Can Brain Volume-Driven Characteristic Features Predict the Response of Alzheimer’s Patients to Repetitive Transcranial Magnetic Stimulation? A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI Data Acquisition
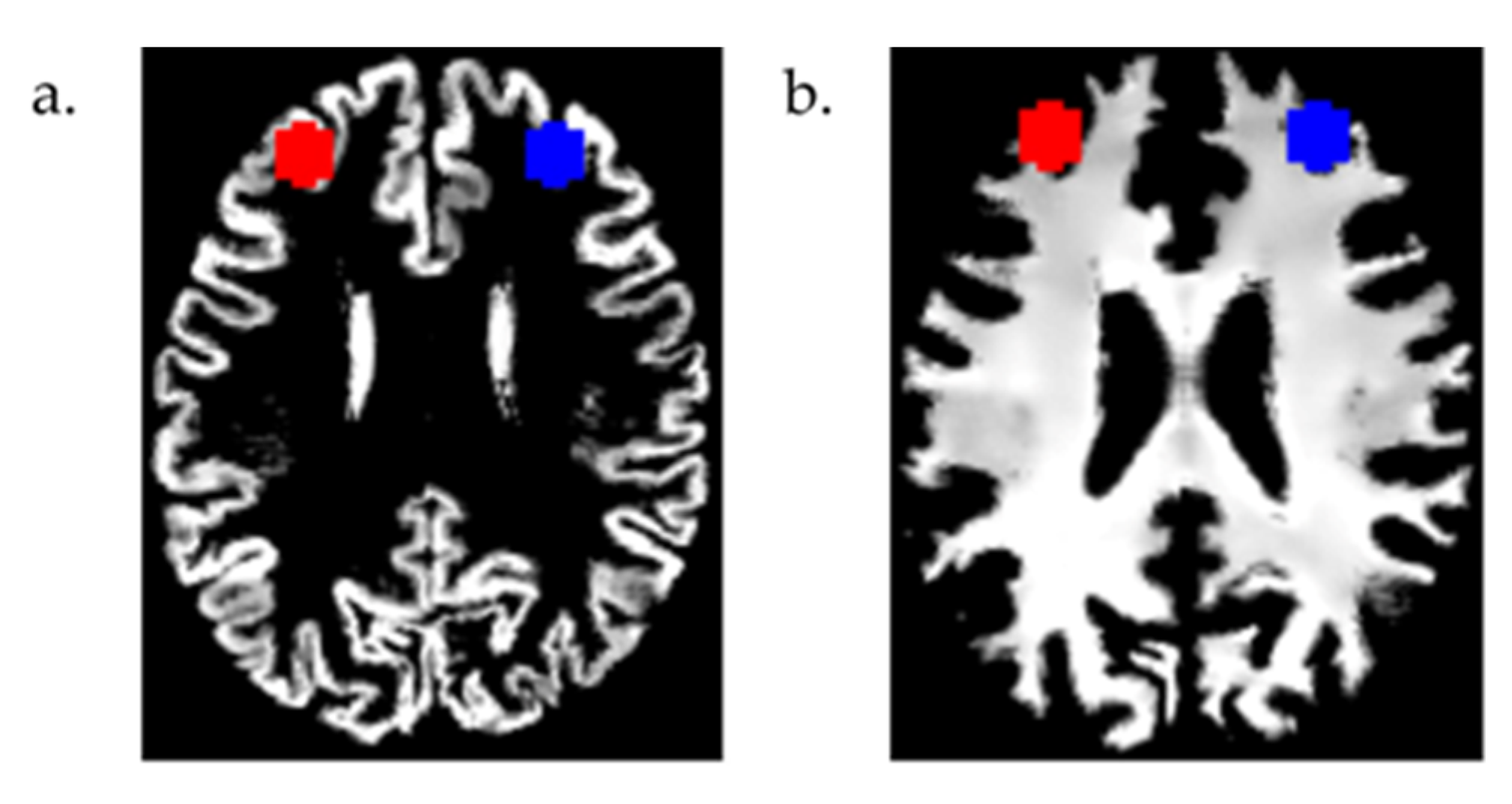
2.3. MRI Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants
3.2. Region of Interest (ROI) Analyses
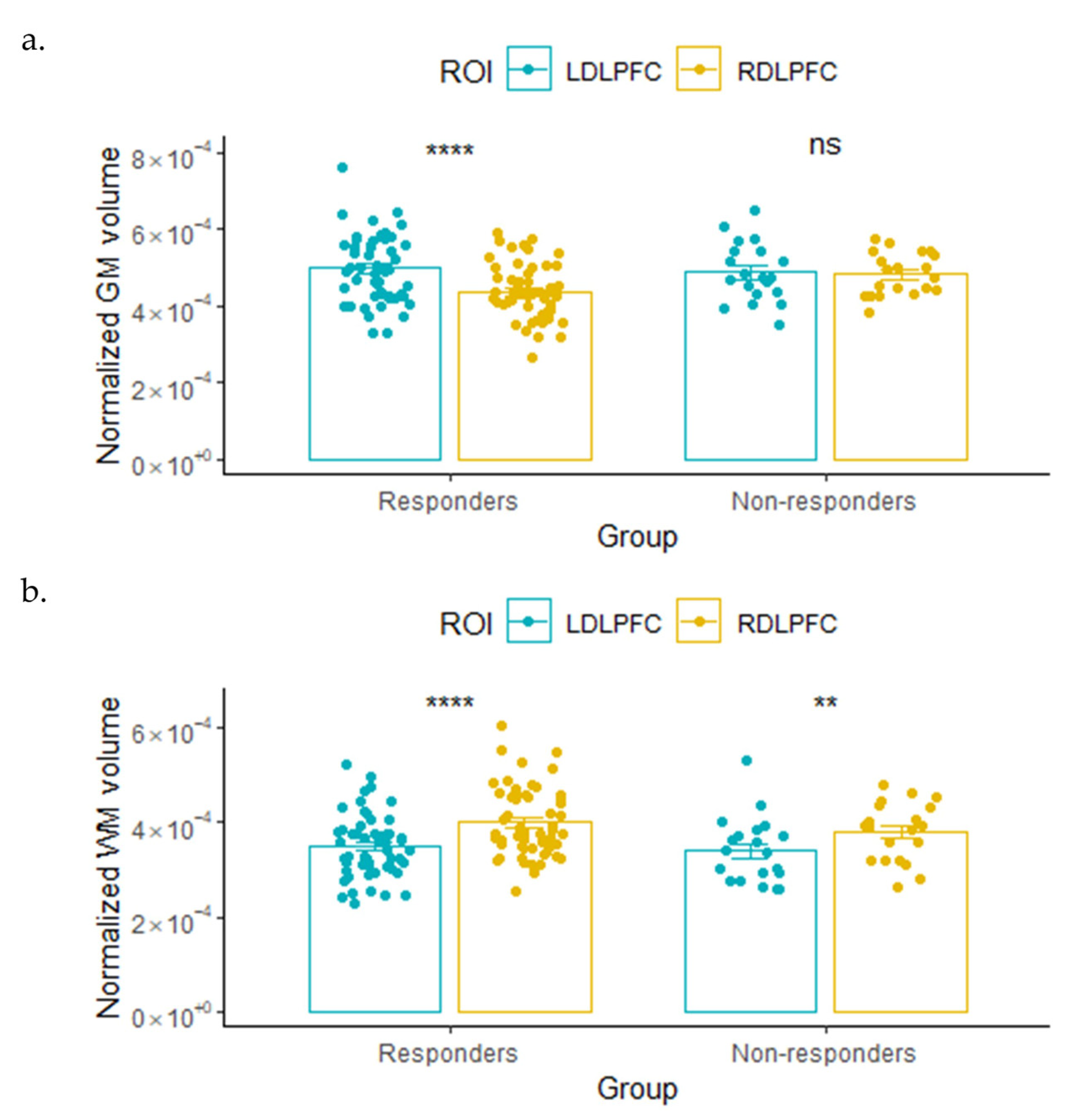
3.2.1. GM and WM Volume
3.2.2. Lateralization and Asymmetry Index
3.3. Predictive Classification Results
3.4. Whole-Brain Analysis Results
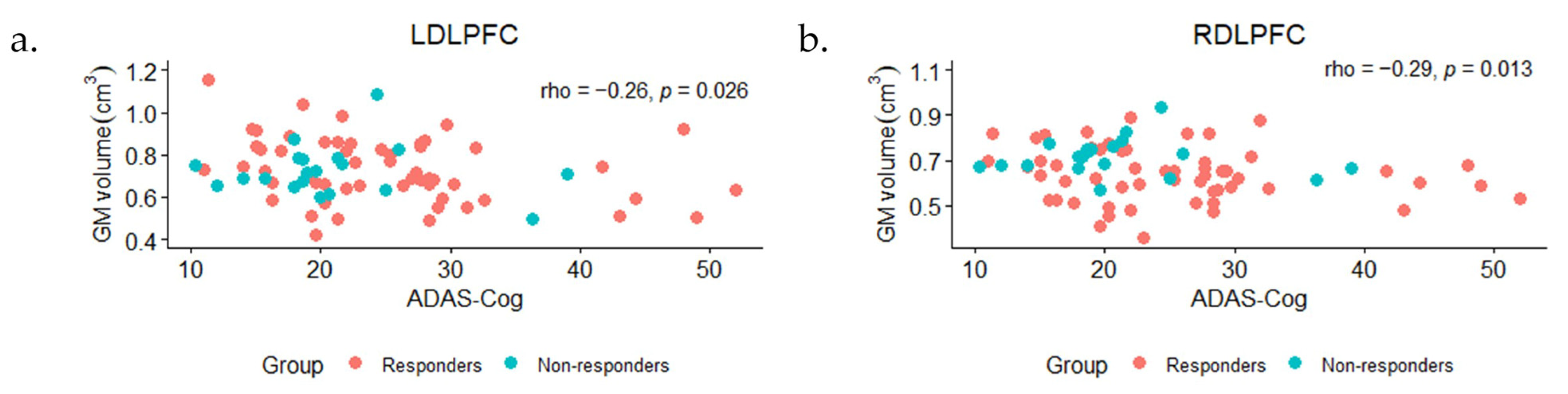
3.5. Correlations between ROI Volumes and Baseline ADAS-Cog Scores
3.6. Results after Adding the 12 Participants Who Received Active Treatment after the Sham 6–7 Months after the Baseline
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cotelli, M.; Manenti, R.; Cappa, S.F.; Geroldi, C.; Zanetti, O.; Rossini, P.M.; Miniussi, C. Effect of Transcranial Magnetic Stimulation on Action Naming in Patients with Alzheimer Disease. Arch. Neurol. 2006, 63, 1602. [Google Scholar] [CrossRef]
- Cotelli, M.; Calabria, M.; Manenti, R.; Rosini, S.; Zanetti, O.; Cappa, S.F.; Miniussi, C. Improved Language Performance in Alzheimer Disease Following Brain Stimulation. J. Neurol. Neurosurg. Psychiatry 2011, 82, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, G.; Lithgow, B.; Moussavi, Z. Short and Long-Term Effects of RTMS Treatment on Alzheimer’s Disease at Different Stages: A Pilot Study. J. Exp. Neurosci. 2015, 9, JEN.S24004. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Casula, E.P.; Bonnì, S.; Borghi, I.; Assogna, M.; Minei, M.; Pellicciari, M.C.; Motta, C.; D’Acunto, A.; Porrazzini, F.; et al. Precuneus Magnetic Stimulation for Alzheimer’s Disease: A Randomized, Sham-Controlled Trial. Brain 2022, 145, 3776–3786. [Google Scholar] [CrossRef]
- Rutherford, G.; Gole, R.; Moussavi, Z. RTMS as a Treatment of Alzheimer’s Disease with and without Comorbidity of Depression: A Review. Neurosci. J. 2013, 2013, 679389. [Google Scholar] [CrossRef] [PubMed]
- Weiler, M.; Stieger, K.C.; Long, J.M.; Rapp, P.R. Transcranial Magnetic Stimulation in Alzheimer’s Disease: Are We Ready? eNeuro 2020, 7, ENEURO.0235-19.2019. [Google Scholar] [CrossRef]
- Rabey, J.M.; Dobronevsky, E.; Aichenbaum, S.; Gonen, O.; Marton, R.G.; Khaigrekht, M. Repetitive Transcranial Magnetic Stimulation Combined with Cognitive Training Is a Safe and Effective Modality for the Treatment of Alzheimer’s Disease: A Randomized, Double-Blind Study. J. Neural Transm. 2013, 120, 813–819. [Google Scholar] [CrossRef]
- Moussavi, Z.; Uehara, M.; Rutherford, G.; Lithgow, B.; Millikin, C.; Wang, X.; Saha, C.; Mansouri, B.; Omelan, C.; Fellows, L.; et al. Repetitive Transcranial Magnetic Stimulation as a Treatment for Alzheimer’s Disease: A Randomized Placebo-Controlled Double-Blind Clinical Trial. Neurotherapeutics 2024, e00331. [Google Scholar] [CrossRef]
- Baxter, L.C.; Sparks, D.L.; Johnson, S.C.; Lenoski, B.; Lopez, J.E.; Connor, D.J.; Sabbagh, M.N. Relationship of Cognitive Measures and Gray and White Matter in Alzheimer’s Disease. J. Alzheimers Dis. 2006, 9, 253–260. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Z.; Li, K.; Li, Z.; Qi, Z.; Jin, Z.; Yao, L.; Chen, K. Voxel-Based Assessment of Gray and White Matter Volumes in Alzheimer’s Disease. Neurosci. Lett. 2010, 468, 146–150. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Bocchetta, M.; Chetelat, G.; Rabinovici, G.D.; de Leon, M.J.; Kaye, J.; Reiman, E.M.; Scheltens, P.; Barkhof, F.; Black, S.E.; et al. Imaging Markers for Alzheimer Disease: Which vs. How. Neurology 2013, 81, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, A.S.; Sun, S.; Taylor, C.; Ward, C.P.; Gamst, A.C.; Petersen, R.C.; Jack, C.R.; Aisen, P.S.; Thal, L.J. Volumetric MRI vs Clinical Predictors of Alzheimer Disease in Mild Cognitive Impairment. Neurology 2008, 70, 191–199. [Google Scholar] [CrossRef]
- Moussavi, Z.; Koski, L.; Fitzgerald, P.B.; Millikin, C.; Lithgow, B.; Jafari-Jozani, M.; Wang, X. Repeated Transcranial Magnetic Stimulation for Improving Cognition in Alzheimer Disease: Protocol for an Interim Analysis of a Randomized Controlled Trial. JMIR Res. Protoc. 2021, 10, e31183. [Google Scholar] [CrossRef]
- Guo, Y.; Dang, G.; Hordacre, B.; Su, X.; Yan, N.; Chen, S.; Ren, H.; Shi, X.; Cai, M.; Zhang, S.; et al. Repetitive Transcranial Magnetic Stimulation of the Dorsolateral Prefrontal Cortex Modulates Electroencephalographic Functional Connectivity in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 679585. [Google Scholar] [CrossRef] [PubMed]
- Hertrich, I.; Dietrich, S.; Blum, C.; Ackermann, H. The Role of the Dorsolateral Prefrontal Cortex for Speech and Language Processing. Front. Hum. Neurosci. 2021, 15, 645209. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.C.K.; Shum, D.; Toulopoulou, T.; Chen, E.Y.H. Assessment of Executive Functions: Review of Instruments and Identification of Critical Issues. Arch. Clin. Neuropsychol. 2008, 23, 201–216. [Google Scholar] [CrossRef]
- Moussavi, Z.; Rutherford, G.; Lithgow, B.; Millikin, C.; Modirrousta, M.; Mansouri, B.; Wang, X.; Omelan, C.; Fellows, L.; Fitzgerald, P.; et al. Repeated Transcranial Magnetic Stimulation for Improving Cognition in Patients with Alzheimer Disease: Protocol for a Randomized, Double-Blind, Placebo-Controlled Trial. JMIR Res. Protoc. 2021, 10, e25144. [Google Scholar] [CrossRef]
- Gaser, C.; Dahnke, R. CAT—A Computational Anatomy Toolbox for the Analysis of Structural MRI Data. HBM 2016, 2016, 336–348. [Google Scholar]
- Ashburner, J.; Friston, K.J. Unified Segmentation. Neuroimage 2005, 26, 839–851. [Google Scholar] [CrossRef]
- Manjón, J.V.; Coupé, P.; Martí-Bonmatí, L.; Collins, D.L.; Robles, M. Adaptive Non-Local Means Denoising of MR Images with Spatially Varying Noise Levels. J. Magn. Reson. Imaging 2010, 31, 192–203. [Google Scholar] [CrossRef]
- Rajapakse, J.C.; Giedd, J.N.; Rapoport, J.L. Statistical Approach to Segmentation of Single-Channel Cerebral MR Images. IEEE Trans. Med. Imaging 1997, 16, 176–186. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Diffeomorphic Registration Using Geodesic Shooting and Gauss–Newton Optimisation. Neuroimage 2011, 55, 954–967. [Google Scholar] [CrossRef]
- Pletzer, B.; Harris, T.A.; Hidalgo-Lopez, E. Subcortical Structural Changes along the Menstrual Cycle: Beyond the Hippocampus. Sci. Rep. 2018, 8, 8–13. [Google Scholar] [CrossRef]
- Brett, M.; Anton, J.-L.L.; Valabregue, R.; Poline, J.-B. Region of Interest Using an SPM Toolbox [Abstract]. In Proceedings of the 8th International Conferance on Functional of the Human Brain, Sendai, Japan, 2–6 June 2002; Available on CD-ROM in NeuroImage. Volume 16. No. 2. [Google Scholar]
- Masina, F.; Vallesi, A.; Di Rosa, E.; Semenzato, L.; Mapelli, D. Possible Role of Dorsolateral Prefrontal Cortex in Error Awareness: Single-Pulse TMS Evidence. Front. Neurosci. 2018, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, E.C.; Zilles, K.; Caspers, S.; Roski, C.; Kellermann, T.S.; Jakobs, O.; Langner, R.; Laird, A.R.; Fox, P.T.; Eickhoff, S.B. Is There One DLPFC in Cognitive Action Control? Evidence for Heterogeneity from Co-Activation-Based Parcellation. Cereb. Cortex 2013, 23, 2677–2689. [Google Scholar] [CrossRef] [PubMed]
- Rorden, C.; Brett, M. Stereotaxic Display of Brain Lesions. Behav. Neurol. 2000, 12, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.Z.; Postema, M.C.; Guadalupe, T.; de Kovel, C.; Boedhoe, P.S.W.; Hoogman, M.; Mathias, S.R.; van Rooij, D.; Schijven, D.; Glahn, D.C.; et al. Mapping Brain Asymmetry in Health and Disease through the ENIGMA Consortium. Hum. Brain Mapp. 2022, 43, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Minkova, L.; Habich, A.; Peter, J.; Kaller, C.P.; Eickhoff, S.B.; Klöppel, S. Gray Matter Asymmetries in Aging and Neurodegeneration: A Review and Meta-Analysis. Hum. Brain Mapp. 2017, 38, 5890–5904. [Google Scholar] [CrossRef] [PubMed]
- Büchel, C.; Raedler, T.; Sommer, M.; Sach, M.; Weiller, C.; Koch, M.A. White Matter Asymmetry in the Human Brain: A Diffusion Tensor MRI Study. Cereb. Cortex 2004, 14, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Liu, H.; Wang, L.; Zhu, Q.; Song, Z.; Chang, R.; Lv, H. A Voxel-Based Morphometric Study of Age- and Sex-Related Changes in White Matter Volume in the Normal Aging Brain. Neuropsychiatr. Dis. Treat. 2016, 12, 453–465. [Google Scholar] [CrossRef]
- Good, C.D.; Johnsrude, I.; Ashburner, J.; Henson, R.N.A.; Friston, K.J.; Frackowiak, R.S.J. Cerebral Asymmetry and the Effects of Sex and Handedness on Brain Structure: A Voxel-Based Morphometric Analysis of 465 Normal Adult Human Brains. Neuroimage 2001, 14, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Sarica, A.; Vasta, R.; Novellino, F.; Vaccaro, M.G.; Cerasa, A.; Quattrone, A. MRI Asymmetry Index of Hippocampal Subfields Increases through the Continuum from the Mild Cognitive Impairment to the Alzheimer’s Disease. Front. Neurosci. 2018, 12, 576. [Google Scholar] [CrossRef] [PubMed]
- Lehtola, S.J.; Tuulari, J.J.; Karlsson, L.; Parkkola, R.; Merisaari, H.; Saunavaara, J.; Lähdesmäki, T.; Scheinin, N.M.; Karlsson, H. Associations of Age and Sex with Brain Volumes and Asymmetry in 2–5-Week-Old Infants. Brain Struct. Funct. 2019, 224, 501–513. [Google Scholar] [CrossRef]
- Kim, H.-Y. Statistical Notes for Clinical Researchers: Analysis of Covariance (ANCOVA). Restor. Dent. Endod. 2018, 43, e43. [Google Scholar] [CrossRef]
- R Core Team: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021.
- RStudio Team. RStudio: Integrated Development Environment for R 2021; RStudio, Inc.: Boston, MA, USA, 2021. [Google Scholar]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority over-Sampling Technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- He, H. Imbalanced Learning. In Self-Adaptive Systems for Machine Intelligence; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 44–107. [Google Scholar]
- Rabinovici, G.D.; Seeley, W.W.; Kim, E.J.; Gorno-Tempini, M.L.; Rascovsky, K.; Pagliaro, T.A.; Allison, S.C.; Halabi, C.; Kramer, J.H.; Johnson, J.K.; et al. Distinct MRI Atrophy Patterns in Autopsy-Proven Alzheimer’s Disease and Frontotemporal Lobar Degeneration. Am. J. Alzheimer’s Dis. Other Dement. 2008, 22, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, L.; Tammewar, G.; Ayakta, N.; Baker, S.L.; Bejanin, A.; Boxer, A.L.; Gorno-Tempini, M.L.; Janabi, M.; Kramer, J.H.; Lazaris, A.; et al. Local and Distant Relationships between Amyloid, Tau and Neurodegeneration in Alzheimer’s Disease. Neuroimage Clin. 2018, 17, 452–464. [Google Scholar] [CrossRef]
- Wagner, T.; Eden, U.; Fregni, F.; Valero-Cabre, A.; Ramos-Estebanez, C.; Pronio-Stelluto, V.; Grodzinsky, A.; Zahn, M.; Pascual-Leone, A. Transcranial Magnetic Stimulation and Brain Atrophy: A Computer-Based Human Brain Model Study. Exp. Brain Res. 2008, 186, 539–550. [Google Scholar] [CrossRef]
- Anderkova, L.; Eliasova, I.; Marecek, R.; Janousova, E.; Rektorova, I. Grey Matter Atrophy in Mild Alzheimer’s Disease Impacts on Cognitive Effects of Noninvasive Brain Stimulation. Clin. Neurophysiol. 2016, 127, e28. [Google Scholar] [CrossRef]
- Esposito, S.; Trojsi, F.; Cirillo, G.; de Stefano, M.; Di Nardo, F.; Siciliano, M.; Caiazzo, G.; Ippolito, D.; Ricciardi, D.; Buonanno, D.; et al. Repetitive Transcranial Magnetic Stimulation (RTMS) of Dorsolateral Prefrontal Cortex May Influence Semantic Fluency and Functional Connectivity in Fronto-Parietal Network in Mild Cognitive Impairment (MCI). Biomedicines 2022, 10, 994. [Google Scholar] [CrossRef]
- Catani, M.; Allin, M.P.G.; Husain, M.; Pugliese, L.; Mesulam, M.M.; Murray, R.M.; Jones, D.K. Symmetries in Human Brain Language Pathways Correlate with Verbal Recall. Proc. Natl. Acad. Sci. USA 2007, 104, 17163–17168. [Google Scholar] [CrossRef]
- Zhou, H.; Tang, Y.; Yuan, Z. White Matter Asymmetries in Patients with Cerebral Small Vessel Disease. J. Integr. Neurosci. 2018, 17, 159–167. [Google Scholar] [CrossRef]
- Allendorfer, J.B.; Storrs, J.M.; Szaflarski, J.P. Changes in White Matter Integrity Follow Excitatory RTMS Treatment of Post-Stroke Aphasia. Restor. Neurol. Neurosci. 2012, 30, 103–113. [Google Scholar] [CrossRef]
- Peng, H.; Zheng, H.; Li, L.; Liu, J.; Zhang, Y.; Shan, B.; Zhang, L.; Yin, Y.; Liu, J.; Li, W.; et al. High-Frequency RTMS Treatment Increases White Matter FA in the Left Middle Frontal Gyrus in Young Patients with Treatment-Resistant Depression. J. Affect. Disord. 2012, 136, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Roiser, J.P.; Linden, D.E.; Gorno-Tempini, M.L.; Moran, R.J.; Dickerson, B.C.; Grafton, S.T. Minimum Statistical Standards for Submissions to Neuroimage: Clinical. Neuroimage Clin. 2016, 12, 1045–1047. [Google Scholar] [CrossRef]
- Wang, W.Y.; Yu, J.T.; Liu, Y.; Yin, R.H.; Wang, H.F.; Wang, J.; Tan, L.; Radua, J.; Tan, L. Voxel-Based Meta-Analysis of Grey Matter Changes in Alzheimer’s Disease. Transl. Neurodegener 2015, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Winston, G.P.; Bartlett, P.; de Tisi, J.; Duncan, J.S.; Focke, N.K. Voxel-Based Magnetic Resonance Image Postprocessing in Epilepsy. Epilepsia 2017, 58, 1653–1664. [Google Scholar] [CrossRef]
- Wei, C.; Gong, S.; Zou, Q.; Zhang, W.; Kang, X.; Lu, X.; Chen, Y.; Yang, Y.; Wang, W.; Jia, L.; et al. A Comparative Study of Structural and Metabolic Brain Networks in Patients with Mild Cognitive Impairment. Front. Aging Neurosci. 2021, 13, 774607. [Google Scholar] [CrossRef]
- Elder, G.J.; Taylor, J.P. Transcranial Magnetic Stimulation and Transcranial Direct Current Stimulation: Treatments for Cognitive and Neuropsychiatric Symptoms in the Neurodegenerative Dementias? Alzheimers Res. Ther. 2014, 6, 74. [Google Scholar] [CrossRef]
| Responders | Non-Responders | Two-Tailed p | |
|---|---|---|---|
| n | 55 | 20 | - |
| Sex (male, female) | 32, 23 | 12, 8 | 0.902 † |
| Age | 72.5 ± 7.9 | 76.2 ± 5.7 | 0.055 †† |
| Handedness (left, right) * | 2, 52 | 1, 19 | 0.680 † |
| CSDD | 4.3 ± 3.7 | 4.6 ± 2.6 | 0.362 ‡ |
| MoCA | 15.3 ± 5.2 | 16.2 ± 4.5 | 0.541 †† |
| CDR | 1.1 ± 0.3 | 1.2 ± 0.4 | 0.535 ‡ |
| ADAS-Cog | 25.2 ± 9.3 | 20.8 ± 7.0 | 0.031 ‡ |
| ROIs | Responders Mean ± SE | Non-Responders Mean ± SE | F * (1, 67) | p * |
|---|---|---|---|---|
| GM | ||||
| Left DLPFC | 0.73 ± 0.02 | 0.72 ± 0.03 | 0.15 | 0.698 |
| Right DLPFC | 0.64 ± 0.02 | 0.72 ± 0.02 | 8.82 | 0.004 |
| WM | ||||
| Left DLPFC | 0.51 ± 0.02 | 0.51 ± 0.03 | 0.03 | 0.859 |
| Right DLPFC | 0.59 ± 0.02 | 0.57 ± 0.03 | 0.14 | 0.713 |
| Volumes | Responders Mean ± SE | Non-Responders Mean ± SE | F * (1, 67) | p * |
|---|---|---|---|---|
| GM | 9.52 ± 0.86 | 5.06 ± 0.79 | 7.17 | 0.009 |
| WM | 9.24 ± 1.09 | 7.58 ± 1.39 | 0.99 | 0.324 |
| Features | AUC | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|
| GM volume | 0.71 | 0.67 | 0.62 | 0.65 |
| Asymmetry index | 0.70 | 0.63 | 0.72 | 0.66 |
| GM volume and asymmetry index | 0.74 | 0.65 | 0.77 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, C.; Figley, C.R.; Lithgow, B.; Fitzgerald, P.B.; Koski, L.; Mansouri, B.; Anssari, N.; Wang, X.; Moussavi, Z. Can Brain Volume-Driven Characteristic Features Predict the Response of Alzheimer’s Patients to Repetitive Transcranial Magnetic Stimulation? A Pilot Study. Brain Sci. 2024, 14, 226. https://doi.org/10.3390/brainsci14030226
Saha C, Figley CR, Lithgow B, Fitzgerald PB, Koski L, Mansouri B, Anssari N, Wang X, Moussavi Z. Can Brain Volume-Driven Characteristic Features Predict the Response of Alzheimer’s Patients to Repetitive Transcranial Magnetic Stimulation? A Pilot Study. Brain Sciences. 2024; 14(3):226. https://doi.org/10.3390/brainsci14030226
Chicago/Turabian StyleSaha, Chandan, Chase R. Figley, Brian Lithgow, Paul B. Fitzgerald, Lisa Koski, Behzad Mansouri, Neda Anssari, Xikui Wang, and Zahra Moussavi. 2024. "Can Brain Volume-Driven Characteristic Features Predict the Response of Alzheimer’s Patients to Repetitive Transcranial Magnetic Stimulation? A Pilot Study" Brain Sciences 14, no. 3: 226. https://doi.org/10.3390/brainsci14030226
APA StyleSaha, C., Figley, C. R., Lithgow, B., Fitzgerald, P. B., Koski, L., Mansouri, B., Anssari, N., Wang, X., & Moussavi, Z. (2024). Can Brain Volume-Driven Characteristic Features Predict the Response of Alzheimer’s Patients to Repetitive Transcranial Magnetic Stimulation? A Pilot Study. Brain Sciences, 14(3), 226. https://doi.org/10.3390/brainsci14030226







