White Matter Integrity and Motor Function Disruption Due to Traumatic Brain Injury in Piglets: Impacts on Motor-Related Brain Fibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Controlled Cortical Impact
2.3. Magnetic Resonance Imaging
- 3D Fast SPoiled GRadient echo (FSPGR) T1-Weighted (T1W) (FA = 9°, number of echoes = 1, TI = 900 ms, receiver bandwidth = 31.25 kHz, FOV = 12.8 × 12.8 × 6.4 cm, slice thickness = 1 mm, and a matrix size of 256 × 256 × 112);
- Fast Spin Echo (FSE) T2W (TR = 5.3 s, TE = 124 ms, echo train length = 17, receiver bandwidth = 20.83 kHz, FOV = 12.8 × 12.8 cm, matrix size of 384 × 224, slice thickness = 3 mm);
- Spin Echo (SE) DTI EPI (TR = 10.0 s, TE = min-full, FOV = 12.8 × 12.8 × 6.4 cm, a matrix size of 64 × 64 × 32, 3 b = 0 images, 30 diffusion weighted images, b = 1000 s/mm2).
2.4. Data Processing
2.5. Gait Analysis
- Stride Length: Distance between successive ground contact of the same hoof.
- Step Length: Distance between corresponding successive points of contact of opposing hooves.
- Step Time: Time from initial contact of a hoof to the initial contact of the opposite hoof.
2.6. Statistical Analysis
3. Results
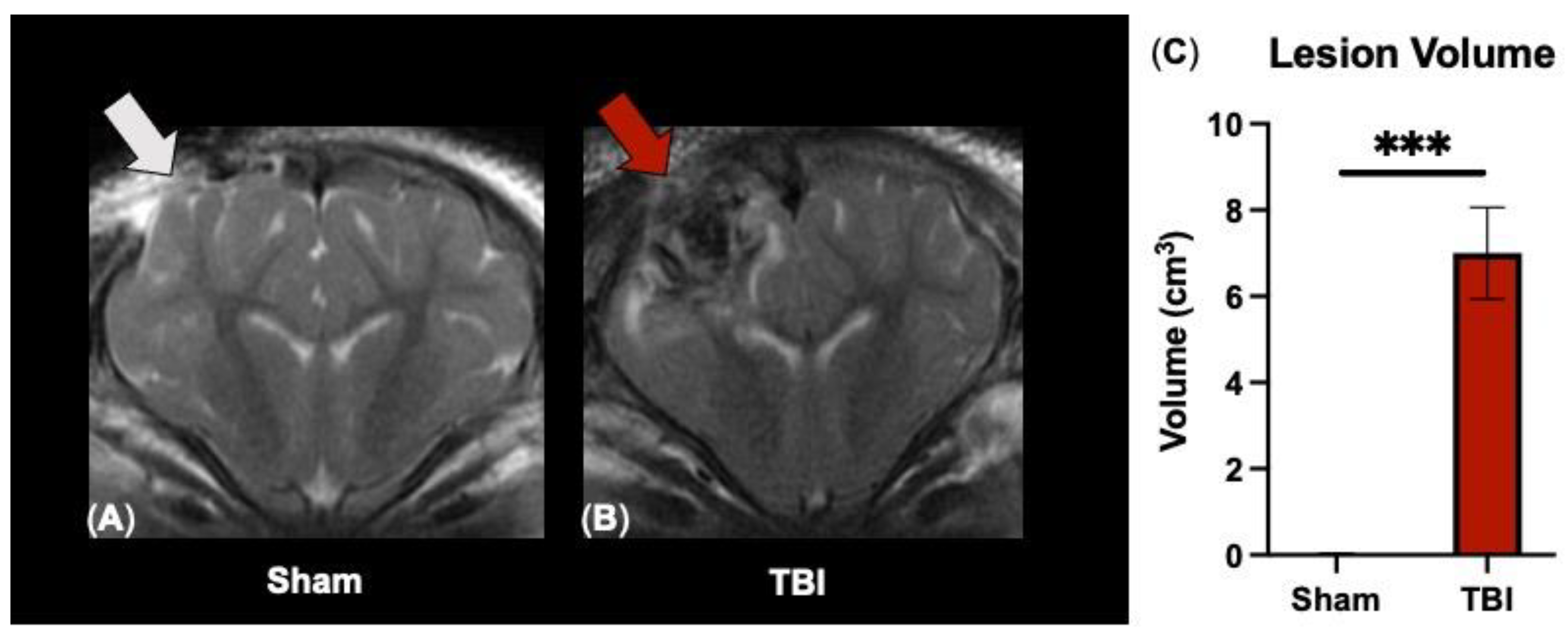
3.1. T2W Sequences Detect Significant Ipsilateral Lesioning following TBI
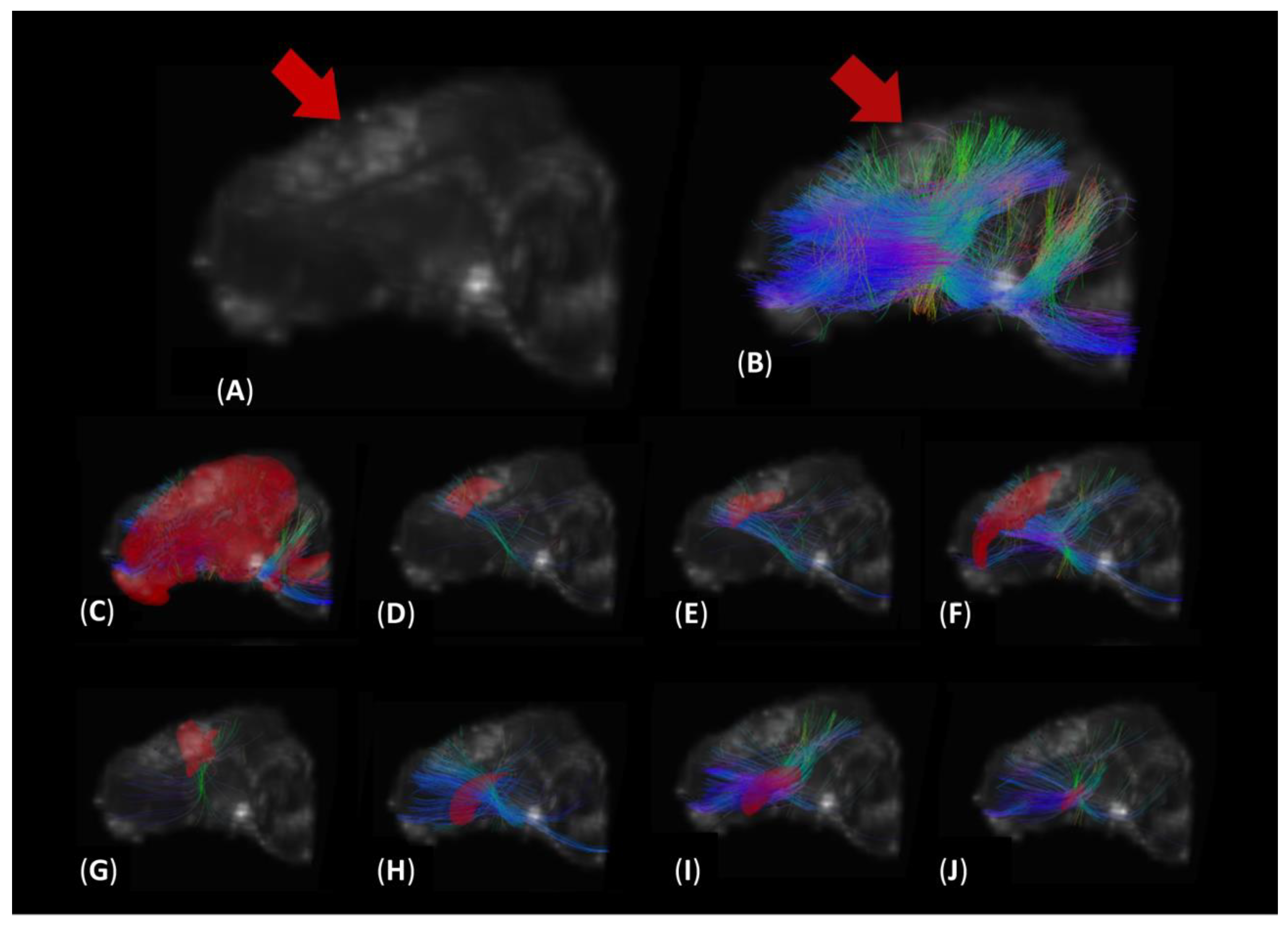
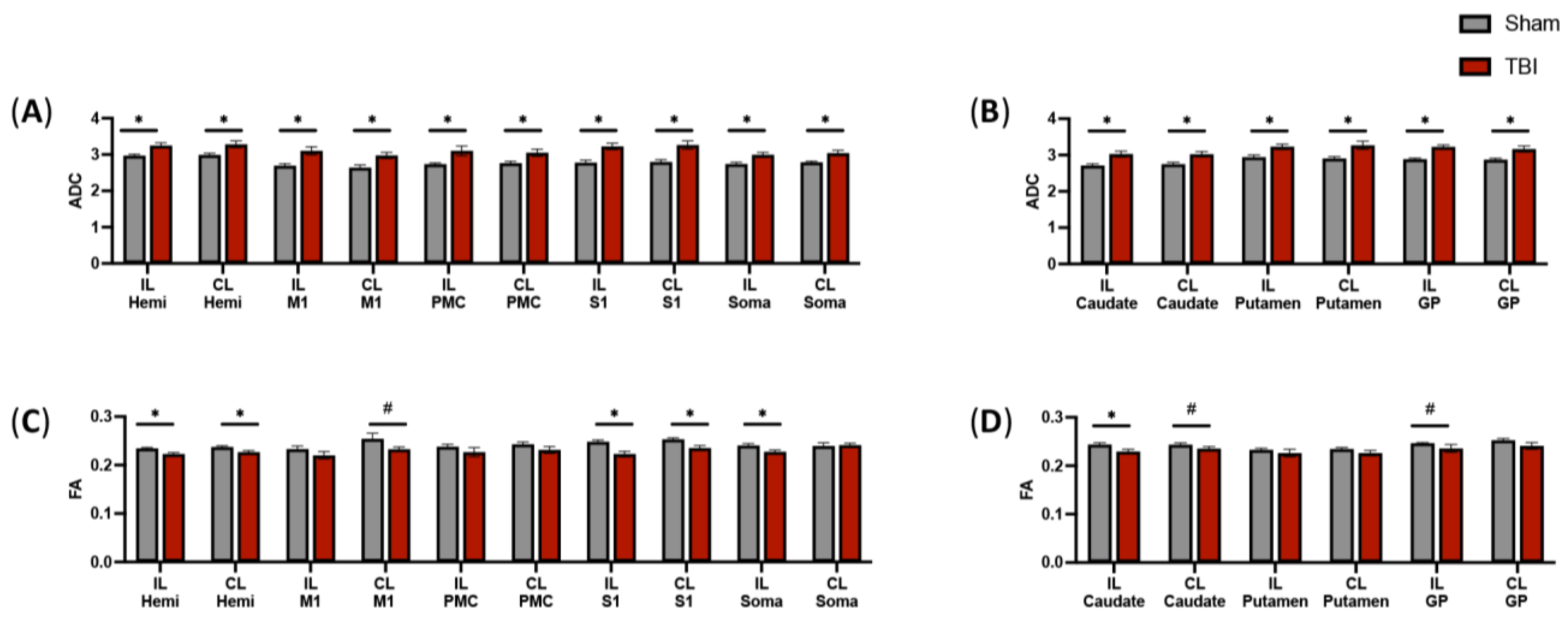
3.2. DTI Tractography Reveals Increased Diffusion in Fibers Intersecting Both Ipsilateral and Contralateral Motor Function-Related Brain Structures
3.3. DTI Scans Indicate Decreased Fractional Anisotropy in the Intersecting Fibers of a Subset of Motor Function-Related Structures
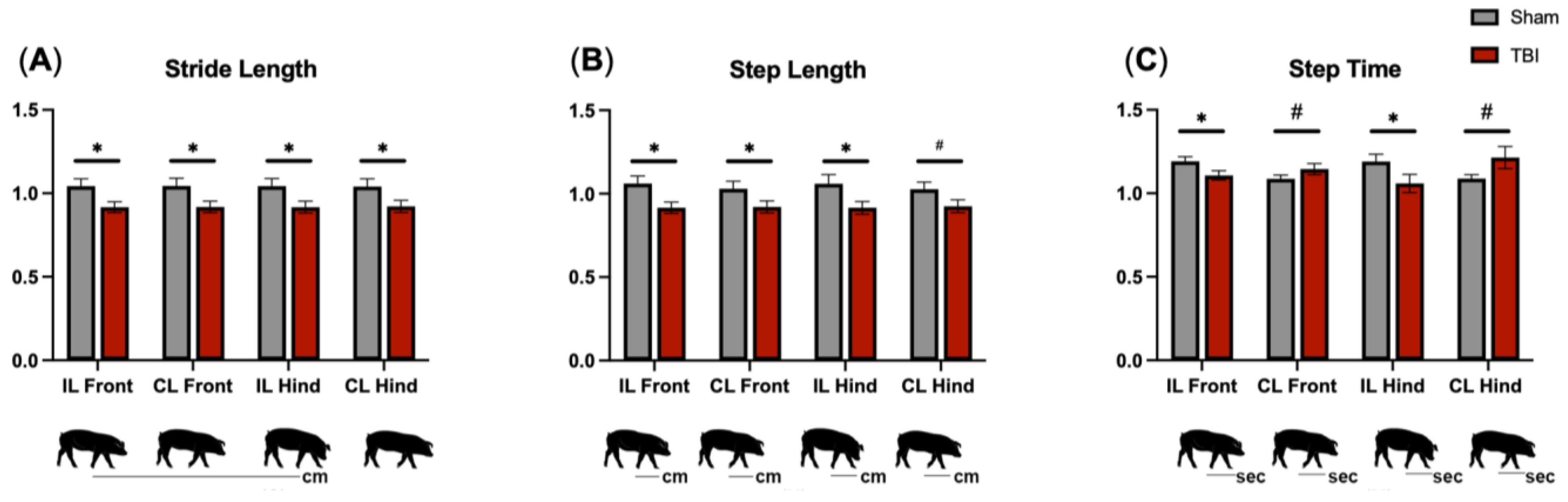
3.4. Gait Analysis Reveals Motor Deficits in All Limbs following TBI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Report to Congress: The Management of Traumatic Brain Injury in Children, National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention: Atlanta, GA, USA, 2018. [Google Scholar]
- Katz-Leurer, M.; Rotem, H.; Lewitus, H.; Keren, O.; Meyer, S. Relationship between Balance Abilities and Gait Characteristics in Children with Post-Traumatic Brain Injury. Brain Inj. 2008, 22, 153–159. [Google Scholar] [CrossRef]
- Beretta, E.; Cimolin, V.; Piccinini, L.; Carla Turconi, A.; Galbiati, S.; Crivellini, M.; Galli, M.; Strazzer, S. Assessment of Gait Recovery in Children after Traumatic Brain Injury. Brain Inj. 2009, 23, 751–759. [Google Scholar] [CrossRef]
- Drijkoningen, D.; Chalavi, S.; Sunaert, S.; Duysens, J.; Swinnen, S.P.; Caeyenberghs, K. Regional Gray Matter Volume Loss Is Associated with Gait Impairments in Young Brain-Injured Individuals. J. Neurotrauma 2017, 34, 1022–1034. [Google Scholar] [CrossRef] [PubMed]
- Caeyenberghs, K.; Leemans, A.; Geurts, M.; Linden, C.V.; Smits-Engelsman, B.C.M.; Sunaert, S.; Swinnen, S.P. Correlations between White Matter Integrity and Motor Function in Traumatic Brain Injury Patients. Neurorehabilit. Neural Repair 2011, 25, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Kim, J. Activation of Less Affected Corticospinal Tract and Poor Motor Outcome in Hemiplegic Pediatric Patients: A Diffusion Tensor Tractography Imaging Study. Neural Regen. Res. 2015, 10, 2054. [Google Scholar] [CrossRef] [PubMed]
- Caeyenberghs, K.; Leemans, A.; Geurts, M.; Taymans, T.; Linden, C.V.; Smits-Engelsman, B.C.M.; Sunaert, S.; Swinnen, S.P. Brain-Behavior Relationships in Young Traumatic Brain Injury Patients: DTI Metrics Are Highly Correlated with Postural Control. Hum. Brain Mapp. 2009, 31, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Caeyenberghs, K.; Leemans, A.; De Decker, C.; Heitger, M.; Drijkoningen, D.; Linden, C.V.; Sunaert, S.; Swinnen, S.P. Brain Connectivity and Postural Control in Young Traumatic Brain Injury Patients: A Diffusion MRI Based Network Analysis. NeuroImage Clin. 2012, 1, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Neil, J.; Miller, J.; Mukherjee, P.; Hüppi, P.S. Diffusion Tensor Imaging of Normal and Injured Developing Human Brain—A Technical Review. NMR Biomed. 2002, 15, 543–552. [Google Scholar] [CrossRef]
- Wang, M.-L.; Li, W.-B. Cognitive Impairment after Traumatic Brain Injury: The Role of MRI and Possible Pathological Basis. J. Neurol. Sci. 2016, 370, 244–250. [Google Scholar] [CrossRef]
- Wang, H.; Baker, E.; Mandal, A. Identification of Predictive MRI and Functional Biomarkers in a Pediatric Piglet Traumatic Brain Injury Model. Neural Regen. Res. 2021, 16, 338. [Google Scholar] [CrossRef]
- Chastain, C.A.; Udochukwu, O.; Zipperman, M.; Joo, E.; Ashwal, S.; Shutter, L.; Tong, K.A. Predicting Outcomes of Traumatic Brain Injury by Imaging Modality and Injury Distribution. J. Neurotrauma 2009, 26, 1183–1196. [Google Scholar] [CrossRef]
- Scheulin, K.M.; Jurgielewicz, B.J.; Spellicy, S.E.; Waters, E.S.; Baker, E.W.; Kinder, H.A.; Simchick, G.A.; Sneed, S.E.; Grimes, J.A.; Zhao, Q.; et al. Exploring the Predictive Value of Lesion Topology on Motor Function Outcomes in a Porcine Ischemic Stroke Model. Sci. Rep. 2021, 11, 3814. [Google Scholar] [CrossRef]
- Jang, S.H. Diagnostic Problems in Diffuse Axonal Injury. Diagnostics 2020, 10, 117. [Google Scholar] [CrossRef]
- Duckworth, J.L.; Stevens, R.D. Imaging Brain Trauma. Curr. Opin. Crit. Care 2010, 16, 92–97. [Google Scholar] [CrossRef]
- Wang, J.; Khamid, B.; Devous, M.D.; Abdi, H.; McColl, R.W.; Moore, C.; Marquez, C.; Ding, K.; Whittemore, A.D.; Babcock, E.E.; et al. Diffusion Tensor Tractography of Traumatic Diffuse Axonal Injury. JAMA Neurol. 2008, 65, 619–626. [Google Scholar] [CrossRef]
- Brandstack, N.; Kurki, T.; Tenovuo, O. Quantitative Diffusion-Tensor Tractography of Long Association Tracts in Patients with Traumatic Brain Injury without Associated Findings at Routine MR Imaging. Radiology 2013, 267, 231–239. [Google Scholar] [CrossRef]
- Jang, S.H.; Seo, Y.S. Difference between Injuries of the Corticospinal Tract and Corticoreticulospinal Tract in Patients with Diffuse Axonal Injury: A Diffusion Tensor Tractography Study. Int. J. Neurosci. 2019, 130, 124–129. [Google Scholar] [CrossRef]
- Levin, H.S. Neuroplasticity Following Non-Penetrating Traumatic Brain Injury. Brain Inj. 2003, 17, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, J.; Krach, L.; Ward, E.; Mueller, B.; Muetzel, R.; Schnoebelen, S.; Kiragu, A.; Lim, K. Neurocognitive and Neuroimaging Correlates of Pediatric Traumatic Brain Injury: A Diffusion Tensor Imaging (DTI) Study. Arch. Clin. Neuropsychol. 2007, 22, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Lebel, C.; Shaywitz, B.; Holahan, J.; Shaywitz, S.; Marchione, K.; Beaulieu, C. Diffusion Tensor Imaging Correlates of Reading Ability in Dysfluent and Non-Impaired Readers. Brain Lang. 2013, 125, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-H.; Kim, S.-H.; Jung, H.-Y. Diffusion-Tensor-Tractography-Based Diagnosis for Injury of Corticospinal Tract in a Patient with Hemiplegia Following Traumatic Brain Injury. Diagnostics 2020, 10, 156. [Google Scholar] [CrossRef]
- Choi, E.B.; Kim, J.Y.; Jang, S.H. Motor Recovery of Hemiparetic Leg by Improvement of Limb-Kinetic Apraxia in a Chronic Patient with Traumatic Brain Injury. Medicine 2020, 99, e20144. [Google Scholar] [CrossRef]
- Kwon, H.G.; Jang, S.H. Delayed Gait Disturbance due to Injury of the Corticoreticular Pathway in a Patient with Mild Traumatic Brain Injury. Brain Inj. 2014, 28, 511–514. [Google Scholar] [CrossRef]
- Ressel, V.; O’Gorman Tuura, R.; Scheer, I.; van Hedel, H.J.A. Diffusion Tensor Imaging Predicts Motor Outcome in Children with Acquired Brain Injury. Brain Imaging Behav. 2016, 11, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Missault, S.; Anckaerts, C.; Blockx, I.; Deleye, S.; Van Dam, D.; Barriche, N.; De Pauw, G.; Aertgeerts, S.; Valkenburg, F.; De Deyn, P.P.; et al. Neuroimaging of Subacute Brain Inflammation and Microstructural Changes Predicts Long-Term Functional Outcome after Experimental Traumatic Brain Injury. J. Neurotrauma 2019, 36, 768–788. [Google Scholar] [CrossRef] [PubMed]
- Kinder, H.A.; Baker, E.W.; West, F.D. The Pig as a Preclinical Traumatic Brain Injury Model: Current Models, Functional Outcome Measures, and Translational Detection Strategies. Neural Regen. Res. 2019, 14, 413. [Google Scholar] [CrossRef]
- Nakamura, M.; Imai, H.; Konno, K.; Kubota, C.; Seki, K.; Puentes, S.; Faried, A.; Hideaki, Y.; Hata, H.; Yoshimoto, Y.; et al. Experimental Investigation of Encephalomyosynangiosis Using Gyrencephalic Brain of the Miniature Pig: Histopathological Evaluation of Dynamic Reconstruction of Vessels for Functional Anastomosis. PubMed 2009, 3, 488–495. [Google Scholar] [CrossRef]
- Vink, R. Large Animal Models of Traumatic Brain Injury. J. Neurosci. Res. 2017, 96, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Kleiven, S. Can Sulci Protect the Brain from Traumatic Injury? J. Biomech. 2009, 42, 2074–2080. [Google Scholar] [CrossRef]
- Watanabe, H.; Andersen, F.; Simonsen, C.Z.; Evans, S.M.; Gjedde, A.; Cumming, P. MR-Based Statistical Atlas of the Göttingen Minipig Brain. NeuroImage 2001, 14, 1089–1096. [Google Scholar] [CrossRef]
- Krafft, P.R.; Bailey, E.L.; Lekic, T.; Rolland, W.B.; Altay, O.; Tang, J.; Wardlaw, J.M.; Zhang, J.H.; Sudlow, C.L.M. Etiology of Stroke and Choice of Models. Int. J. Stroke 2012, 7, 398–406. [Google Scholar] [CrossRef]
- Kaiser, E.; West, F. Large Animal Ischemic Stroke Models: Replicating Human Stroke Pathophysiology. Neural Regen. Res. 2020, 15, 1377. [Google Scholar] [CrossRef]
- Ahmad, A.S.; Satriotomo, I.; Fazal, J.; Nadeau, S.E.; Doré, S. Considerations for the Optimization of Induced White Matter Injury Preclinical Models. Front. Neurol. 2015, 6, 172. [Google Scholar] [CrossRef]
- Simchick, G.; Scheulin, K.M.; Sun, W.; Sneed, S.E.; Fagan, M.M.; Cheek, S.R.; West, F.D.; Zhao, Q. Detecting Functional Connectivity Disruptions in a Translational Pediatric Traumatic Brain Injury Porcine Model Using Resting-State and Task-Based FMRI. Sci. Rep. 2021, 11, 12406. [Google Scholar] [CrossRef]
- Flynn, T.J. Developmental Changes of Myelin-Related Lipids in Brain of Miniature Swine. Neurochem. Res. 1984, 9, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.S.; Dilger, R.N.; Johnson, R.W. Brain Growth of the Domestic Pig(Sus Scrofa)from 2 to 24 Weeks of Age: A Longitudinal MRI Study. Dev. Neurosci. 2012, 34, 291–298. [Google Scholar] [CrossRef]
- Baker, E.W.; Kinder, H.A.; Hutcheson, J.M.; Duberstein, K.J.J.; Platt, S.R.; Howerth, E.W.; West, F.D. Controlled Cortical Impact Severity Results in Graded Cellular, Tissue, and Functional Responses in a Piglet Traumatic Brain Injury Model. J. Neurotrauma 2019, 36, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Kinder, H.A.; Baker, E.W.; Wang, S.; Fleischer, C.C.; Howerth, E.W.; Duberstein, K.J.; Mao, H.; Platt, S.R.; West, F.D. Traumatic Brain Injury Results in Dynamic Brain Structure Changes Leading to Acute and Chronic Motor Function Deficits in a Pediatric Piglet Model. J. Neurotrauma 2019, 36, 2930–2942. [Google Scholar] [CrossRef] [PubMed]
- Fagan, M.M.; Welch, C.B.; Scheulin, K.M.; Sneed, S.E.; Jeon, J.H.; Golan, M.E.; Cheek, S.R.; Barany, D.A.; Oeltzschner, G.; Callaway, T.R.; et al. Fecal microbial transplantation limits neural injury severity and functional deficits in a pediatric piglet traumatic brain injury model. Front. Neurosci. 2023, 17, 1249539. [Google Scholar] [CrossRef]
- Kinder, H.A.; Baker, E.W.; Howerth, E.W.; Duberstein, K.J.; West, F.D. Controlled Cortical Impact Leads to Cognitive and Motor Function Deficits That Correspond to Cellular Pathology in a Piglet Traumatic Brain Injury Model. J. Neurotrauma 2019, 36, 2810–2826. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Morgan, P.S.; Ashburner, J.; Smith, J.; Rorden, C. The First Step for Neuroimaging Data Analysis: DICOM to NIfTI Conversion. J. Neurosci. Methods 2016, 264, 47–56. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Buzsaki, G. Neuronal Oscillations in Cortical Networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef] [PubMed]
- Saikali, S.; Meurice, P.; Sauleau, P.; Eliat, P.-A.; Bellaud, P.; Randuineau, G.; Vérin, M.; Malbert, C.-H. A Three-Dimensional Digital Segmented and Deformable Brain Atlas of the Domestic Pig. J. Neurosci. Methods 2010, 192, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E. The Brain’s Dark Energy. Science 2006, 314, 1249–1250. [Google Scholar] [CrossRef] [PubMed]
- Kenet, T.; Bibitchkov, D.; Tsodyks, M.; Grinvald, A.; Arieli, A. Spontaneously Emerging Cortical Representations of Visual Attributes. Nature 2003, 425, 954–956. [Google Scholar] [CrossRef] [PubMed]
- Kuhtz-Buschbeck, J.P.; Stolze, H.; Gölge, M.G.; Ritz, A. Analyses of Gait, Reaching, and Grasping in Children after Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2003, 84, 424–430. [Google Scholar] [CrossRef]
- Pischiutta, F.; Micotti, E.; Hay, J.R.; Marongiu, I.; Sammali, E.; Tolomeo, D.; Vegliante, G.; Stocchetti, N.; Forloni, G.; De Simoni, M.-G.; et al. Single Severe Traumatic Brain Injury Produces Progressive Pathology with Ongoing Contralateral White Matter Damage One Year after Injury. Exp. Neurol. 2018, 300, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.G.; Verley, D.R.; Gutman, B.A.; Sutton, R.L. Bi-Directional Changes in Fractional Anisotropy after Experiment TBI: Disorganization and Reorganization? NeuroImage 2016, 133, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Riemann, B.L.; Lephart, S.M. The Sensorimotor System, Part I: The Physiologic Basis of Functional Joint Stability. J. Athl. Train. 2002, 37, 71–79. [Google Scholar]
- Azim, E.; Seki, K. Gain Control in the Sensorimotor System. Curr. Opin. Physiol. 2019, 8, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Jang, S. The Corticospinal Tract from the Viewpoint of Brain Rehabilitation. J. Rehabil. Med. 2014, 46, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.D.; Sullivan, P.G.; Gibson, T.R.; Pavel, K.M.; Thompson, B.M.; Scheff, S.W. Spatial and Temporal Characteristics of Neurodegeneration after Controlled Cortical Impact in Mice: More than a Focal Brain Injury. J. Neurotrauma 2005, 22, 252–265. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fagan, M.M.; Scheulin, K.M.; Sneed, S.E.; Sun, W.; Welch, C.B.; Cheek, S.R.; Kaiser, E.E.; Zhao, Q.; Duberstein, K.J.; West, F.D. White Matter Integrity and Motor Function Disruption Due to Traumatic Brain Injury in Piglets: Impacts on Motor-Related Brain Fibers. Brain Sci. 2024, 14, 247. https://doi.org/10.3390/brainsci14030247
Fagan MM, Scheulin KM, Sneed SE, Sun W, Welch CB, Cheek SR, Kaiser EE, Zhao Q, Duberstein KJ, West FD. White Matter Integrity and Motor Function Disruption Due to Traumatic Brain Injury in Piglets: Impacts on Motor-Related Brain Fibers. Brain Sciences. 2024; 14(3):247. https://doi.org/10.3390/brainsci14030247
Chicago/Turabian StyleFagan, Madison M., Kelly M. Scheulin, Sydney E. Sneed, Wenwu Sun, Christina B. Welch, Savannah R. Cheek, Erin E. Kaiser, Qun Zhao, Kylee J. Duberstein, and Franklin D. West. 2024. "White Matter Integrity and Motor Function Disruption Due to Traumatic Brain Injury in Piglets: Impacts on Motor-Related Brain Fibers" Brain Sciences 14, no. 3: 247. https://doi.org/10.3390/brainsci14030247
APA StyleFagan, M. M., Scheulin, K. M., Sneed, S. E., Sun, W., Welch, C. B., Cheek, S. R., Kaiser, E. E., Zhao, Q., Duberstein, K. J., & West, F. D. (2024). White Matter Integrity and Motor Function Disruption Due to Traumatic Brain Injury in Piglets: Impacts on Motor-Related Brain Fibers. Brain Sciences, 14(3), 247. https://doi.org/10.3390/brainsci14030247





