–5871. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.W.; Cheung, C.Y.; Lim, G.; Tan, G.S.W.; Quang, N.D.; Gan, A.; Hamzah, H.; Garcia-Franco, R.; San Yeo, I.Y.; Lee, S.Y.; et al. Development and Validation of a Deep Learning System for Diabetic Retinopathy and Related Eye Diseases Using Retinal Images from Multiethnic Populations with Diabetes. JAMA 2017, 318, 2211–2223. [Google Scholar] [CrossRef]
- Yellapragada, B.; Hornauer, S.; Snyder, K.; Yu, S.; Yiu, G. Self-Supervised Feature Learning and Phenotyping for Assessing Age-Related Macular Degeneration Using Retinal Fundus Images. Ophthalmol. Retin. 2022, 6, 116–129. [Google Scholar] [CrossRef]
- Heo, T.Y.; Kim, K.M.; Min, H.K.; Gu, S.M.; Kim, J.H.; Yun, J.; Min, J.K. Development of a Deep-Learning-Based Artificial Intelligence Tool for Differential Diagnosis between Dry and Neovascular Age-Related Macular Degeneration. Diagnostics 2020, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.G.; Park, S.; Park, C.M.; Jeon, Y.K.; Chung, D.H.; Goo, J.M.; Kim, Y.T.; Kim, H. Histopathologic Basis for a Chest CT Deep Learning Survival Prediction Model in Patients with Lung Adenocarcinoma. Radiology 2022, 305, 441–451. [Google Scholar] [CrossRef]
| Classification | Category | Stage | Definition |
|---|---|---|---|
| Control | 1 | No AMD | No drusen or only small drusen 63 mm, and no pigment abnormalities |
| 2 | Early AMD | Medium drusen > 63 mm and ≤125 mm, and no pigment abnormalities | |
| Referable AMD | 3 | Intermediate AMD | Large drusen > 125 mm or any pigment abnormalities |
| 4 | Advanced AMD | Neovascular AMD or geographical atrophy |
| Category | Primary Dataset | Validation Datasets | |||
|---|---|---|---|---|---|
| Sub-Dataset 1 * | Sub-Dataset 2 * | Total | |||
| Referable AMD | 453 (30.61) | 75 (44.64) | 14 (20.00) | 89 (37.39) | |
| NVAMD | 213 (14.39) | 30 (17.86) | 11(15.71) | 41 (17.23) | |
| Non-NVAMD | 240 (16.22) | 45 (26.78) | 3 (4.29) | 48 (20.16) | |
| Control | 1027 (69.39) | 93 (55.36) | 56 (80.00) | 149 (62.61) | |
| Total | 1480 (100) | 168(100) | 70 (100) | 238 (100) | |
| Se (%) | Sp (%) | Acc (%) | |
|---|---|---|---|
| Referable AMD vs. Control | 97.4 | 96.8 | 97.0 |
| NVAMD vs. (Non-NVAMD + Control) | 98.1 | 96.1 | 96.4 |
| Confusion Matrix | Se | Sp | Acc | AUC | p | |||
|---|---|---|---|---|---|---|---|---|
| Referable AMO vs. Control | 1 | 0 | ||||||
| 1 | 76 | 4 | 85.4 | 97.3 | 92.9 | 0.967 | - | |
| 0 | 13 | 145 | ||||||
| Subgroups | 1 | 0 | 0.704 | |||||
| Sub-dataset 1 | 1 | 64 | 3 | 85.3 | 96.8 | 91.7 | 0.968 | |
| 0 | 11 | 90 | ||||||
| Sub-dataset 2 | 1 | 12 | 1 | 85.7 | 98.2 | 95.7 | 0.950 | |
| 0 | 2 | 55 | ||||||
| NVAMD vs. (Non-NVA + Control) | 1 | 0 | ||||||
| 1 | 38 | 12 | 92.7 | 93.9 | 93.7 | 0.967 | - | |
| 0 | 3 | 185 | ||||||
| Subgroups | 1 | 0 | 0.213 | |||||
| Sub-dataset 1 | 1 | 27 | 11 | 90.0 | 92.0 | 91.7 | 0.967 | |
| 0 | 3 | 127 | ||||||
| Sub-dataset 2 | 1 | 11 | 1 | 100.0 | 98.3 | 98.6 | 0.996 | |
| 0 | 0 | 58 | ||||||
| Global Acc | Mean Acc | Mean IoU | Weighted IoU | Mean BF Score | |
|---|---|---|---|---|---|
| Segmentation | 93.03% | 91.83% | 68.7% | 89.63% | 67.77% |
| Data Source | Method | Performance | |
|---|---|---|---|
| CNV Delineation | |||
| Tsai et al. [40] | Fluorescein angiography images | Random walk algorithm | Accuracy = 83.26% |
| Wang et al. [42] | Projection-resolved optical coherence tomographic angiography (PR-OCTA) | Convolutional neural networks (CNNs) | Mean intersection over the union = 0.88 |
| Our study | Colour fundus retinal images | Global accuracy = 93.03%; Weighted IoU = 89.63% | |
| Referable AMD Detection | |||
| Agurto et al. [44] | Retinal digital photographs | A computer-aided algorithm | Sensitivity = 0.94; Specificity = 0.50; AUC = 0.84 |
| Burlina et al. [10]. | Fundus images | Deep convolutional neural networks | Accuracy: 88.4% to 91.6%; AUC: 0.94 to 0.96 |
| Ting et al. [45] | Retinal images | Deep learning system (DLS) | Sensitivity = 93.2%; Specificity = 88.7%; AUC = 0.931 |
| Yellapragada et al. [46] | Fundus photographs | Deep neural network with self-supervised Non-Parametric Instance Discrimination (NPID) | Self-supervised-trained network: Accuracy = 87%; Supervised-trained network: Accuracy = 90% |
| Our study | Colour fundus retinal images | AI-based Retinal Image Analysis (ARIA) | Sensitivity = 97.4%; Specificity = 96.8%; Accuracy = 97.0% |
| Neovascular AMD Detection | |||
| Heo et al. [47] | Fundus photographs | Visual Geometry Group with 16 layers (VGG16) model of convolutional neural networks | Normal vs. nAMD: accuracy = 0.9099; dAMD vs. nAMD: accuracy = 0.7601 |
| Our study | Colour fundus retinal images | AI-based Retinal Image Analysis (ARIA) | nAMD vs. (Non-nAMD+Normal): Sensitivity = 98.1%; Specificity = 96.1%; Accuracy = 96.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, C.; Lee, J.; Shi, D.; Wang, G.; Yuan, F.; Lai, T.Y.Y.; Liu, J.; Lu, Y.; Liu, D.; Qin, B.; et al. AI-Based Retinal Image Analysis for the Detection of Choroidal Neovascular Age-Related Macular Degeneration (AMD) and Its Association with Brain Health. Brain Sci. 2025, 15, 1249. https://doi.org/10.3390/brainsci15111249
Shi C, Lee J, Shi D, Wang G, Yuan F, Lai TYY, Liu J, Lu Y, Liu D, Qin B, et al. AI-Based Retinal Image Analysis for the Detection of Choroidal Neovascular Age-Related Macular Degeneration (AMD) and Its Association with Brain Health. Brain Sciences. 2025; 15(11):1249. https://doi.org/10.3390/brainsci15111249
Chicago/Turabian StyleShi, Chuying, Jack Lee, Di Shi, Gechun Wang, Fei Yuan, Timothy Y. Y. Lai, Jingwen Liu, Yijie Lu, Dongcheng Liu, Bo Qin, and et al. 2025. "AI-Based Retinal Image Analysis for the Detection of Choroidal Neovascular Age-Related Macular Degeneration (AMD) and Its Association with Brain Health" Brain Sciences 15, no. 11: 1249. https://doi.org/10.3390/brainsci15111249
APA StyleShi, C., Lee, J., Shi, D., Wang, G., Yuan, F., Lai, T. Y. Y., Liu, J., Lu, Y., Liu, D., Qin, B., & Zee, B. C.-Y. (2025). AI-Based Retinal Image Analysis for the Detection of Choroidal Neovascular Age-Related Macular Degeneration (AMD) and Its Association with Brain Health. Brain Sciences, 15(11), 1249. https://doi.org/10.3390/brainsci15111249






