Expectancies of the Effects of Cannabis Use in Individuals with Social Anxiety Disorder (SAD)
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Procedures
2.3. Outcome Measures
2.4. In-Depth Interviews
2.5. Data Analysis
3. Results
3.1. Demographic and Clinical Characteristics
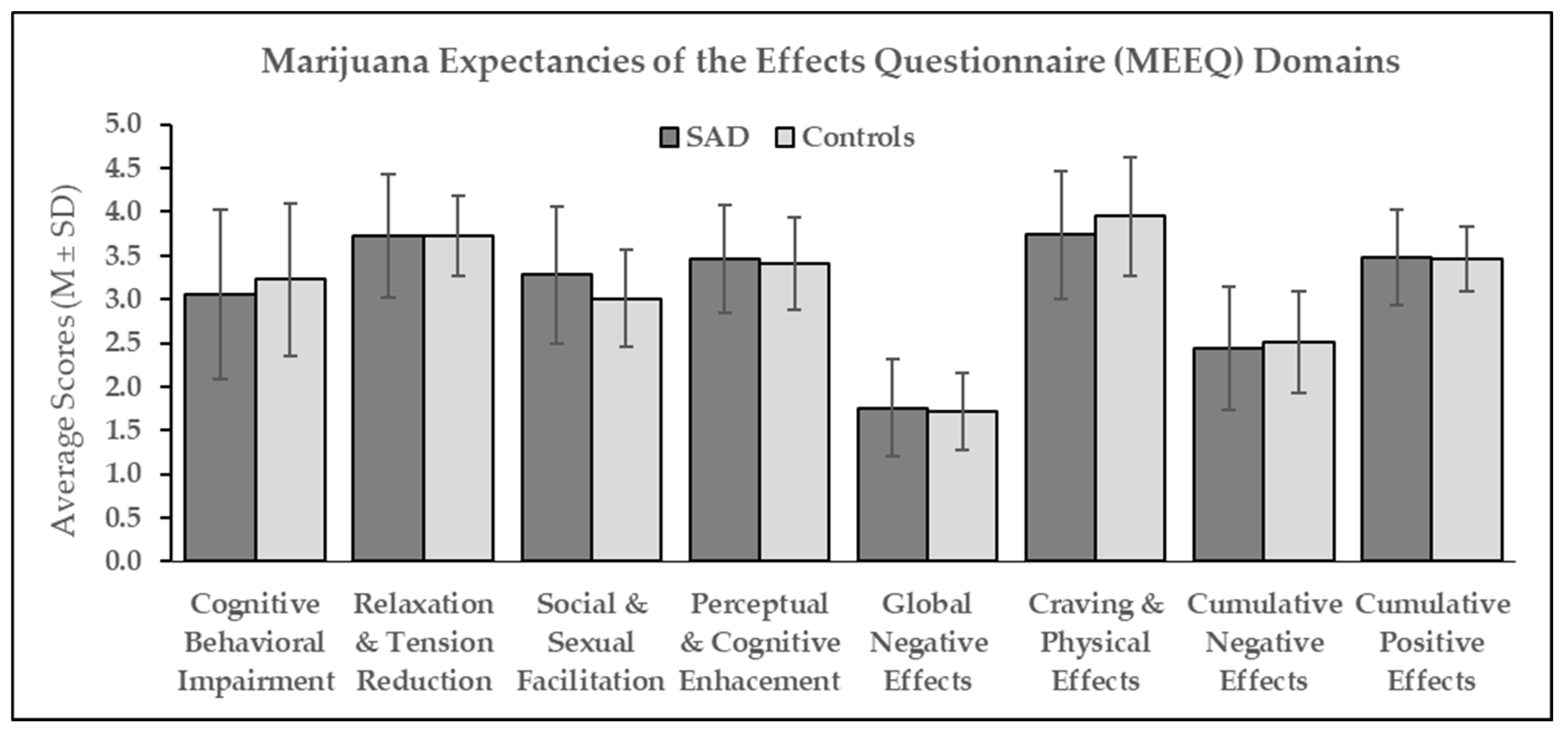
3.2. Expectancies of the Effects of Repeated Cannabis Use
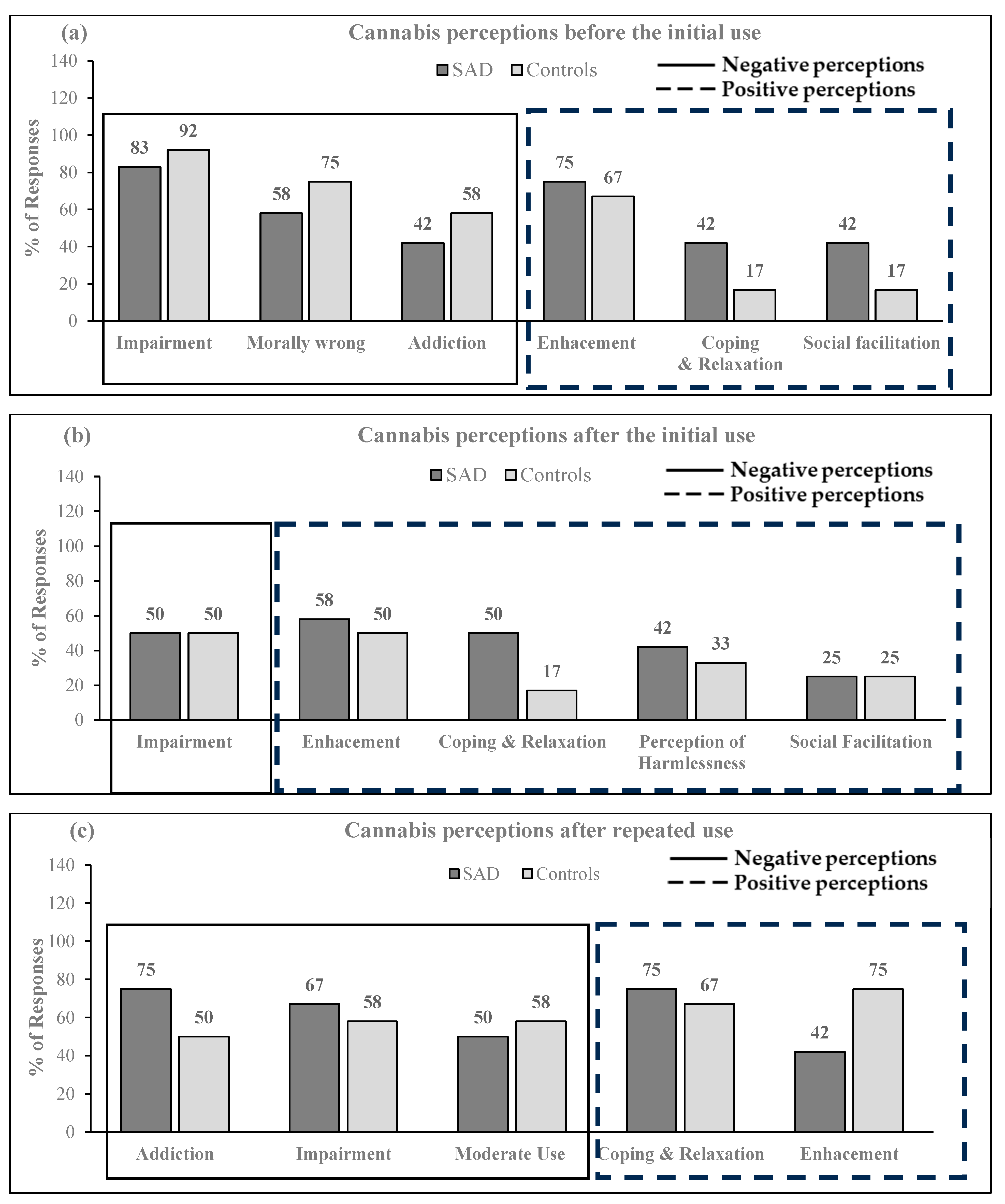
3.3. The Trajectory of Cannabis Use Perceptions Based on Qualitative Analysis
- Stage I: Cannabis use perceptions before the initial use
“I thought it might make me a lot more inebriated and unable to hold a conversation or something or I also maybe just thought it would be more of an intense high where you’re very out of reality.”
“The only thing I knew about it before using it [cannabis] was stuff I’d see in movies like Cheech and Chong, or stuff like that, or just sitting around being stupid.”
“I just thought I was going to laugh. I just thought I was going to laugh and have a good time…”
- Stage II: Cannabis use perceptions immediately after the initial use
“So I guess I just was enlightened to know what it actually meant, and what the euphoria was. So my perception became. This isn’t a bad thing. This is a thing that can make me feel happy, and giggly, and what not.”
“I’m not staying up anymore thinking about whatever happened earlier in the day and I’m distracted. I can focus on my Netflix or whatever, or I can take the notes from this lecture much easier. If that makes sense.”
“But I learned that, that was quite actually misinformation. It’s not the whole truth of it, eventually. I thought that it was something that not serious people did, I guess…. So I was like. It’s not a big deal. All these people are doing it, and they show up at school the next day. What’s the big deal? So I think it just became disillusioned to me, as not a big deal, it wasn’t a hard drug. It’s not like they were doing cocaine or anything. It was just a joint.”
“So the first time that I did it, we were planning to go out and I thought to myself, okay, it makes you free, or it makes you less stuck in your head. If I do it, maybe I’ll be able to go out with my group, maybe have a fun time, but that didn’t happen. It just elevated my anxiety even more, and I had to go back home.”
- Stage III: Cannabis use perceptions after repeated use
“I would have graduated school 10 years ago. It [cannabis use] is kind of dangerous like that.”
“I think I have to smoke more now to manage the anxiety than I did before. It’s really something that it’s more of a cyclical effect”
“And yeah, I’m more conscious of cannabis’ addictive potential, because before I thought it wasn’t addictive, so no. I’m more aware that it is addictive. Yeah, like the effect is not the exact same as the first day….Then, I used to use it because it takes a little bit more, but before I used to use 0.75 grams in one day. Now I use 1 gram.”
“I also think it can be abused and I think it can be harmful in a way to people….You should try to reduce, like once in a while is good”
“You have to find the right fit for yourself, like and what strains to do, because not all the effects… Something can be enjoyable for one person and have a really stressful effect on somebody else. …So right now I feel like you should make an informed decision about whatever you’re going to try and don’t force it on yourself, something just because everyone else was doing it…..I know what strains I like now, and what strains might give me more anxiety than I already have.”
4. Discussion
4.1. Expectancies of the Effects after Repeated Use
4.2. The Time Trajectory of Cannabis Use Perceptions
4.3. Scientific Significance
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agosti, V.; Nunes, E.; Levin, F. Rates of Psychiatric Comorbidity Among, U.S. Residents with Lifetime Cannabis Dependence. Am. J. Drug Alcohol Abus. 2002, 28, 643–652. [Google Scholar] [CrossRef]
- Leichsenring, F.; Leweke, F. Social Anxiety Disorder. N. Engl. J. Med. 2017, 376, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, M.B.; Fowler, K.F. Social anxiety disorder in the Canadian population: Exploring gender differences in sociodemographic profile. J. Anxiety Disord. 2013, 27, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Shields, M. Social anxiety disorder--beyond shyness. Health Rep. 2004, 15 Suppl., 45–61. [Google Scholar]
- Stein, D.J.; Lim, C.C.W.; Roest, A.M.; de Jonge, P.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Benjet, C.; Bromet, E.J.; Bruffaerts, R.; et al. The cross-national epidemiology of social anxiety disorder: Data from the World Mental Health Survey Initiative. BMC Med. 2017, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- Bruffaerts, R.; Harris, M.G.; Kazdin, A.E.; Vigo, D.V.; Sampson, N.A.; Chiu, W.T.; Al-Hamzawi, A.; Alonso, J.; Benjet, C.; de Girolamo, G.; et al. Perceived helpfulness of treatment for social anxiety disorder: Findings from the WHO World Mental Health Surveys. Soc. Psychiatry Psychiatr. Epidemiol. 2022, 57, 2079–2095. [Google Scholar] [CrossRef] [PubMed]
- Buckner, J.D.; Mallott, M.A.; Schmidt, N.B.; Taylor, J. Peer influence and gender differences in problematic cannabis use among individuals with social anxiety. J. Anxiety Disord. 2006, 20, 1087–1102. [Google Scholar] [CrossRef] [PubMed]
- Buckner, J.D.; Schmidt, N.B.; Bobadilla, L.; Taylor, J. Social anxiety and problematic cannabis use: Evaluating the moderating role of stress reactivity and perceived coping. Behav. Res. Ther. 2006, 44, 1007–1015. [Google Scholar] [CrossRef]
- Buckner, J.D.; Schmidt, N.B. Marijuana effect expectancies: Relations to social anxiety and marijuana use problems. Addict. Behav. 2008, 33, 1477–1483. [Google Scholar] [CrossRef]
- Buckner, J.D.; Schmidt, N.B. Social anxiety disorder and marijuana use problems: The mediating role of marijuana effect expectancies. Depress. Anxiety 2009, 26, 864–870. [Google Scholar] [CrossRef]
- Buckner, J.D.; Heimberg, R.G.; Matthews, R.A.; Silgado, J. Marijuana-related problems and social anxiety: The role of marijuana behaviors in social situations. Psychol. Addict. Behav. 2012, 26, 151–156. [Google Scholar] [CrossRef]
- Buckner, J.D.; Zvolensky, M.J.; Ecker, A.H.; Jeffries, E.R.; Lemke, A.W.; Dean, K.E.; Businelle, M.S.; Gallagher, M.W. Anxiety and cannabis-related problem severity among dually diagnosed outpatients: The impact of false safety behaviors. Addict. Behav. 2017, 70, 49–53. [Google Scholar] [CrossRef]
- Cloutier, R.M.; Blumenthal, H.; Mischel, E.R. An Examination of Social Anxiety in Marijuana and Cigarette Use Motives Among Adolescents. Subst. Use Misuse 2016, 51, 408–418. [Google Scholar] [CrossRef]
- Elsaid, S.; Wang, R.; Kloiber, S.; Le Foll, B.; Hassan, A.N. Motivations for Cannabis Use in Individuals with Social Anxiety Disorder (SAD). Brain Sci. 2023, 13, 1698. [Google Scholar] [CrossRef]
- Patel, T.A.; Schubert, F.T.; Hom, M.A.; Cougle, J.R. Correlates of treatment seeking in individuals with social anxiety disorder: Findings from a nationally representative sample. J. Anxiety Disord. 2022, 91, 102616. [Google Scholar] [CrossRef]
- Single, A.; Bilevicius, E.; Ho, V.; Theule, J.; Buckner, J.D.; Mota, N.; Keough, M.T. Cannabis use and social anxiety in young adulthood: A meta-analysis. Addict. Behav. 2022, 129, 107275. [Google Scholar] [CrossRef]
- Stinson, F.S.; Ruan, W.J.; Pickering, R.; Grant, B.F. Cannabis use disorders in the USA: Prevalence, correlates and co-morbidity. Psychol. Med. 2006, 36, 1447–1460. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.S.; Del Boca, F.K.; Darkes, J. Alcohol expectancy theory: The application of cognitive neuroscience. In Psychological Theories of Drinking and Alcoholism, 2nd ed.; The Guilford Press: New York, NY, USA, 1999; pp. 203–246. [Google Scholar]
- Schafer, J.; Brown, S.A. Marijuana and cocaine effect expectancies and drug use patterns. J. Consult. Clin. Psychol. 1991, 59, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Aarons, G.A.; Brown, S.A.; Stice, E.; Coe, M.T. Psychometric evaluation of the marijuana and stimulant effect expectancy questionnaires for adolescents. Addict. Behav. 2001, 26, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Koob, G.F.; McLellan, A.T. Neurobiologic Advances from the Brain Disease Model of Addiction. N. Engl. J. Med. 2016, 374, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.W.; Ecker, A.H.; Zvolensky, M.J.; Buckner, J.D. Social Anxiety and Cannabis Cravings: The Influences of Parent Injunctive Norms and Tension Reduction Expectancies. J. Soc. Clin. Psychol. 2015, 34, 731–746. [Google Scholar] [CrossRef]
- Berglas, S.; Jones, E.E. Drug choice as a self-handicapping strategy in response to noncontingent success. J. Pers. Soc. Psychol. 1978, 36, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Hanson, W.E.; Clark, V.L.P.; Petska, K.S.; Creswell, J.D. Mixed methods research designs in counseling psychology. J. Couns. Psychol. 2005, 52, 224. [Google Scholar] [CrossRef]
- Guetterman, T.C.; Fetters, M.D.; Creswell, J.W. Integrating Quantitative and Qualitative Results in Health Science Mixed Methods Research Through Joint Displays. Ann. Fam. Med. 2015, 13, 554. [Google Scholar] [CrossRef] [PubMed]
- Creswell, J.W.; Fetters, M.D.; Plano Clark, V.L.; Morales, A. Mixed methods intervention trials. In Mixed Methods Research for Nursing and the Health Sciences; Wiley: Hoboken, NJ, USA, 2009; pp. 161–180. [Google Scholar]
- Liebowitz, M.R. Social Phobia. Mod. Probl. Pharm. 1987, 22, 141–173. [Google Scholar] [CrossRef]
- First, M.B.; Williams, J.B.W.; Karg, R.S.; Spitzer, R.L. Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version, SCID-5-RV; American Psychiatric Association: Arlington, VA, USA, 2015. [Google Scholar]
- Bashford, J.; Flett, R.; Copeland, J. The Cannabis Use Problems Identification Test (CUPIT): Development, reliability, concurrent and predictive validity among adolescents and adults. Addiction 2010, 105, 615–625. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Baker, S.L.; Heinrichs, N.; Kim, H.-J.; Hofmann, S.G. The Liebowitz social anxiety scale as a self-report instrument: A pre-liminary psychometric analysis. Behav. Res. Ther. 2002, 40, 701–715. [Google Scholar] [PubMed]
- Enkema, M.C.; Hallgren, K.A.; Larimer, M.E. Craving is impermanent and it matters: Investigating craving and cannabis use among young adults with problematic use interested in reducing use. Drug Alcohol Depend. 2020, 210, 107957. [Google Scholar] [CrossRef] [PubMed]
- Serre, F.; Fatseas, M.; Swendsen, J.; Auriacombe, M. Ecological momentary assessment in the investigation of craving and substance use in daily life: A systematic review. Drug Alcohol Depend. 2015, 148, 1–20. [Google Scholar] [CrossRef]
- Enkema, M.C.; Hallgren, K.A.; Bowen, S.; Lee, C.M.; Larimer, M.E. Craving management: Exploring factors that influence momentary craving-related risk of cannabis use among young adults. Addict. Behav. 2021, 115, 106750. [Google Scholar] [CrossRef]
- Moore, R.C.; Depp, C.A.; Wetherell, J.L.; Lenze, E.J. Ecological momentary assessment versus standard assessment instruments for measuring mindfulness, depressed mood, and anxiety among older adults. J. Psychiatr. Res. 2016, 75, 116–123. [Google Scholar] [CrossRef]
- Enkema, M.C.; Bowen, S. Mindfulness practice moderates the relationship between craving and substance use in a clinical sample. Drug Alcohol Depend. 2017, 179, 1–7. [Google Scholar] [CrossRef]
- Nosen, E.; Woody, S.R. Applying Lessons Learned from Obsessions: Metacognitive Processes in Smoking Cessation. Cogn. Ther. Res. 2009, 33, 241–254. [Google Scholar] [CrossRef]
- Creswell, J.D. Mindfulness Interventions. Annu. Rev. Psychol. 2017, 68, 491–516. [Google Scholar] [CrossRef]
- Nosen, E.; Woody, S.R. Acceptance of cravings: How smoking cessation experiences affect craving beliefs. Behav. Res. Ther. 2014, 59, 71–81. [Google Scholar] [CrossRef]
- Amiet, D.; Youssef, G.J.; Hagg, L.J.; Lorenzetti, V.; Parkes, L.; Solowij, N.; Yücel, M. Young Adults With Higher Motives and Expectancies of Regular Cannabis Use Show Poorer Psychosocial Functioning. Front. Psychiatry 2020, 11, 599365. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Krumholz, H.M.; Allore, H.G. Using Latent Class Analysis to Identify Hidden Clinical Phenotypes. JAMA 2020, 324, 700–701. [Google Scholar] [CrossRef] [PubMed]
- Buckner, J.D.; Heimberg, R.G.; Ecker, A.H.; Vinci, C. A Biopsychosocial model of social anxiety and substance use. Depress. Anxiety 2012, 30, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Buckner, J.D.; Morris, P.E.; Abarno, C.N.; Glover, N.I.; Lewis, E.M. Biopsychosocial model social anxiety and substance use Revised. Curr. Psychiatry Rep. 2021, 23(6), 35. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. 2022 Canadian Cannabis Survey (CCS), Government of Canada. 2022. Available online: https://publications.gc.ca/collections/collection_2022/sc-hc/H21-312-2022-2-eng.pdf (accessed on 19 October 2023).
- Crippa, J.A.S.; Derenusson, G.N.; Ferrari, T.B.; Wichert-Ana, L.; Duran, F.L.; Martin-Santos, R.; Simões, M.V.; Bhattacharyya, S.; Fusar-Poli, P.; Atakan, Z.; et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report. J. Psychopharmacol. 2011, 25, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, M.M.; Queiroz, R.H.C.; Chagas, M.H.N.; de Oliveira, D.C.G.; De Martinis, B.S.; Kapczinski, F.; Quevedo, J.; Roesler, R.; Schröder, N.; E Nardi, A.; et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology 2011, 36, 1219–1226. [Google Scholar] [CrossRef]
- Masataka, N. Anxiolytic effects of repeated cannabidiol treatment in teenagers with social anxiety disorders. Front. Psychol. 2019, 10, 2466. [Google Scholar] [CrossRef]
- Elsaid, S.; Kloiber, S.; Le Foll, B. Effects of cannabidiol (CBD) in neuropsychiatric disorders: A review of pre-clinical and clinical findings. Prog. Mol. Biol. Transl. Sci. 2019, 167, 25–75. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, S.; Le Foll, B. The complexity of pharmacology of cannabidiol (CBD) and its implications in the treatment of brain disorders. Neuropsychopharmacology 2020, 45, 229–230. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.C.; Perry, E.; MacDougall, L.; Ammerman, Y.; Cooper, T.; Wu, Y.-T.; Braley, G.; Gueorguieva, R.; Krystal, J.H. The psychotomimetic effects of intravenous Delta-9-tetrahydrocannabinol in healthy individuals: Implications for psychosis. Neuropsychopharmacology 2004, 29, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.C.; Ranganathan, M.; Braley, G.; Gueorguieva, R.; Zimolo, Z.; Cooper, T.; Perry, E.; Krystal, J. Blunted Psychotomimetic and Amnestic Effects of Δ-9-Tetrahydrocannabinol in Frequent Users of Cannabis. Neuropsychopharmacology 2008, 33, 2505–2516. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, L.; Sinclair, J.; Kramer, A.; de Manincor, M.; Sarris, J. Cannabis, a cause for anxiety? A critical appraisal of the anxiogenic and anxiolytic properties. J. Transl. Med. 2020, 18, 374. [Google Scholar] [CrossRef] [PubMed]
- Wingo, T.; Nesil, T.; Choi, J.-S.; Li, M.D. Novelty seeking and drug addiction in humans and animals: From behavior to molecules. J. Neuroimmune Pharmacol. 2016, 11, 456–470. [Google Scholar] [PubMed]
- Zehra, A.; Burns, J.; Liu, C.K.; Manza, P.; Wiers, C.E.; Volkow, N.D.; Wang, G.-J. Cannabis addiction and the brain: A review. J. Neuroimmune Pharmacol. 2018, 13, 438–452. [Google Scholar] [CrossRef]
- Landmark, C.J.; Brandl, U. Pharmacology and drug interactions of cannabinoids. Epileptic Disord. 2020, 22, S16–S22. [Google Scholar] [CrossRef]
- Mason, N.L.; Theunissen, E.L.; Hutten, N.R.; Tse, D.H.; Toennes, S.W.; Jansen, J.F.; Stiers, P.; Ramaekers, J.G. Reduced responsiveness of the reward system is associated with tolerance to cannabis impairment in chronic users. Addict. Biol. 2021, 26, e12870. [Google Scholar] [CrossRef]
- Le Strat, Y.; Dubertret, C.; Le Foll, B. Impact of age at onset of cannabis use on cannabis dependence and driving under the influence in the United States. Accid. Anal. Prev. 2015, 76, 1–5. [Google Scholar] [CrossRef]
- Garrison, E.; Gilligan, C.; Ladd, B.O.; Anderson, K.G. Social Anxiety, Cannabis Use Motives, and Social Context’s Impact on Willingness to Use Cannabis. Int. J. Environ. Res. Public Health 2021, 18, 4882. [Google Scholar] [CrossRef]
- Edalati, H.; Conrod, P.J. A Review of Personality-Targeted Interventions for Prevention of Substance Misuse and Related Harm in Community Samples of Adolescents. Front. Psychiatry 2019, 9, 770. [Google Scholar] [CrossRef]
- Conrod, P.J.; O’leary-Barrett, M.; Newton, N.; Topper, L.; Castellanos-Ryan, N.; Mackie, C.; Girard, A. Effectiveness of a Selective, Personality-Targeted Prevention Program for Adolescent Alcohol Use and Misuse: A Cluster Randomized Controlled Trial. JAMA Psychiatry 2013, 70, 334–342. [Google Scholar] [CrossRef]
- Conrod, P.J.; Castellanos-Ryan, N.; Mackie, C. Long-term effects of a personality-targeted intervention to reduce alcohol use in adolescents. J. Consult. Clin. Psychol. 2011, 79, 296. [Google Scholar] [CrossRef] [PubMed]
- Meffert, B.N.; Morabito, D.M.; Mosich, M.K.; Loflin, M.J.; Sottile, J.; Heinz, A.J. Navigating Blind in the Green Rush: Clinical Considerations and Harm Reduction Practices for Cannabis. Curr. Drug Res. Rev. 2019, 11, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.A.; Pate, D.W.; Clark, R.D.; Davies, N.M.; El-Kadi, A.O.; Löbenberg, R. Phytocannabinoid drug-drug interactions and their clinical implications. Pharmacol. Ther. 2020, 215, 107621. [Google Scholar] [CrossRef]
- Kruger, D.J.; Kruger, J.S.; Collins, R.L. Frequent cannabis users demonstrate low knowledge of cannabinoid content and dosages. Drugs Educ. Prev. Policy 2021, 28, 97–103. [Google Scholar] [CrossRef]
- Buckner, J.D.; Schmidt, N.B.; Lang, A.R.; Small, J.W.; Schlauch, R.C.; Lewinsohn, P.M. Specificity of social anxiety disorder as a risk factor for alcohol and cannabis dependence. J. Psychiatr. Res. 2008, 42, 230–239. [Google Scholar] [CrossRef]
- Villarosa-Hurlocker, M.C.; Bravo, A.J.; Pearson, M.R.; Prince, M.A.; Madson, M.B.; Henson, J.M.; Looby, A.; Gonzalez, V.M.; Henslee, A.M.; Cuttler, C.; et al. The Relationship Between Social Anxiety and Alcohol and Marijuana Use Outcomes Among Concurrent Users: A Motivational Model of Substance Use. Alcohol. Clin. Exp. Res. 2019, 43, 732–740. [Google Scholar] [CrossRef] [PubMed]
| SAD Group (n = 26) | Control Group (n = 26) | |||||
|---|---|---|---|---|---|---|
| M or % | SD | M of % | SD | t or χ2 | d or Phi | |
| Age (years) | 27.92 | 7.34 | 27.35 | 6.69 | 0.30 | 0.08 |
| Age of onset of cannabis use | 16.63 | 3.15 | 20.12 | 4.74 | −3.12 * | 0.87 |
| Age of highest cannabis use | 23.05 | 8.48 | 23.02 | 4.50 | 0.02 | 0.01 |
| Sex (% female) | 53.8 | 50.0 | 0.08 | 0.04 | ||
| Race (% Caucasian) | 76.9 | 57.7 | 2.19 | 0.21 | ||
| Education (% university or higher) | 46.2 | 65.4 | 1.95 | 0.19 | ||
| Employed or student (%) | 73.1 | 92.3 | 3.36 | 0.25 | ||
| Last year’s income ≥ %50,000 | 40.0 | 66.7 | 3.50 | 0.27 | ||
| Married/common law (%) | 23.1 | 19.2 | 0.12 | 0.05 | ||
| Cannabis use (times/week) | 5.06 | 2.19 | 3.96 | 2.28 | 1.77 | 0.49 |
| Cannabis use (g/week) | 7.57 | 9.37 | 2.00 | 1.81 | 2.92 † | 0.82 |
| Alcohol use (drinks 1/week) | 2.22 | 3.78 | 1.93 | 1.85 | 0.35 | 0.10 |
| The severity of SAD (per total LSAS) | 92.65 | 20.82 | 9.46 | 6.52 | 19.45 ‡ | 5.39 |
| LSAS Anxiety scores | 48.92 | 9.63 | 6.27 | 4.58 | 20.39 ‡ | 5.66 |
| LSAS Avoidance scores | 43.73 | 12.05 | 3.19 | 3.46 | 16.49 ‡ | 4.57 |
| Cannabis problem severity (per CUPIT) | 36.54 | 12.35 | 26.31 | 9.80 | 3.31 † | 0.92 |
| SAD comorbidities (%): | ||||||
| Current CUD | 57.7 | 46.2 | 0.70 | 0.12 | ||
| Lifetime CUD | 80.8 | 53.8 | 4.28 † | 0.29 | ||
| Past MDD | 34.6 | |||||
| Current GAD | 26.9 | |||||
| Other 2 | 30.8 | |||||
| Dependent Variables | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive & Behavioral Impairment | Relaxation & Tension Reduction | Social & Sexual Facilitation | Perceptual & Cognitive Enhancement | Global Negative Effects | Craving & Physical Effects | Cumulative Negative Effects | Cumulative Positive Effects | |||||||||
|
Predictor variables (n = 52) | B (SE) | β | B (SE) | β | B (SE) | β | B (SE) | β | B (SE) | β | B (SE) | β | B (SE) | β | B (SE) | β |
| 1 SAD status | −0.14 (0.24) | −0.08 | −0.03 (0.17) | −0.03 | 0.21 (0.19) | 0.15 | 0.05 (0.16) | 0.04 | 0.05 (0.14) | 0.05 | −0.17 (0.18) | −0.12 | −0.05 (0.16) | −0.04 | 0.01 (0.13) | 0.01 |
| 1 Sex | −0.14 (0.24) | −0.08 | 0.05 (0.17) | 0.04 | 0.13 (0.19) | 0.10 | −0.13 (0.15) | −0.12 | 0.02 (0.14) | 0.14 | −0.17 (0.18) | −0.12 | −0.07 (0.16) | −0.05 | 0.06 (0.13) | 0.06 |
| Age | −0.06 (0.02) | −0.45 ‡ | 0.01 (0.01) | 0.07 | −0.01 (0.01) | −0.11 | −0.02 (0.01) | −0.25 | −0.02 (0.01) | −0.26 | −0.04 (0.01) | −0.44 † | −0.04 (0.01) | −0.44 † | −0.01 (0.01) | −0.20 |
| 1 Current CUD | 0.07 (0.24) | 0.04 | 0.28 (0.17) | 0.24 | 0.29 (0.19) | 0.22 | 0.26 (0.16) | 0.23 | 0.06 (0.14) | 0.06 | −0.01 (0.18) | −0.01 | 0.07 (0.17) | 0.05 | 0.19 (0.13) | 0.21 |
| Predictor Variables: (n = 52) | Dependent Variable: Problematic Cannabis Use (CUPIT) | |||||
|---|---|---|---|---|---|---|
| B | SE | β | R2 | Adj R2 | F | |
| SAD 1 | 9.92 | 2.82 | 0.41 ‡ | 0.60 | 0.31 | 6.67 ‡ |
| Age | 0.20 | 0.20 | 0.17 | |||
| Sex 1 | 3.98 | 2.81 | 0.11 | |||
| MEEQ Tension Reduction and Relaxation | 7.47 | 2.39 | 0.37 † | |||
| SAD 1 | 9.72 | 3.14 | 0.40 † | 0.48 | 0.23 | 3.54 † |
| Age | 0.23 | 0.23 | 0.23 | |||
| Sex 1 | 4.34 | 3.11 | 0.18 | |||
| MEEQ Social and Sexual Facilitation | 0.89 | 2.35 | 0.05 | |||
| SAD 1 | 9.81 | 3.10 | 0.41 † | 0.49 | 0.24 | 3.60 † |
| Age | 0.26 | 0.23 | 0.15 | |||
| Sex 1 | 4.67 | 3.10 | 0.19 | |||
| MEEQ Perceptual and Cognitive Enhancement | 1.64 | 2.85 | 0.08 | |||
| SAD 1 | 10.26 | 3.05 | 0.43 † | 0.51 | 0.26 | 4.06 † |
| Age | 0.37 | 0.25 | 0.21 | |||
| Sex 1 | 4.81 | 3.05 | 0.20 | |||
| MEEQ Cognitive Behavioral Impairment | 2.49 | 1.89 | 0.19 | |||
| SAD 1 | 9.69 | 3.04 | 0.40 † | 0.51 | 9.26 | 4.10 † |
| Age | 0.31 | 0.23 | 0.18 | |||
| Sex 1 | 4.40 | 3.04 | 0.18 | |||
| MEEQ Global Negative Effects | 4.38 | 3.30 | 0.18 | |||
| SAD 1 | 9.58 | 3.10 | 0.40 † | 0.49 | 0.24 | 3.71 † |
| Age | 0.13 | 0.25 | 0.08 | |||
| Sex 1 | 4.13 | 3.10 | 0.17 | |||
| MEEQ Craving and Physical Effects | −0.20 | 2.49 | −0.12 | |||
| SAD 1 | 9.80 | 3.06 | 0.41 † | 0.50 | 0.25 | 3.92 † |
| Age | 0.28 | 0.23 | 0.16 | |||
| Sex 1 | 4.20 | 3.06 | 0.17 | |||
| MEEQ Higher-order Positive Effects | 3.96 | 3.45 | 0.15 | |||
| SAD 1 | 10.11 | 3.02 | 0.42 † | 0.52 | 0.27 | 4.28 † |
| Age | 0.39 | 0.25 | 0.23 | |||
| Sex 1 | 4.73 | 3.02 | 0.20 | |||
| MEEQ Higher-order Negative Effects | 4.20 | 2.70 | 0.22 | |||
| (n = 26) | Age | Sex 1 | Current CUD 1 | Lifetime MDD 1 | Current GAD 1 | Educ. 1 | Race (WC) 1 | Race (EA) 1 | Income 1 | Occ. Status 1 | Marital Status 1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total LSAS | −0.16 | −0.06 | 0.28 | 0.24 | −0.16 | −0.09 | −0.18 | −0.03 | −0.29 | −0.26 | −0.18 |
| LSAS Anxiety | −0.28 | 0.03 | 0.30 | 0.30 | −0.11 | −0.01 | −0.22 | −0.06 | −0.23 | −0.21 | −0.24 |
| LSAS Avoidance | −0.06 | −0.12 | 0.24 | 0.17 | −0.18 | −0.15 | −0.14 | 0.01 | −0.31 | −0.29 | −0.12 |
| Weekly cannabis use frequency | 0.14 | 0.21 | 0.60 † | 0.18 | 0.33 | −0.31 | 0.55 † | 0.34 | −0.28 | −0.07 | −0.06 |
| The weekly amount of cannabis use (g) | 0.13 | 0.23 | 0.42 * | −0.01 | 0.30 | −0.38 | 0.24 | 0.18 | −0.35 | −0.09 | 0.01 |
| The weekly alcohol frequency (drinks 2/week) | 0.16 | −0.08 | −0.19 | −0.12 | −0.23 | −0.34 | 0.08 | −0.18 | −0.02 | 0.19 | 0.07 |
| CUPIT | 0.13 | 0.16 | 0.64 † | 0.19 | 0.35 | −0.35 | 0.27 | 0.20 | −0.55 † | −0.13 | −0.21 |
| MEEQ Total | −0.59 † | 0.07 | 0.19 | 0.03 | 0.12 | 0.34 | −0.06 | 0.05 | 0.07 | 0.05 | −0.51 † |
| MEEQ Cognitive & Behavioral Impairment | −0.44 * | −0.17 | 0.05 | −0.12 | 0.15 | 0.33 | −0.17 | −0.07 | 0.04 | 0.03 | −0.15 |
| MEEQ Relaxation & Tension Reduction | 0.01 | 0.18 | 0.21 | 0.04 | −0.06 | −0.18 | −0.03 | 0.12 | −0.21 | 0.09 | −0.52 † |
| MEEQ Social & Sexual Facilitation | −0.35 | 0.36 | 0.21 | 0.25 | 0.09 | 0.07 | 0.18 | 0.11 | 0.04 | 0.21 | −0.62 † |
| MEEQ Perceptual & Cognitive Enhancement | −0.35 | −0.06 | 0.12 | −0.07 | −0.07 | 0.20 | 0.17 | 0.28 | 0.12 | 0.12 | −0.53 † |
| MEEQ Global Negative Effects | −0.37 | −0.20 | 0.02 | 0.02 | −0.07 | 0.30 | −0.15 | −0.24 | 0.05 | −0.11 | 0.04 |
| MEEQ Craving & Physical Effects | −0.44 * | 0.05 | −0.03 | −0.12 | 0.02 | 0.50 * | −0.22 | 0.03 | 0.23 | −0.22 | 0.13 |
| MEEQ Cumulative Negative Effects | −0.46 * | −0.20 | 0.05 | −0.12 | 0.13 | 0.36 | −0.18 | −0.15 | 0.05 | −0.02 | −0.10 |
| MEEQ Cumulative Positive Effects | −0.32 | 0.37 | 0.15 | 0.20 | 0.04 | 0.10 | −0.12 | 0.13 | 0.08 | 0.08 | −0.52 † |
| (n = 26) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||
| 0.95 † | ||||||||||||||
| 0.97 † | 0.84 † | |||||||||||||
| 0.16 | 0.10 | 0.21 | ||||||||||||
| 0.28 | 0.24 | 0.30 | 0.56 † | |||||||||||
| 0.01 | −0.07 | 0.07 | 0.13 | −0.25 | ||||||||||
| 0.37 | 0.28 | 0.42 * | 0.77 † | 0.70 † | 0.04 | |||||||||
| 0.28 | 0.36 | 0.19 | −0.15 | −0.22 | −0.16 | −0.04 | ||||||||
| 0.01 | −0.01 | 0.01 | −0.30 | −0.38 | −0.04 | −0.13 | 0.72 † | |||||||
| 0.44 * | 0.49 * | 0.37 | 0.23 | 0.30 | −0.17 | 0.39 | 0.42 * | −0.10 | ||||||
| 0.25 | 0.40 * | 0.12 | 0.18 | 0.18 | −0.17 | 0.03 | 0.46 * | −0.18 | 0.56 † | |||||
| 0.05 | 0.07 | 0.03 | 0.06 | −0.22 | −0.08 | −0.06 | 0.72 † | 0.47 * | 0.39 | 0.28 | ||||
| 0.19 | 0.18 | 0.18 | −0.23 | −0.12 | 0.18 | −0.02 | 0.48 * | 0.56 † | −0.15 | −0.20 | 0.22 | |||
| 0.00 | 0.05 | −0.04 | −0.51 † | −0.53 † | −0.32 | −0.36 | 0.53 † | 0.62 † | −0.21 | −0.03 | 0.15 | 0.22 | ||
| 0.07 | 0.06 | 0.08 | −0.31 | −0.32 | 0.04 | −0.10 | 0.72 † | 0.95 † | −0.13 | −0.21 | 0.43 * | 0.79 † | 0.54 † | |
| 0.37 | 0.50 † | 0.25 | 0.06 | 0.07 | −0.28 | 0.06 | 0.63 † | 0.03 | 0.69 † | 0.88 † | 0.39 * | −0.19 | 0.25 | −0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsaid, S.; Wang, R.; Kloiber, S.; Haines-Saah, R.; Hassan, A.N.; Le Foll, B. Expectancies of the Effects of Cannabis Use in Individuals with Social Anxiety Disorder (SAD). Brain Sci. 2024, 14, 246. https://doi.org/10.3390/brainsci14030246
Elsaid S, Wang R, Kloiber S, Haines-Saah R, Hassan AN, Le Foll B. Expectancies of the Effects of Cannabis Use in Individuals with Social Anxiety Disorder (SAD). Brain Sciences. 2024; 14(3):246. https://doi.org/10.3390/brainsci14030246
Chicago/Turabian StyleElsaid, Sonja, Ruoyu Wang, Stefan Kloiber, Rebecca Haines-Saah, Ahmed N. Hassan, and Bernard Le Foll. 2024. "Expectancies of the Effects of Cannabis Use in Individuals with Social Anxiety Disorder (SAD)" Brain Sciences 14, no. 3: 246. https://doi.org/10.3390/brainsci14030246
APA StyleElsaid, S., Wang, R., Kloiber, S., Haines-Saah, R., Hassan, A. N., & Le Foll, B. (2024). Expectancies of the Effects of Cannabis Use in Individuals with Social Anxiety Disorder (SAD). Brain Sciences, 14(3), 246. https://doi.org/10.3390/brainsci14030246







