Alzheimer’s Disease-Related Cerebrospinal Fluid Biomarkers in Progressive Supranuclear Palsy
Abstract
Highlights
- Progressive Supranuclear Palsy (PSP) presents with various clinical phenotypes, making accurate diagnosis difficult.
- No biomarker systems, such as the AT(N) system for Alzheimer’s Disease (AD), have been established yet.
- In PSP, core AD cerebrospinal fluid biomarkers show a unique pattern, where Aβ42, Aβ40, p-tau, and t-tau levels are decreased while NfL levels are remarkably increased.
Abstract
1. Difficulties in the Antemortem Diagnosis of PSP
2. AT(N) Biomarkers in Alzheimer’s Disease
3. Changes in Core AD biomarkers in PSP
3.1. Aβ: Aβ42, Aβ40

3.2. Tau: Total Tau (t-tau), Phosphorylated Tau (p-tau181)
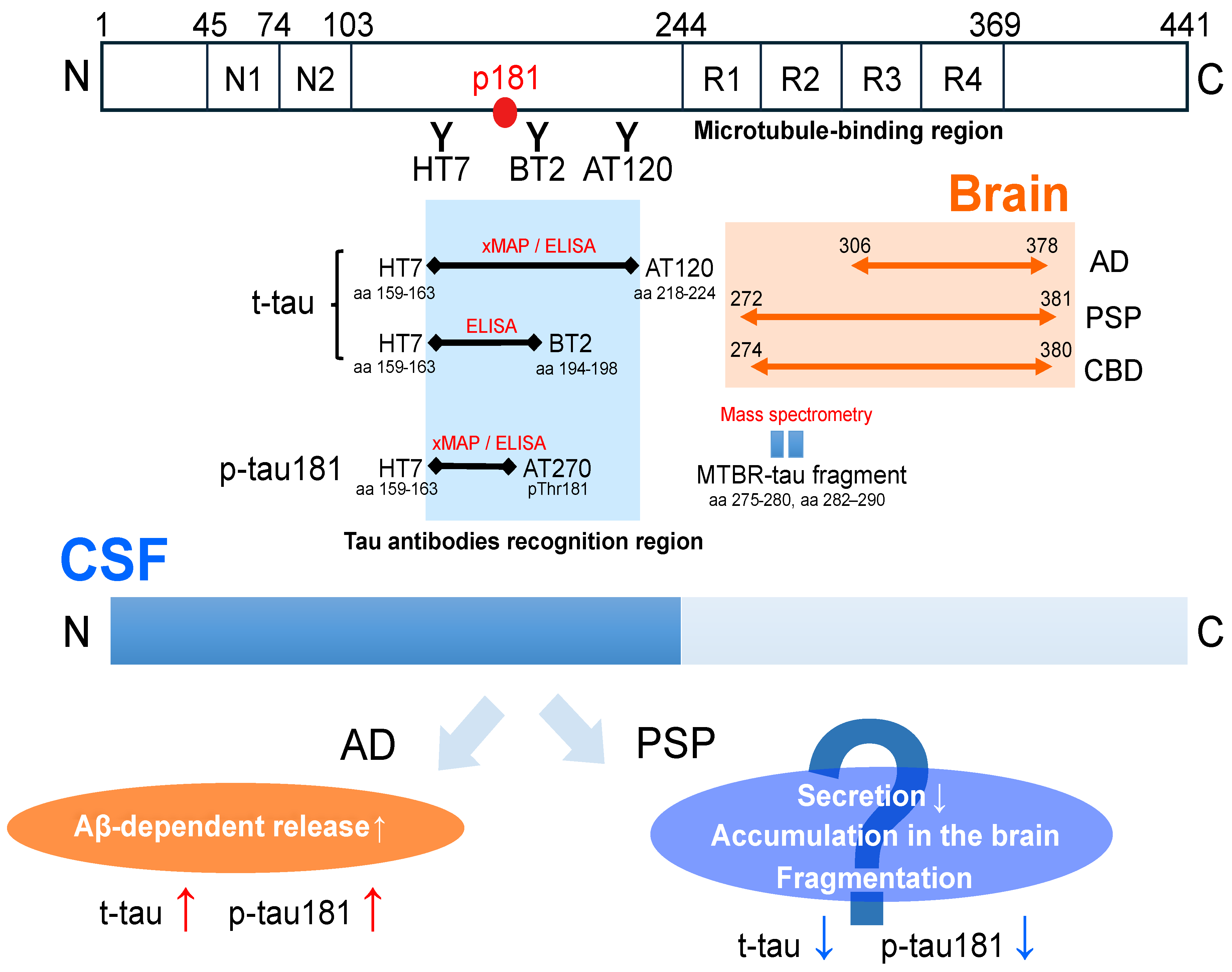
3.3. NfL
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boxer, A.L.; Yu, J.T.; Golbe, L.I.; Litvan, I.; Lang, A.E.; Höglinger, G.U. Advances in progressive supranuclear palsy: New diagnostic criteria, biomarkers, and therapeutic approaches. Lancet Neurol. 2017, 16, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.C.; Richardson, J.C.; Olszewski, J. Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch. Neurol. 1964, 10, 333–359. [Google Scholar] [CrossRef] [PubMed]
- Höglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R.; de Silva, R.; Paviour, D.C.; Pittman, A.; Watt, H.C.; Kilford, L.; Holton, J.L.; Revesz, T.; Lees, A.J. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSP-parkinsonism. Brain 2005, 128, 1247–1258. [Google Scholar] [CrossRef]
- Williams, D.R.; Lees, A.J. What features improve the accuracy of the clinical diagnosis of progressive supranuclear palsy-parkinsonism (PSP-P)? Mov. Disord. 2010, 25, 357–362. [Google Scholar] [CrossRef]
- Respondek, G.; Stamelou, M.; Kurz, C.; Ferguson, L.W.; Rajput, A.; Chiu, W.Z.; van Swieten, J.C.; Troakes, C.; Al Sarraj, S.; Gelpi, E.; et al. The phenotypic spectrum of progressive supranuclear palsy: A retrospective multicenter study of 100 definite cases. Mov. Disord. 2014, 29, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- Donker Kaat, L.; Boon, A.J.; Kamphorst, W.; Ravid, R.; Duivenvoorden, H.J.; van Swieten, J.C. Frontal presentation in progressive supranuclear palsy. Neurology 2007, 69, 723–729. [Google Scholar] [CrossRef]
- Han, H.J.; Kim, H.; Park, J.H.; Shin, H.W.; Kim, G.U.; Kim, D.S.; Lee, E.J.; Oh, H.E.; Park, S.H.; Kim, Y.J. Behavioral changes as the earliest clinical manifestation of progressive supranuclear palsy. J. Clin. Neurol. 2010, 6, 148–151. [Google Scholar] [CrossRef][Green Version]
- Boeve, B.; Dickson, D.; Duffy, J.; Bartleson, J.; Trenerry, M.; Petersen, R. Progressive nonfluent aphasia and subsequent aphasic dementia associated with atypical progressive supranuclear palsy pathology. Eur. Neurol. 2003, 49, 72–78. [Google Scholar] [CrossRef]
- Josephs, K.A.; Duffy, J.R.; Strand, E.A.; Whitwell, J.L.; Layton, K.F.; Parisi, J.E.; Hauser, M.F.; Witte, R.J.; Boeve, B.F.; Knopman, D.S.; et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 2006, 129, 1385–1398. [Google Scholar] [CrossRef]
- Tsuboi, Y.; Josephs, K.A.; Boeve, B.F.; Litvan, I.; Caselli, R.J.; Caviness, J.N.; Uitti, R.J.; Bott, A.D.; Dickson, D.W. Increased tau burden in the cortices of progressive supranuclear palsy presenting with corticobasal syndrome. Mov. Disord. 2005, 20, 982–988. [Google Scholar] [CrossRef]
- Josephs, K.A.; Petersen, R.C.; Knopman, D.S.; Boeve, B.F.; Whitwell, J.L.; Duffy, J.R.; Parisi, J.E.; Dickson, D.W. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology 2006, 66, 41–48. [Google Scholar] [CrossRef]
- Compta, Y.; Valldeoriola, F.; Tolosa, E.; Rey, M.J.; Martí, M.J.; Valls-Solé, J. Long lasting pure freezing of gait preceding progressive supranuclear palsy: A clinicopathological study. Mov. Disord. 2007, 22, 1954–1958. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R.; Holton, J.L.; Strand, K.; Revesz, T.; Lees, A.J. Pure akinesia with gait freezing: A third clinical phenotype of progressive supranuclear palsy. Mov. Disord. 2007, 22, 2235–2241. [Google Scholar] [CrossRef]
- Litvan, I.; Agid, Y.; Calne, D.; Campbell, G.; Dubois, B.; Duvoisin, R.C.; Goetz, C.G.; Golbe, L.I.; Grafman, J.; Growdon, J.H.; et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): Report of the NINDS-SPSP international workshop. Neurology 1996, 47, 1–9. [Google Scholar] [CrossRef]
- Kurz, C.; Ebersbach, G.; Respondek, G.; Giese, A.; Arzberger, T.; Höglinger, G.U. An autopsy-confirmed case of progressive supranuclear palsy with predominant postural instability. Acta Neuropathol. Commun. 2016, 4, 120. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, M.; Shimohata, T.; Toyoshima, Y.; Tada, M.; Kakita, A.; Morita, T.; Ozawa, T.; Takahashi, H.; Nishizawa, M. Cerebellar involvement in progressive supranuclear palsy: A clinicopathological study. Mov. Disord. 2009, 24, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, M.; Tada, M.; Onodera, O.; Takahashi, H.; Nishizawa, M.; Shimohata, T. Early clinical features of patients with progressive supranuclear palsy with predominant cerebellar ataxia. Park. Relat. Disord. 2013, 19, 1149–1151. [Google Scholar] [CrossRef]
- Ando, S.; Kanazawa, M.; Onodera, O. Progressive Supranuclear Palsy with Predominant Cerebellar Ataxia. J. Mov. Disord. 2020, 13, 20–26. [Google Scholar] [CrossRef]
- Ali, F.; Martin, P.R.; Botha, H.; Ahlskog, J.E.; Bower, J.H.; Masumoto, J.Y.; Maraganore, D.; Hassan, A.; Eggers, S.; Boeve, B.F.; et al. Sensitivity and Specificity of Diagnostic Criteria for Progressive Supranuclear Palsy. Mov. Disord. 2019, 34, 1144–1153. [Google Scholar] [CrossRef]
- Olfati, N.; Shoeibi, A.; Litvan, I. Clinical Spectrum of Tauopathies. Front. Neurol. 2022, 13, 944806. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.J.; Respondek, G.; Stamelou, M.; Arzberger, T.; Ferguson, L.; Gelpi, E.; Giese, A.; Grossman, M.; Irwin, D.J.; Pantelyat, A.; et al. How to apply the movement disorder society criteria for diagnosis of progressive supranuclear palsy. Mov. Disord. 2019, 34, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Respondek, G.; Roeber, S.; Kretzschmar, H.; Troakes, C.; Al-Sarraj, S.; Gelpi, E.; Gaig, C.; Chiu, W.Z.; van Swieten, J.C.; Oertel, W.H.; et al. Accuracy of the National Institute for Neurological Disorders and Stroke/Society for Progressive Supranuclear Palsy and neuroprotection and natural history in Parkinson plus syndromes criteria for the diagnosis of progressive supranuclear palsy. Mov. Disord. 2013, 28, 504–509. [Google Scholar] [CrossRef]
- Grimm, M.J.; Respondek, G.; Stamelou, M.; Arzberger, T.; Ferguson, L.; Gelpi, E.; Giese, A.; Grossman, M.; Irwin, D.J.; Pantelyat, A.; et al. Clinical Conditions “Suggestive of Progressive Supranuclear Palsy”-Diagnostic Performance. Mov. Disord. 2020, 35, 2301–2313. [Google Scholar] [CrossRef] [PubMed]
- Dam, T.; Boxer, A.L.; Golbe, L.I.; Höglinger, G.U.; Morris, H.R.; Litvan, I.; Lang, A.E.; Corvol, J.C.; Aiba, I.; Grundman, M.; et al. Safety and efficacy of anti-tau monoclonal antibody gosuranemab in progressive supranuclear palsy: A phase 2, randomized, placebo-controlled trial. Nat. Med. 2021, 27, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Höglinger, G.U.; Litvan, I.; Mendonca, N.; Wang, D.; Zheng, H.; Rendenbach-Mueller, B.; Lon, H.K.; Jin, Z.; Fisseha, N.; Budur, K.; et al. Safety and efficacy of tilavonemab in progressive supranuclear palsy: A phase 2, randomised, placebo-controlled trial. Lancet Neurol. 2021, 20, 182–192. [Google Scholar] [CrossRef]
- Guasp, M.; Molina-Porcel, L.; Painous, C.; Caballol, N.; Camara, A.; Perez-Soriano, A.; Sánchez-Gómez, A.; Garrido, A.; Muñoz, E.; Marti, M.J.; et al. Association of PSP phenotypes with survival: A brain-bank study. Park. Relat. Disord. 2021, 84, 77–81. [Google Scholar] [CrossRef]
- Street, D.; Malpetti, M.; Rittman, T.; Ghosh, B.C.P.; Murley, A.G.; Coyle-Gilchrist, I.; Passamonti, L.; Rowe, J.B. Clinical progression of progressive supranuclear palsy: Impact of trials bias and phenotype variants. Brain Commun. 2021, 3, fcab206. [Google Scholar] [CrossRef]
- Respondek, G.; Kurz, C.; Arzberger, T.; Compta, Y.; Englund, E.; Ferguson, L.W.; Gelpi, E.; Giese, A.; Irwin, D.J.; Meissner, W.G.; et al. Which ante mortem clinical features predict progressive supranuclear palsy pathology? Mov. Disord. 2017, 32, 995–1005. [Google Scholar] [CrossRef]
- Jecmenica Lukic, M.; Kurz, C.; Respondek, G.; Grau-Rivera, O.; Compta, Y.; Gelpi, E.; Troakes, C.; van Swieten, J.C.; Giese, A.; Roeber, S.; et al. Copathology in Progressive Supranuclear Palsy: Does It Matter? Mov. Disord. 2020, 35, 984–993. [Google Scholar] [CrossRef]
- Montine, T.J.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; Mirra, S.S.; et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol. 2012, 123, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Olsson, B.; Lautner, R.; Andreasson, U.; Öhrfelt, A.; Portelius, E.; Bjerke, M.; Hölttä, M.; Rosén, C.; Olsson, C.; Strobel, G.; et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol. 2016, 15, 673–684. [Google Scholar] [CrossRef]
- Toledo, J.B.; Brettschneider, J.; Grossman, M.; Arnold, S.E.; Hu, W.T.; Xie, S.X.; Lee, V.M.; Shaw, L.M.; Trojanowski, J.Q. CSF biomarkers cutoffs: The importance of coincident neuropathological diseases. Acta Neuropathol. 2012, 124, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; van der Kant, R.; Hansson, O. Tau biomarkers in Alzheimer’s disease: Towards implementation in clinical practice and trials. Lancet Neurol. 2022, 21, 726–734. [Google Scholar] [CrossRef]
- Bridel, C.; van Wieringen, W.N.; Zetterberg, H.; Tijms, B.M.; Teunissen, C.E.; Alvarez-Cermeño, J.C.; Andreasson, U.; Axelsson, M.; Bäckström, D.C.; Bartos, A.; et al. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol. 2019, 76, 1035–1048. [Google Scholar] [CrossRef]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef]
- Kasuga, K.; Tsukie, T.; Kikuchi, M.; Tokutake, T.; Washiyama, K.; Shimizu, S.; Yoshizawa, H.; Kuroha, Y.; Yajima, R.; Mori, H.; et al. The clinical application of optimized AT(N) classification in Alzheimer’s clinical syndrome (ACS) and non-ACS conditions. Neurobiol. Aging 2023, 127, 23–32. [Google Scholar] [CrossRef]
- Hansson, O.; Blennow, K.; Zetterberg, H.; Dage, J. Blood biomarkers for Alzheimer’s disease in clinical practice and trials. Nat. Aging 2023, 3, 506–519. [Google Scholar] [CrossRef]
- Teunissen, C.E.; Verberk, I.M.W.; Thijssen, E.H.; Vermunt, L.; Hansson, O.; Zetterberg, H.; van der Flier, W.M.; Mielke, M.M.; Del Campo, M. Blood-based biomarkers for Alzheimer’s disease: Towards clinical implementation. Lancet Neurol. 2022, 21, 66–77. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 2024. [Google Scholar] [CrossRef] [PubMed]
- Magdalinou, N.K.; Paterson, R.W.; Schott, J.M.; Fox, N.C.; Mummery, C.; Blennow, K.; Bhatia, K.; Morris, H.R.; Giunti, P.; Warner, T.T.; et al. A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Öhrfelt, A.; Constantinescu, R.; Andreasson, U.; Surova, Y.; Bostrom, F.; Nilsson, C.; Håkan, W.; Decraemer, H.; Någga, K.; et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch. Neurol. 2012, 69, 1445–1452. [Google Scholar] [CrossRef]
- Hansson, O.; Janelidze, S.; Hall, S.; Magdalinou, N.; Lees, A.J.; Andreasson, U.; Norgren, N.; Linder, J.; Forsgren, L.; Constantinescu, R.; et al. Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology 2017, 88, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Wagshal, D.; Sankaranarayanan, S.; Guss, V.; Hall, T.; Berisha, F.; Lobach, I.; Karydas, A.; Voltarelli, L.; Scherling, C.; Heuer, H.; et al. Divergent CSF τ alterations in two common tauopathies: Alzheimer’s disease and progressive supranuclear palsy. J. Neurol. Neurosurg. Psychiatry 2015, 86, 244–250. [Google Scholar] [CrossRef]
- Thijssen, E.H.; La Joie, R.; Wolf, A.; Strom, A.; Wang, P.; Iaccarino, L.; Bourakova, V.; Cobigo, Y.; Heuer, H.; Spina, S.; et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med. 2020, 26, 387–397. [Google Scholar] [CrossRef]
- Urakami, K.; Wada, K.; Arai, H.; Sasaki, H.; Kanai, M.; Shoji, M.; Ishizu, H.; Kashihara, K.; Yamamoto, M.; Tsuchiya-Ikemoto, K.; et al. Diagnostic significance of tau protein in cerebrospinal fluid from patients with corticobasal degeneration or progressive supranuclear palsy. J. Neurol. Sci. 2001, 183, 95–98. [Google Scholar] [CrossRef]
- Holmberg, B.; Johnels, B.; Blennow, K.; Rosengren, L. Cerebrospinal fluid Abeta42 is reduced in multiple system atrophy but normal in Parkinson’s disease and progressive supranuclear palsy. Mov. Disord. 2003, 18, 186–190. [Google Scholar] [CrossRef]
- Noguchi, M.; Yoshita, M.; Matsumoto, Y.; Ono, K.; Iwasa, K.; Yamada, M. Decreased beta-amyloid peptide42 in cerebrospinal fluid of patients with progressive supranuclear palsy and corticobasal degeneration. J. Neurol. Sci. 2005, 237, 61–65. [Google Scholar] [CrossRef]
- Aerts, M.B.; Esselink, R.A.; Bloem, B.R.; Verbeek, M.M. Cerebrospinal fluid tau and phosphorylated tau protein are elevated in corticobasal syndrome. Mov. Disord. 2011, 26, 169–173. [Google Scholar] [CrossRef]
- Schoonenboom, N.S.; Reesink, F.E.; Verwey, N.A.; Kester, M.I.; Teunissen, C.E.; van de Ven, P.M.; Pijnenburg, Y.A.; Blankenstein, M.A.; Rozemuller, A.J.; Scheltens, P.; et al. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology 2012, 78, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Scherling, C.S.; Hall, T.; Berisha, F.; Klepac, K.; Karydas, A.; Coppola, G.; Kramer, J.H.; Rabinovici, G.; Ahlijanian, M.; Miller, B.L.; et al. Cerebrospinal fluid neurofilament concentration reflects disease severity in frontotemporal degeneration. Ann. Neurol. 2014, 75, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Bäckström, D.C.; Eriksson Domellöf, M.; Linder, J.; Olsson, B.; Öhrfelt, A.; Trupp, M.; Zetterberg, H.; Blennow, K.; Forsgren, L. Cerebrospinal Fluid Patterns and the Risk of Future Dementia in Early, Incident Parkinson Disease. JAMA Neurol. 2015, 72, 1175–1182. [Google Scholar] [CrossRef]
- Constantinides, V.C.; Paraskevas, G.P.; Emmanouilidou, E.; Petropoulou, O.; Bougea, A.; Vekrellis, K.; Evdokimidis, I.; Stamboulis, E.; Kapaki, E. CSF biomarkers β-amyloid, tau proteins and a-synuclein in the differential diagnosis of Parkinson-plus syndromes. J. Neurol. Sci. 2017, 382, 91–95. [Google Scholar] [CrossRef]
- Jeppsson, A.; Wikkelsö, C.; Blennow, K.; Zetterberg, H.; Constantinescu, R.; Remes, A.M.; Herukka, S.K.; Rauramaa, T.; Nagga, K.; Leinonen, V.; et al. CSF biomarkers distinguish idiopathic normal pressure hydrocephalus from its mimics. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1117–1123. [Google Scholar] [CrossRef]
- Borroni, B.; Malinverno, M.; Gardoni, F.; Alberici, A.; Parnetti, L.; Premi, E.; Bonuccelli, U.; Grassi, M.; Perani, D.; Calabresi, P.; et al. Tau forms in CSF as a reliable biomarker for progressive supranuclear palsy. Neurology 2008, 71, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Snellman, A.; Lantero-Rodriguez, J.; Emeršič, A.; Vrillon, A.; Karikari, T.K.; Ashton, N.J.; Gregorič Kramberger, M.; Čučnik, S.; Paquet, C.; Rot, U.; et al. N-terminal and mid-region tau fragments as fluid biomarkers in neurological diseases. Brain 2022, 145, 2834–2848. [Google Scholar] [CrossRef]
- Patterson, B.W.; Elbert, D.L.; Mawuenyega, K.G.; Kasten, T.; Ovod, V.; Ma, S.; Xiong, C.; Chott, R.; Yarasheski, K.; Sigurdson, W.; et al. Age and amyloid effects on human central nervous system amyloid-beta kinetics. Ann. Neurol. 2015, 78, 439–453. [Google Scholar] [CrossRef]
- Grothe, M.J.; Moscoso, A.; Ashton, N.J.; Karikari, T.K.; Lantero-Rodriguez, J.; Snellman, A.; Zetterberg, H.; Blennow, K.; Schöll, M. Associations of Fully Automated CSF and Novel Plasma Biomarkers With Alzheimer Disease Neuropathology at Autopsy. Neurology 2021, 97, e1229–e1242. [Google Scholar] [CrossRef]
- Mattsson-Carlgren, N.; Grinberg, L.T.; Boxer, A.; Ossenkoppele, R.; Jonsson, M.; Seeley, W.; Ehrenberg, A.; Spina, S.; Janelidze, S.; Rojas-Martinex, J.; et al. Cerebrospinal Fluid Biomarkers in Autopsy-Confirmed Alzheimer Disease and Frontotemporal Lobar Degeneration. Neurology 2022, 98, e1137–e1150. [Google Scholar] [CrossRef]
- Hansson, O.; Lehmann, S.; Otto, M.; Zetterberg, H.; Lewczuk, P. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimers Res. Ther. 2019, 11, 34. [Google Scholar] [CrossRef]
- Kurihara, M.; Matsubara, T.; Morimoto, S.; Arakawa, A.; Ohse, K.; Kanemaru, K.; Iwata, A.; Murayama, S.; Saito, Y. Neuropathological changes associated with aberrant cerebrospinal fluid p-tau181 and Aβ42 in Alzheimer’s disease and other neurodegenerative diseases. Acta Neuropathol. Commun. 2024, 12, 48. [Google Scholar] [CrossRef]
- Harris, S.S.; Wolf, F.; De Strooper, B.; Busche, M.A. Tipping the Scales: Peptide-Dependent Dysregulation of Neural Circuit Dynamics in Alzheimer’s Disease. Neuron 2020, 107, 417–435. [Google Scholar] [CrossRef]
- Roemer, S.N.; Brendel, M.; Gnörich, J.; Malpetti, M.; Zaganjori, M.; Quattrone, A.; Gross, M.; Steward, A.; Dewenter, A.; Wagner, F.; et al. Subcortical tau is linked to hypoperfusion in connected cortical regions in 4-repeat tauopathies. Brain 2024, 147, 2428–2439. [Google Scholar] [CrossRef]
- Graff-Radford, N.R. Alzheimer CSF biomarkers may be misleading in normal-pressure hydrocephalus. Neurology 2014, 83, 1573–1575. [Google Scholar] [CrossRef]
- Espay, A.J.; Herrup, K.; Kepp, K.P.; Daly, T. The proteinopenia hypothesis: Loss of Aβ(42) and the onset of Alzheimer’s Disease. Ageing Res. Rev. 2023, 92, 102112. [Google Scholar] [CrossRef]
- Yamada, K.; Holth, J.K.; Liao, F.; Stewart, F.R.; Mahan, T.E.; Jiang, H.; Cirrito, J.R.; Patel, T.K.; Hochgräfe, K.; Mandelkow, E.M.; et al. Neuronal activity regulates extracellular tau in vivo. J. Exp. Med. 2014, 211, 387–393. [Google Scholar] [CrossRef]
- Pooler, A.M.; Phillips, E.C.; Lau, D.H.; Noble, W.; Hanger, D.P. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 2013, 14, 389–394. [Google Scholar] [CrossRef]
- Sato, C.; Barthélemy, N.R.; Mawuenyega, K.G.; Patterson, B.W.; Gordon, B.A.; Jockel-Balsarotti, J.; Sullivan, M.; Crisp, M.J.; Kasten, T.; Kirmess, K.M.; et al. Tau Kinetics in Neurons and the Human Central Nervous System. Neuron 2018, 97, 1284–1298.e1287. [Google Scholar] [CrossRef]
- Chen, Z.; Mengel, D.; Keshavan, A.; Rissman, R.A.; Billinton, A.; Perkinton, M.; Percival-Alwyn, J.; Schultz, A.; Properzi, M.; Johnson, K.; et al. Learnings about the complexity of extracellular tau aid development of a blood-based screen for Alzheimer’s disease. Alzheimers Dement. 2019, 15, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Kaeser, S.A.; Häsler, L.M.; Lambert, M.; Bergmann, C.; Bottelbergs, A.; Theunis, C.; Mercken, M.; Jucker, M. CSF p-tau increase in response to Aβ-type and Danish-type cerebral amyloidosis and in the absence of neurofibrillary tangles. Acta Neuropathol. 2022, 143, 287–290. [Google Scholar] [CrossRef]
- Fitzpatrick, A.W.P.; Falcon, B.; He, S.; Murzin, A.G.; Murshudov, G.; Garringer, H.J.; Crowther, R.A.; Ghetti, B.; Goedert, M.; Scheres, S.H.W. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 2017, 547, 185–190. [Google Scholar] [CrossRef]
- Cicognola, C.; Brinkmalm, G.; Wahlgren, J.; Portelius, E.; Gobom, J.; Cullen, N.C.; Hansson, O.; Parnetti, L.; Constantinescu, R.; Wildsmith, K.; et al. Novel tau fragments in cerebrospinal fluid: Relation to tangle pathology and cognitive decline in Alzheimer’s disease. Acta Neuropathol. 2019, 137, 279–296. [Google Scholar] [CrossRef]
- Constantinides, V.C.; Paraskevas, G.P.; Boufidou, F.; Bourbouli, M.; Pyrgelis, E.S.; Stefanis, L.; Kapaki, E. CSF Aβ42 and Aβ42/Aβ40 Ratio in Alzheimer’s Disease and Frontotemporal Dementias. Diagnostics 2023, 13, 783. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.C.; Bang, J.; Lobach, I.V.; Tsai, R.M.; Rabinovici, G.D.; Miller, B.L.; Boxer, A.L. CSF neurofilament light chain and phosphorylated tau 181 predict disease progression in PSP. Neurology 2018, 90, e273–e281. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, W.; Yang, Y.; Murzin, A.G.; Falcon, B.; Kotecha, A.; van Beers, M.; Tarutani, A.; Kametani, F.; Garringer, H.J.; et al. Structure-based classification of tauopathies. Nature 2021, 598, 359–363. [Google Scholar] [CrossRef]
- Zhang, W.; Tarutani, A.; Newell, K.L.; Murzin, A.G.; Matsubara, T.; Falcon, B.; Vidal, R.; Garringer, H.J.; Shi, Y.; Ikeuchi, T.; et al. Novel tau filament fold in corticobasal degeneration. Nature 2020, 580, 283–287. [Google Scholar] [CrossRef]
- Zhao, Y.; Tseng, I.C.; Heyser, C.J.; Rockenstein, E.; Mante, M.; Adame, A.; Zheng, Q.; Huang, T.; Wang, X.; Arslan, P.E.; et al. Appoptosin-Mediated Caspase Cleavage of Tau Contributes to Progressive Supranuclear Palsy Pathogenesis. Neuron 2015, 87, 963–975. [Google Scholar] [CrossRef]
- Horie, K.; Barthélemy, N.R.; Spina, S.; VandeVrede, L.; He, Y.; Paterson, R.W.; Wright, B.A.; Day, G.S.; Davis, A.A.; Karch, C.M.; et al. CSF tau microtubule-binding region identifies pathological changes in primary tauopathies. Nat. Med. 2022, 28, 2547–2554. [Google Scholar] [CrossRef]
- Saijo, E.; Metrick, M.A., 2nd; Koga, S.; Parchi, P.; Litvan, I.; Spina, S.; Boxer, A.; Rojas, J.C.; Galasko, D.; Kraus, A.; et al. 4-Repeat tau seeds and templating subtypes as brain and CSF biomarkers of frontotemporal lobar degeneration. Acta Neuropathol. 2020, 139, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Schonhaut, D.R.; McMillan, C.T.; Spina, S.; Dickerson, B.C.; Siderowf, A.; Devous, M.D., Sr.; Tsai, R.; Winer, J.; Russell, D.S.; Litvan, I.; et al. (18) F-flortaucipir tau positron emission tomography distinguishes established progressive supranuclear palsy from controls and Parkinson disease: A multicenter study. Ann. Neurol. 2017, 82, 622–634. [Google Scholar] [CrossRef]
- Tagai, K.; Ono, M.; Kubota, M.; Kitamura, S.; Takahata, K.; Seki, C.; Takado, Y.; Shinotoh, H.; Sano, Y.; Yamamoto, Y.; et al. High-Contrast In Vivo Imaging of Tau Pathologies in Alzheimer’s and Non-Alzheimer’s Disease Tauopathies. Neuron 2021, 109, 42–58.e48. [Google Scholar] [CrossRef]
- Brendel, M.; Barthel, H.; van Eimeren, T.; Marek, K.; Beyer, L.; Song, M.; Palleis, C.; Gehmeyr, M.; Fietzek, U.; Respondek, G.; et al. Assessment of 18F-PI-2620 as a Biomarker in Progressive Supranuclear Palsy. JAMA Neurol. 2020, 77, 1408–1419. [Google Scholar] [CrossRef]
- Therriault, J.; Vermeiren, M.; Servaes, S.; Tissot, C.; Ashton, N.J.; Benedet, A.L.; Karikari, T.K.; Lantero-Rodriguez, J.; Brum, W.S.; Lussier, F.Z.; et al. Association of Phosphorylated Tau Biomarkers With Amyloid Positron Emission Tomography vs. Tau Positron Emission Tomography. JAMA Neurol. 2023, 80, 188–199. [Google Scholar] [CrossRef]
- Kasuga, K.; Kikuchi, M.; Tsukie, T.; Suzuki, K.; Ihara, R.; Iwata, A.; Hara, N.; Miyashita, A.; Kuwano, R.; Iwatsubo, T.; et al. Different AT(N) profiles and clinical progression classified by two different N markers using total tau and neurofilament light chain in cerebrospinal fluid. BMJ Neurol. Open 2022, 4, e000321. [Google Scholar] [CrossRef]
- Van Hulle, C.; Jonaitis, E.M.; Betthauser, T.J.; Batrla, R.; Wild, N.; Kollmorgen, G.; Andreasson, U.; Okonkwo, O.; Bendlin, B.B.; Asthana, S.; et al. An examination of a novel multipanel of CSF biomarkers in the Alzheimer’s disease clinical and pathological continuum. Alzheimers Dement. 2021, 17, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Rubinski, A.; Franzmeier, N.; Dewenter, A.; Luan, Y.; Smith, R.; Strandberg, O.; Ossenkoppele, R.; Dichgans, M.; Hansson, O.; Ewers, M. Higher levels of myelin are associated with higher resistance against tau pathology in Alzheimer’s disease. Alzheimers Res. Ther. 2022, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.G.; Cristiani, C.M.; Scaramuzzino, L.; Sarica, A.; Augimeri, A.; Chimento, I.; Buonocore, J.; Parrotta, E.I.; Quattrone, A.; Cuda, G. Combined blood Neurofilament light chain and third ventricle width to differentiate Progressive Supranuclear Palsy from Parkinson’s Disease: A machine learning study. Park. Relat. Disord. 2024, 123, 106978. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef]
- Coughlin, D.G.; Litvan, I. Progressive supranuclear palsy: Advances in diagnosis and management. Park. Relat. Disord. 2020, 73, 105–116. [Google Scholar] [CrossRef] [PubMed]
| Study [Reference] | Platform | Cohort Sizes (n) | |||
|---|---|---|---|---|---|
| CTRL | AD | CBS/CBD | PSP | ||
| Magdalinou NK et al. [42] | ELISA | 30 | 26 | 14 | 33 |
| Hall S et al. [43] | xMAP | 107 | 48 | 12 | 45 |
| Hansson O et al. (original) [44] | ELISA | 53 | n/a | 5 | 19 |
| Hansson O et al. (validation) [44] | ELISA | 26 | n/a | 12 | 29 |
| Wagshal D et al. (original) [45] | xMAP | 26 | 37 | n/a | 24 |
| Thijssen EH et al. [46] | xMAP | 69 | 56 | 39 | 48 |
| Urakami K et al. [47] | ELISA | 36 | n/a | 27 | 30 |
| Holmberg B et al. [48] | ELISA | 32 | n/a | n/a | 15 |
| Noguchi M et al. [49] | ELISA | 43 | 69 | 9 | 18 |
| Aerts MB et al. [50] | ELISA | 49 | n/a | 12 | 21 |
| Schoonenboom NS et al. [51] | ELISA | 275 | 512 | 16 | 20 |
| Scherling CS et al. [52] | xMAP | 47 | 50 | 17 | 22 |
| Bäckström DC et al. [53] | ELISA | 30 | n/a | n/a | 12 |
| Constantinides VC et al. [54] | ELISA | 18 | n/a | 17 | 19 |
| Jeppsson A et al. [55] | Aβ42: ECL | 54 | 50 | 15 | 34 |
| t-tau, p-tau181: ELISA | |||||
| Borroni B et al. [56] | ELISA | 27 | 29 | 20 | 21 |
| Snellman A et al. [57] | ELISA | 22 | 22 | n/a | 22 |
| Study [Reference] | AD | CBS/CBD | PSP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Aβ42 | t-tau | p-tau181 | Aβ42 | t-tau | p-tau181 | Aβ42 | t-tau | p-tau181 | |
| Magdalinou NK et al. [42] | ↓ * | ↑ * | ↑ * | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| Hall S et al. [43] | ↓ ‡ | ↑ ‡ | ↑ ‡ | ↔ | ↔ | ↔ | ↔ | ↔ | ↓ ‡ |
| Hansson O et al. (original) [44] | n/a | n/a | n/a | ↔ | ↔ | ↔ | ↓ * | ↓ * | ↓ † |
| Hansson O et al. (validation) [44] | n/a | n/a | n/a | ↔ | ↔ | ↔ | ↓ * | ↔ | ↓ † |
| Wagshal D et al. (original) [45] | ↓ * | ↑ * | ↑ * | n/a | n/a | n/a | ↓ * | ↓ * | ↓ * |
| Thijssen EH et al. [46] | n/a | n/a | ↑ * | n/a | n/a | ↔ | n/a | n/a | ↔ |
| Urakami K et al. [47] | n/a | n/a | n/a | n/a | ↑ ‡ | n/a | n/a | ↔ | n/a |
| Holmberg B et al. [48] | n/a | n/a | n/a | n/a | n/a | n/a | ↔ | n/a | n/a |
| Noguchi M et al. [49] | ↓ ‡ | ↑ ‡ | ↑ ‡ | ↓ * | ↔ | ↔ | ↓ ‡ | ↔ | ↔ |
| Aerts MB et al. [50] | n/a | n/a | n/a | ↔ | ↑ ‡ | ↑ † | ↔ | ↑ * | ↔ |
| Schoonenboom NS et al. [51] | ↓ * | ↑ * | ↑ * | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| Scherling CS et al. [52] | ↓‡ | ↑ * | ↑‡ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| Bäckström DC et al. [53] | n/a | n/a | n/a | n/a | n/a | n/a | ↓ * | ↔ | ↔ |
| Constantinides VC et al. [54] | n/a | n/a | n/a | ↔ | ↑ * | ↔ | ↔ | ↔ | ↔ |
| Jeppsson A et al. [55] | ↓ ‡ | ↑ ‡ | ↑ ‡ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| Borroni B et al. [56] | n/a | ↑ ‡ | ↑ † | n/a | ↔ | ↔ | n/a | ↔ | ↔ |
| Snellman A et al. [57] | ↓‡ | ↑ ‡ | ↑ ‡ | n/a | n/a | n/a | ↔ | ↔ | ↔ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishiguro, T.; Kasuga, K. Alzheimer’s Disease-Related Cerebrospinal Fluid Biomarkers in Progressive Supranuclear Palsy. Brain Sci. 2024, 14, 859. https://doi.org/10.3390/brainsci14090859
Ishiguro T, Kasuga K. Alzheimer’s Disease-Related Cerebrospinal Fluid Biomarkers in Progressive Supranuclear Palsy. Brain Sciences. 2024; 14(9):859. https://doi.org/10.3390/brainsci14090859
Chicago/Turabian StyleIshiguro, Takanobu, and Kensaku Kasuga. 2024. "Alzheimer’s Disease-Related Cerebrospinal Fluid Biomarkers in Progressive Supranuclear Palsy" Brain Sciences 14, no. 9: 859. https://doi.org/10.3390/brainsci14090859
APA StyleIshiguro, T., & Kasuga, K. (2024). Alzheimer’s Disease-Related Cerebrospinal Fluid Biomarkers in Progressive Supranuclear Palsy. Brain Sciences, 14(9), 859. https://doi.org/10.3390/brainsci14090859







