Early Biomedical and Environmental Factors Associated with Developmental Coordination Disorder in a Brazilian Preterm Cohort
Abstract
1. Introduction
2. Materials and Methods
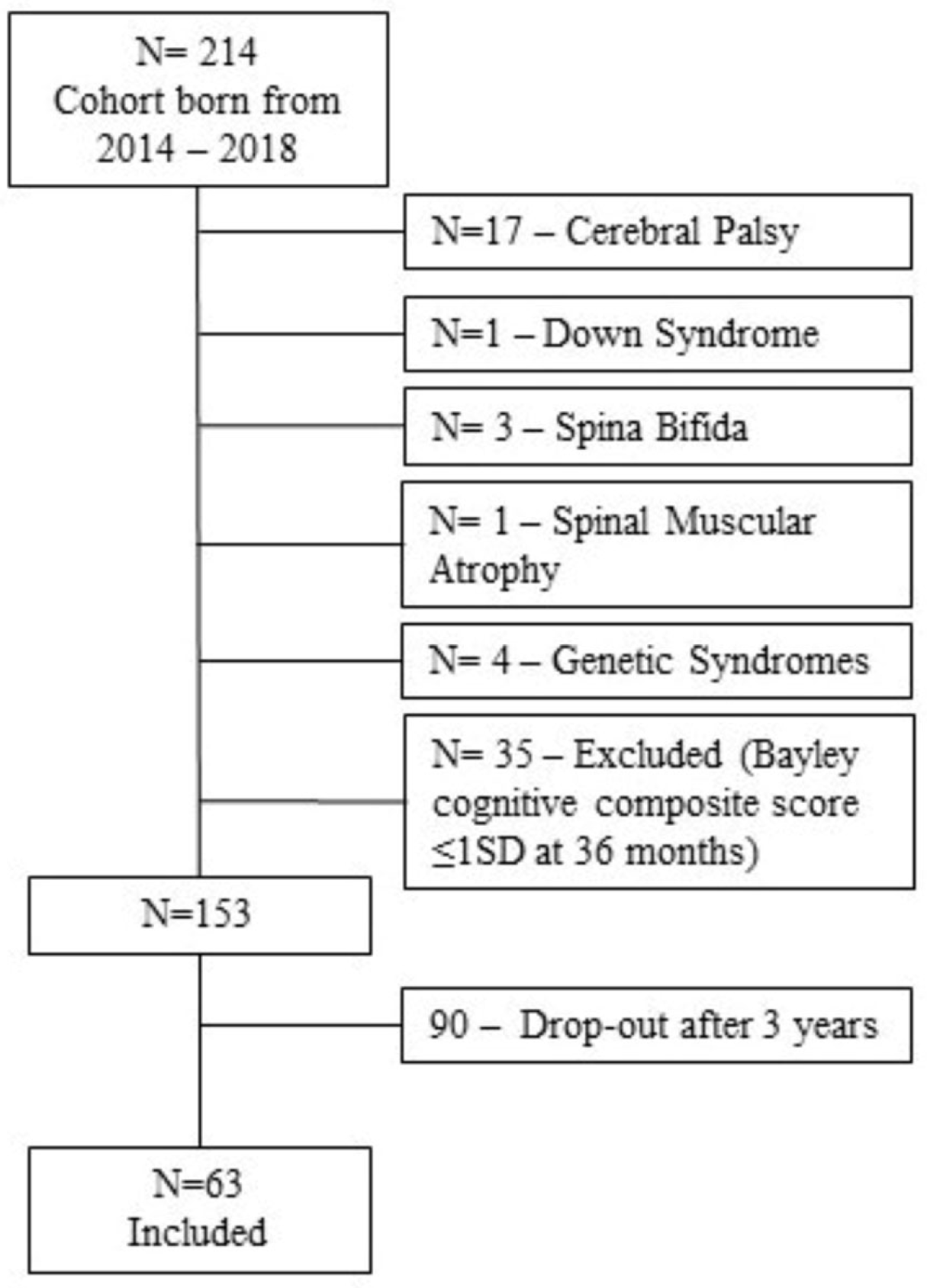
2.1. Context and Participants
2.2. Classification of DCD
2.3. Assessments
2.4. Procedures
2.5. Data Analyses
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DCD | Developmental Coordination Disorder |
References
- Blank, R.; Barnett, A.L.; Cairney, J.; Green, D.; Kirby, A.; Polatajko, H.; Rosenblum, S.; Smits-Engelsman, B.; Sugden, D.; Wilson, P.; et al. International clinical practice recommendations on the definition, diagnosis, assessment, intervention, and psychosocial aspects of developmental coordination disorder. Dev. Med. Child Neurol. 2019, 61, 242–285. [Google Scholar] [CrossRef]
- Spittle, A.J.; Dewey, D.; Nguyen, T.N.; Ellis, R.; Burnett, A.; Kwong, A.; Lee, K.; Cheong, J.; Doyle, L.W.; Anderson, P.J. Rates of Developmental Coordination Disorder in Children Born Very Preterm. J. Pediatr. 2021, 231, 61–67. [Google Scholar] [CrossRef]
- APA—American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2014. [Google Scholar]
- Harrowell, I.; Hollén, L.; Lingam, R.; Emond, A. Mental health outcomes of developmental coordination disorder in late adolescence. Dev. Med. Child Neurol. 2017, 59, 973–979. [Google Scholar] [CrossRef]
- Sartori, R.F.; Nobre, G.C.; Fonseca, R.P.; Valentini, N.C. Do executive functions and gross motor skills predict writing and mathematical performance in children with developmental coordination disorder? Appl. Neuropsychol. Child 2021, 11, 825–839. [Google Scholar] [CrossRef]
- Harris, S.; Wilmut, K.; Rathbone, C. Anxiety, confidence and self-concept in adults with and without developmental coordination disorder. Res. Dev. Disabil. 2021, 119, 104119. [Google Scholar] [CrossRef] [PubMed]
- Engan, M.; Engeseth, M.S.; Fevang, S.; Vollsæter, M.; Eide, G.E.; Røksund, O.D.; Halvorsen, T.; Clemm, H. Predicting physical activity in a national cohort of children born extremely preterm. Early Hum. Dev. 2020, 145, 105037. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, J.G.; Yoon, S.W.; Mackay, M.; Petrie-Thomas, J.; Rogers, M.; Synnes, A.R. Perinatal and neonatal predictors of developmental coordination disorder in very low birthweight children. Arch. Dis. Child. 2013, 98, 118–122. [Google Scholar] [CrossRef] [PubMed]
- van Hoorn, J.F.; Schoemaker, M.M.; Stuive, I.; Dijkstra, P.U.; Rodrigues Trigo Pereira, F.; van der Sluis, C.K.; Hadders-Algra, M. Risk factors in early life for developmental coordination disorder: A scoping review. Dev. Med. Child Neurol. 2021, 63, 511–519. [Google Scholar] [CrossRef]
- Panceri, C.; Sbruzzi, G.; Zanella, L.W.; Wiltgen, A.; Procianoy, R.S.; Silveira, R.C.; Valentini, N.C. Developmental coordination disorder in preterm children: A systematic review and meta-analysis. Eur. J. Neurosci. 2024, 60, 4128–4147. [Google Scholar] [CrossRef]
- Zoia, S.; Biancotto, M.; Caravale, B.; Valletti, A.; Montelisciani, L.; Croci, I.; Voller, F.; Rusconi, F.; Carrozzi, M.; Chiandotto, V.; et al. Early factors associated with risk of developmental coordination disorder in very preterm children: A prospective area-based cohort study in Italy. Paediatr. Perinat. Epidemiol. 2022, 36, 683–695. [Google Scholar] [CrossRef]
- Hua, J.; Gu, G.; Jiang, P.; Zhang, L.; Zhu, L.; Meng, W. The prenatal, perinatal, and neonatal risk factors for children’s developmental coordination disorder: A population study in mainland China. Res. Dev. Disabil. 2014, 35, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Dewey, D.; Thompson, D.K.; Kelly, C.E.; Spittle, A.J.; Cheong, J.L.Y.; Doyle, L.W.; Anderson, P.J. Very preterm children at risk for developmental coordination disorder have brain alterations in motor areas. Acta Paediatr 2019, 108, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Ke, L.; Wang, Y.; Hua, J.; Duan, W.; Barnett, A.L. The prenatal, postnatal, neonatal, and family environmental risk factors for Developmental Coordination Disorder: A study with a national representative sample. Res. Dev. Disabil. 2020, 104, 103699. [Google Scholar] [CrossRef]
- Wu, M.-Q.; Wu, D.-Q.; Hu, C.-P.; Iao, L.-S. Studies on Children with Developmental Coordination Disorder in the Past 20 Years: A Bibliometric Analysis via CiteSpace. Front. Psychiatry 2021, 12, 776883. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.P.; Wachs, T.D.; Grantham-McGregor, S.; Black, M.M.; Nelson, C.A.; Huffman, S.L.; Baker-Henningham, H.; Chang, S.M.; Hamadani, J.D.; Lozoff, B.; et al. Inequality in early childhood: Risk and protective factors for early child development. Lancet 2011, 378, 1325–1338. [Google Scholar] [CrossRef]
- Henderson, L.; Sugden, D.A.; Barnett, A. Movement Assessment Battery for Children—Second Edition; Harcourt Assessment: San Antonio, TX, USA, 2017. [Google Scholar]
- Bolk, J.; Farooqi, A.; Hafström, M.; Åden, U.; Serenius, F. Developmental Coordination Disorder and Its Association with Developmental Comorbidities at 6.5 Years in Apparently Healthy Children Born Extremely Preterm. JAMA Pediatr. 2018, 172, 765–774. [Google Scholar] [CrossRef]
- 19 de Kieviet, J.F.; Pouwels, P.J.; Lafeber, H.N.; Vermeulen, R.J.; van Elburg, R.M.; Oosterlaan, J. A crucial role of altered fractional anisotropy in motor problems of very preterm children. Eur. J. Paediatr. Neurol. 2014, 18, 126–133. [Google Scholar] [CrossRef]
- Kwok, C.; Mackay, M.; Agnew, J.A.; Synnes, A.; Zwicker, J.G. Does the Movement Assessment Battery for Children-2 at 3 years of age predict developmental coordination disorder at 4.5 years of age in children born very preterm? Res. Dev. Disabil. 2019, 84, 36–42. [Google Scholar] [CrossRef]
- Bayley, N. Bayley Scales of Infant and Toddler Development, (Bayley-III), 3rd ed.; Harcourt Assessment: San Antonio, TX, USA, 2005. [Google Scholar]
- Valentini, N.C.; Ramalho, M.H.; Oliveira, M.A. Movement assessment battery for children-2: Translation, reliability, and validity for Brazilian children. Res. Dev. Disabil. 2014, 35, 733–740. [Google Scholar] [CrossRef]
- Nobre-Lima, L.; da Luz Vale-Dias, M.; Mendes, T.V.; Mónico, L.; MacPhee, D. The Portuguese version of the Knowledge of Infant Development Inventory-P (KIDI-P). Eur. J. Dev. Psychol. 2014, 11, 740–745. [Google Scholar] [CrossRef]
- Anme, T. Manual of Interaction Rating Scale; Pediatric Press: Tokyo, Japan, 2009. [Google Scholar]
- Caçola, P.M.; Gabbard, C.; Montebelo, M.I.L.; Santos, D.C.C. Further development and validation of the Affordances in the Home Environment for Motor Development—Infant Scale (AHEMD-IS). Phys. Ther. 2015, 95, 901–923. [Google Scholar] [CrossRef] [PubMed]
- Vittinghoff, E.; McCulloch, C.E. Relaxing the rule of ten events per variable in logistic and Cox regression. Am. J. Epidemiol. 2007, 165, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Costello, A.B.; Osborne, J.W. Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Pract. Assess. Res. Eval. 2005, 10, 7. [Google Scholar]
- Deshmukh, A.A.; Sahu, V.; Deshpande, M.S. Prevalence of suspected Developmental Coordination Disorder and its association with preterm and low birth weight in 5–10-year old children. Med. J. Armed Forces India 2024, 80, 153–160. [Google Scholar] [CrossRef]
- Bolk, J.; Källén, K.; Farooqi, A.; Hafström, M.; Fellman, V.; Åden, U.; Serenius, F. Perinatal risk factors for developmental coordination disorder in children born extremely preterm. Acta Paediatr. 2023, 112, 675–685. [Google Scholar] [CrossRef]
- Panceri, C.; Valentini, N.C.; Silveira, R.C.; Smith, B.A.; Procianoy, R.S. Neonatal Adverse Outcomes, Neonatal Birth Risks, and Socioeconomic Status: Combined Influence on Preterm Infants’ Cognitive, Language, and Motor Development in Brazil. J. Child Neurol. 2020, 35, 989–998. [Google Scholar] [CrossRef]
- Zayat, N.; Truffert, P.; Drumez, E.; Duhamel, A.; Labreuche, J.; Zemlin, M.; Milligan, D.; Maier, R.F.; Jarreau, P.-H.; Torchin, H.; et al. Systemic Steroids in Preventing Bronchopulmonary Dysplasia (BPD): Neurodevelopmental Outcome According to the Risk of BPD in the EPICE Cohort. Int. J. Environ. Res. Public Health 2022, 19, 5600. [Google Scholar] [CrossRef]
- Zhu, J.L.; Olsen, J.; Olesen, A.W. Risk for Developmental Coordination Disorder Correlates with Gestational Age at Birth. Paediatr. Perinat. Epidemiol. 2012, 26, 572–577. [Google Scholar] [CrossRef]
| Biomedical Characteristics | t-Test | Univariate Logistic Regression | ||||
|---|---|---|---|---|---|---|
| DCD (n = 33) | Non-DCD (n = 30) | p | Total (n = 63) | OR (95% CI) | p | |
| Child’s Biomedical Outcomes | ||||||
| Sex N(%) | ||||||
| Boys | 26 (78.8) | 11(36.7) | 0.001 | 37 (58.7) | 0.156 (0.048–0.458) | 0.001 |
| Girls | 7 (21.2) | 19 (63.3) | 26 (41.3) | |||
| Gestational age—weeks M (SD) | 29.16 (2.56) | 30.13 (2.19) | 0.115 | 29.62 (2.42) | 1.189 (0.963–1.496) | 0.118 |
| Birth weight—grams M (SD) | 1226.00 (440.54) | 1311.36 (361.90) | 0.407 | 1266.64 (404.08) | 1.001 (0.999–1.002) | 0.401 |
| Birth length—cm M (SD) | 37.59 (4.08) | 38.41 (3.65) | 0.403 | 37.98 (3.88) | 1.058 (0.930–1.210) | 0.394 |
| Head circumference—cm M (SD) | 26.74 (3.35) | 27.03 (2.39) | 0.695 | 26.88 (2.91) | 1.035 (0.872–1.233) | 0.693 |
| APGAR 5th minute M (SD) | 7.15 (2.10) | 7.80 (1.24) | 0.139 | 7.46 (1.76) | 1.252 (0.934–1.749) | 0.136 |
| NICU (days) M (SD) | 73.85 (40.10) | 57.53 (25.11) | 0.056 | 66.08 (34.53) | 0.985 (0.969–1.000) | 0.066 |
| Ventilatory support (days) M (SD) | 33.75 (36.13) | 13.20 (16.02) | 0.006 | 52.76 (16.27) | 0.969 (0.941–0.991) | 0.017 |
| Bronchopulmonary dysplasia N(%) | 13 (39.4) | 3 (10.0) | 0.010 | 16 (25.4) | 5.850 (1.628–28.051) | 0.012 |
| Sepsis N(%) | 27 (81.8) | 23 (76.7) | 0.525 | 50 (79.4) | 1.704 (0.481–6.442) | 0.412 |
| Necrotizing enterocolitis N (%) | 3 (9.1) | 0 | 0.119 | 3 (4.8) | -- | -- |
| Intraventricular hemorrhage N (%) | ||||||
| Grade 0 | 20 (60.6) | 24 (80.0) | 0.154 | 44 (69.8) | ||
| Grades 1 and 2 | 11 (33.3) | 6 (20.0) | 17 (26.9) | -- | -- | |
| Grades 3 and 4 | 2 (6.1) | 0 | 2 (3.2) | |||
| Periventricular leukomalacia N (%) | 0 | 2 (6.7) | 0.216 | 2 (3.2) | ||
| Parenteral nutrition M (SD) | 16.18 (14.86) | 13.83 (11.02) | 0.483 | 15.06 (13.12) | 0.986 (0.946–1.024) | 0.472 |
| Mother’s health | ||||||
| Antenatal steroids N (%) | 30 (90.9) | 30 (100) | 0.244 | 60 (95.2) | - | - |
| Prenatal appointments M (SD) | 4.88 (2.45) | 5.30 (1.51) | 0.422 | 5.08 (2.05) | 1.108 (0.868–1.439) | 0.412 |
| Pre-eclampsia N (%) | 12 (36.4) | 10 (33.3) | 0.999 | 22 (34.9) | 0.875 (0.305–2.475) | 0.801 |
| Biomedical Characteristics | t-Test | Univariate Logistic Regression | ||||
|---|---|---|---|---|---|---|
| DCD (n = 33) | Non-DCD (n = 30) | p | Total (n = 63) | OR (95% CI) | p | |
| Environmental | ||||||
| Mother’s age at infant’s birth M (SD) | 28.39 (7.11) | 30.80 (5.70) | 0.146 | 29.54 (6.54) | 1.061 (0.981–1.153) | 0.147 |
| Father’s age at infant’s birth M (SD) | 32.82 (8.74) | 33.72 (8.56) | 0.544 | 33.25 (8.58) | 1.020 (0.958–1.088) | 0.538 |
| Family income (BRL) M (SD) | 2012.85 (1144.62) | 2843.52 (1673.67) | 0.013 | 2412.21 (1470.28) | 1.001 (1.000–1.001) | 0.016 |
| Mother’s formal education N (%) | ||||||
| Less than High School | 18 (54.5) | 15 (50.0) | 33 (52.4) | |||
| High School | 11 (33.3) | 8 (26.7) | 0.560 | 19 (30.2) | 0.476 (0.107–1.890) | 0.488 |
| College | 4 (12.1) | 7 (23.3) | 11 (17.5) | |||
| Father’s formal education N (%) | ||||||
| Less than High Scholl | 23 (69.7) | 19 (63.3) | 0.208 | 42 (66.7) | ||
| Completed High School | 10 (30.3) | 8 (26.7) | 18 (28.6) | -- | -- | |
| College | -- | 3 (10.0) | 3 (4.8) | |||
| Siblings N (%) | 14 (42.4) | 9 (30) | 0.436 | 23 (36.5) | 1.1842 (0.397–9.973) | 0.587 |
| KIDI—parents’ knowledge M (SD) | 0.60 (0.08) | 0.63 (0.08) | 0.142 | 0.61 (0.09) | 99.063 (0.253–72,701.109) | 0.134 |
| IRS—Interaction Rating Scale M (SD) | 53.57 (14.58) | 54.50 (13.14) | 0.793 | 54.01 (13.81) | 1.005 (0.969–1.043) | 0.789 |
| AHEM—Home Affordances for Development M (SD) | 54.57 (17.39) | 50.00 (14.52) | 0.405 | 52.76 (16.27) | 0.982 (0.940–1.023) | 0.389 |
| Risk Factors | B | Exponential (B) | Wald X2 | p | OR (95% CI) | R2 | p | Hosmer- Lemeshow |
|---|---|---|---|---|---|---|---|---|
| Sex male | 2.44 | 11.472 | 9.76 | 0.002 | 11.47 (2.48–53.02) | 0.524 | 0.006 | 0.929 |
| Ventilatory support | −0.006 | 0.99 | 0.062 | 0.803 | 0.994 (0.95–1.04) | |||
| BPD | 3.11 | 22.54 | 4.75 | 0.029 | 22.54 (1.37–370.61) | |||
| Family Income | 0.001 | 1.00 | 6.86 | 0.009 | 101(1.00–1.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panceri, C.; Procianoy, R.S.; Silveira, R.d.C.; Valentini, N.C. Early Biomedical and Environmental Factors Associated with Developmental Coordination Disorder in a Brazilian Preterm Cohort. Brain Sci. 2025, 15, 1250. https://doi.org/10.3390/brainsci15121250
Panceri C, Procianoy RS, Silveira RdC, Valentini NC. Early Biomedical and Environmental Factors Associated with Developmental Coordination Disorder in a Brazilian Preterm Cohort. Brain Sciences. 2025; 15(12):1250. https://doi.org/10.3390/brainsci15121250
Chicago/Turabian StylePanceri, Carolina, Renato Soibelmann Procianoy, Rita de Cássia Silveira, and Nadia Cristina Valentini. 2025. "Early Biomedical and Environmental Factors Associated with Developmental Coordination Disorder in a Brazilian Preterm Cohort" Brain Sciences 15, no. 12: 1250. https://doi.org/10.3390/brainsci15121250
APA StylePanceri, C., Procianoy, R. S., Silveira, R. d. C., & Valentini, N. C. (2025). Early Biomedical and Environmental Factors Associated with Developmental Coordination Disorder in a Brazilian Preterm Cohort. Brain Sciences, 15(12), 1250. https://doi.org/10.3390/brainsci15121250






