Cognitive-Motor Training Improves Reading-Related Executive Functions: A Randomized Clinical Trial Study in Dyslexia
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Design
2.2. Study Measures
2.3. Training Programs
2.4. Analysis
3. Results
| Outcomes | TMT-A | TCT | PVFT | SVFT | SCT | SWT | SCWT | SCWI |
|---|---|---|---|---|---|---|---|---|
| BDS | 0.23 (0.241) | 0.42 * (0.030) | 0.39 * (0.046) | 0.30 (0.124) | 0.30 (0.882) | 0.02 (0.941) | −0.24 (0.235) | −0.25 (0.200) |
| TMT-A | - | 0.33 (0.241) | 0.18 (0.367) | 0.23 (0.243) | 0.02 (0.894) | 0.20 (0.307) | 0.16 (0.423) | 0.07 (0.745) |
| TCT | - | - | 0.36 (0.062) | 0.26 (0.183) | −0.08 (0.697) | −0.02 (0.916) | −0.49 * (0.010) | 0.50 * (0.008) |
| PVFT | - | - | - | 0.28 (0.157) | 0.00 (0.985) | 0.29 (0.149) | −0.02 (0.907) | −0.17 (0.403) |
| SVFT | - | - | - | - | −0.10 (0.604) | 0.21 (0.292) | −0.39 * (0.049) | 0.51 ** (0.007) |
| SCT | - | - | - | - | - | 0.70 ** (<0.001) | 0.54 ** (0.004) | 0.21 (0.289) |
| SWT | - | - | - | - | - | - | 0.33 (0.095) | −0.16 (0.430) |
| SCWT | - | - | - | - | - | - | - | 0.88 ** (<0.001) |
| Outcomes | NWRT | CWT | PDT |
|---|---|---|---|
| WRT | 0.39 * (0.048) | 0.72 ** (<0.001) | 0.46 * (0.017) |
| NWRT | - | 0.45 * (0.018) | 0.34 (0.086) |
| CWT | - | - | 0.44 * (0.021) |
4. Discussion
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. CONSORT Checklist
| Section/Topic | Item No | Checklist Item | Reported on Page No |
| Title and abstract | |||
| 1a | Identification as a randomized trial in the title | 1 | |
| 1b | Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts) | 1 | |
| Introduction | |||
| Background and objectives | 2a | Scientific background and explanation of rationale | 1–3 |
| 2b | Specific objectives or hypotheses | 3 | |
| Methods | |||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | 3–4 |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | N/A | |
| Participants | 4a | Eligibility criteria for participants | 4 |
| 4b | Settings and locations where the data were collected | 3 | |
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | 5–6 |
| Outcomes | 6a | Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed | 4–5 |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | N/A | |
| Sample size | 7a | How sample size was determined | 3 |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | N/A | |
| Randomization: | |||
| Sequence generation | 8a | Method used to generate the random allocation sequence | 4 |
| 8b | Type of randomization; details of any restriction (such as blocking and block size) | 4 | |
| Allocation concealment mechanism | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | 3–4 |
| Implementation | 10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions | 4 |
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how | 4 |
| 11b | If relevant, description of the similarity of interventions | 5–6 | |
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | 6–7 |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | 6–7 | |
| Results | |||
| Participant flow (a diagram is strongly recommended) | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analyzed for the primary outcome | Figure 1 |
| 13b | For each group, losses and exclusions after randomization, together with reasons | Figure 1 | |
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | N/A |
| 14b | Why the trial ended or was stopped | N/A | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | Table 1 |
| Numbers analyzed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | Figure 1 |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) | Figure 2, Figure 3, Figure 4 and Figure 5 Table 2 |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | Table 2 | |
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory | N/A |
| Harms | 19 | All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) | N/A |
| Discussion | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | 13 |
| Generalizability | 21 | Generalizability (external validity, applicability) of the trial findings | 13 |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | 7–13 |
| Other information | |||
| Registration | 23 | Registration number and name of trial registry | 13 |
| Protocol | 24 | Where the full trial protocol can be accessed, if available | 13 |
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders | 13 |
References
- Tunmer, W.; Greaney, K. Defining dyslexia. J. Learn. Disabil. 2010, 43, 229–243. [Google Scholar] [CrossRef]
- Ramezani, M.; Behzadipour, S.; Pourghayoomi, E.; Joghataei, M.T.; Shirazi, E.; Fawcett, A.J. Evaluating a new verbal working memory-balance program: A double-blind, randomized controlled trial study on Iranian children with dyslexia. BMC Neurosci. 2021, 22, 55. [Google Scholar] [CrossRef] [PubMed]
- Boyle, C.A.; Boulet, S.; Schieve, L.A.; Cohen, R.A.; Blumberg, S.J.; Yeargin-Allsopp, M.; Visser, S.; Kogan, M.D. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics 2011, 127, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Chiarenza, G.A. Motor-perceptual function in children with developmental reading disorders: Neuropsychophysiological analysis. J. Learn. Disabil. 1990, 23, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Chiarenza, G.A.; Di Pietro, S.F.; Casarotto, S. The psychophysiology of reading. Int. J. Psychophysiol. 2014, 94, 111–119. [Google Scholar] [CrossRef]
- Nicolson, R.I.; Fawcett, A.J. Automaticity: A new framework for dyslexia research? Cognition 1990, 35, 159–182. [Google Scholar] [CrossRef]
- Nicolson, R.I.; Fawcett, A.J. Comparison of deficits in cognitive and motor skills among children with dyslexia. Ann. Dyslexia 1994, 44, 147–164. [Google Scholar] [CrossRef]
- Lachmann, T.; van Leeuwen, C. Reading as functional coordination: Not recycling but a novel synthesis. Front. Psychol. 2014, 5, 1046. [Google Scholar] [CrossRef] [PubMed]
- Rusiak, P.; Lachmann, T.; Jaskowski, P.; van Leeuwen, C. Mental rotation of letters and shapes in developmental dyslexia. Perception 2007, 36, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Stein, J. What is developmental dyslexia? Brain Sci. 2018, 8, 26. [Google Scholar] [CrossRef]
- Stein, J. The magnocellular theory of developmental dyslexia. Dyslexia 2001, 7, 12–36. [Google Scholar] [CrossRef]
- Lachmann, T.; Berti, S.; Kujala, T.; Schröger, E. Diagnostic subgroups of developmental dyslexia have different deficits in neural processing of tones and phonemes. Int. J. Psychophysiol. 2005, 56, 105–120. [Google Scholar] [CrossRef]
- Lachmann, T.; van Leeuwen, C. Paradoxical enhancement of letter recognition in developmental dyslexia. Dev. Neuropsychol. 2007, 31, 61–77. [Google Scholar] [CrossRef]
- Nergård-Nilssen, T.; Hulme, C. Developmental dyslexia in adults: Behavioural manifestations and cognitive correlates. Dyslexia 2014, 20, 191–207. [Google Scholar] [CrossRef]
- Valdois, S.; Bosse, M.L.; Tainturier, M.J. The cognitive deficits responsible for developmental dyslexia: Review of evidence for a selective visual attentional disorder. Dyslexia 2004, 10, 339–363. [Google Scholar] [CrossRef]
- Ellis, A.W. The cognitive neuropsychology of developmental (and acquired) dyslexia: A critical survey. Cogn. Neuropsychol. 1985, 2, 169–205. [Google Scholar] [CrossRef]
- Butterfuss, R.; Kendeou, P. The role of executive functions in reading comprehension. Educ. Psychol. Rev. 2018, 30, 801–826. [Google Scholar] [CrossRef]
- Follmer, D.J. Executive function and reading comprehension: A meta-analytic review. Educ. Psychol. 2018, 53, 42–60. [Google Scholar] [CrossRef]
- Helland, T.; Asbjørnsen, A. Executive functions in dyslexia. Child Neuropsychol. 2000, 6, 37–48. [Google Scholar] [CrossRef]
- Meltzer, L. Executive Function in Education: From Theory to Practice; Guilford Publications: New York, NY, USA, 2018. [Google Scholar]
- Nouwens, S.; Groen, M.A.; Kleemans, T.; Verhoeven, L. How executive functions contribute to reading comprehension. Br. J. Educ. Psychol. 2021, 91, 169–192. [Google Scholar] [CrossRef]
- Pasqualotto, A.; Venuti, P. A multifactorial model of dyslexia: Evidence from executive functions and phonological-based treatments. Learn. Disabil. Res. Pract. 2020, 35, 150–164. [Google Scholar] [CrossRef]
- Smith-Spark, J.H.; Henry, L.A.; Messer, D.J.; Edvardsdottir, E.; Zięcik, A.P. Executive functions in adults with developmental dyslexia. Res. Dev. Disabil. 2016, 53, 323–341. [Google Scholar] [CrossRef]
- Lonergan, A.; Doyle, C.; Cassidy, C.; MacSweeney Mahon, S.; Roche, R.A.; Boran, L.; Bramham, J. A meta-analysis of executive functioning in dyslexia with consideration of the impact of comorbid ADHD. J. Cogn. Psychol. 2019, 31, 725–749. [Google Scholar] [CrossRef]
- Bater, L.R.; Jordan, S.S. Selective attention. In Encyclopedia of Personality and Individual Differences; Springer: Cham, Switzerland, 2019; pp. 1–4. [Google Scholar]
- Facoetti, A.; Paganoni, P.; Turatto, M.; Marzola, V.; Mascetti, G.G. Visual-spatial attention in developmental dyslexia. Cortex 2000, 36, 109–123. [Google Scholar] [CrossRef]
- Neill, W.T.; Valdes, L.A.; Terry, K.M. Selective attention and the inhibitory control of cognition. In Interference and Inhibition in Cognition; Academic Press: Cambridge, MA, USA, 1995; pp. 207–261. [Google Scholar]
- Wang, L.-C.; Tasi, H.-J.; Yang, H.-M. Cognitive inhibition in students with and without dyslexia and dyscalculia. Res. Dev. Disabil. 2012, 33, 1453–1461. [Google Scholar] [CrossRef]
- Wang, L.C.; Yang, H.M. Diverse inhibition and working memory of word recognition for dyslexic and typically developing children. Dyslexia 2015, 21, 162–176. [Google Scholar] [CrossRef]
- Van Reybroeck, M.; De Rom, M. Children with dyslexia show an inhibition domain-specific deficit in reading. Read. Writ. 2020, 33, 907–933. [Google Scholar] [CrossRef]
- Baddeley, A. Working memory. Science 1992, 255, 556–559. [Google Scholar] [CrossRef]
- Smith-Spark, J.H.; Fisk, J.E. Working memory functioning in developmental dyslexia. Memory 2007, 15, 34–56. [Google Scholar] [CrossRef]
- Smith-Spark, J.; Fisk, J.; Fawcett, A.; Nicolson, R. Investigating the central executive in adult dyslexics: Evidence from phonological and visuospatial working memory performance. Eur. J. Cogn. Psychol. 2003, 15, 567–587. [Google Scholar] [CrossRef]
- Berninger, V.W.; Raskind, W.; Richards, T.; Abbott, R.; Stock, P. A multidisciplinary approach to understanding developmental dyslexia within working-memory architecture: Genotypes, phenotypes, brain, and instruction. Dev. Neuropsychol. 2008, 33, 707–744. [Google Scholar] [CrossRef]
- Swanson, H.L. Working memory in learning disability subgroups. J. Exp. Child Psychol. 1993, 56, 87–114. [Google Scholar] [CrossRef] [PubMed]
- Menghini, D.; Finzi, A.; Carlesimo, G.A.; Vicari, S. Working memory impairment in children with developmental dyslexia: Is it just a phonological deficity? Dev. Neuropsychol. 2011, 36, 199–213. [Google Scholar] [CrossRef]
- Bowers, P.G.; Wolf, M. Theoretical links among naming speed, precise timing mechanisms and orthographic skill in dyslexia. Read. Writ. 1993, 5, 69–85. [Google Scholar] [CrossRef]
- Stefanac, N.; Spencer-Smith, M.; Brosnan, M.; Vangkilde, S.; Castles, A.; Bellgrove, M. Visual processing speed as a marker of immaturity in lexical but not sublexical dyslexia. Cortex 2019, 120, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, C.J.; Stein, J.F. A processing speed deficit in dyslexic adults? Evidence from a peg-moving task. Neurosci. Lett. 2006, 399, 264–267. [Google Scholar] [CrossRef]
- Badian, N.A. Dyslexia and the double deficit hypothesis. Ann. Dyslexia 1997, 47, 69–87. [Google Scholar] [CrossRef]
- Bexkens, A.; van den Wildenberg, W.P.; Tijms, J. Rapid automatized naming in children with dyslexia: Is inhibitory control involved? Dyslexia 2015, 21, 212–234. [Google Scholar] [CrossRef]
- Salthouse, T.A. The processing-speed theory of adult age differences in cognition. Psychol. Rev. 1996, 103, 403. [Google Scholar] [CrossRef]
- Vinogradov, S.; Kirkland, J.; Poole, J.H.; Drexler, M.; Ober, B.A.; Shenaut, G.K. Both processing speed and semantic memory organization predict verbal fluency in schizophrenia. Schizophr. Res. 2003, 59, 269–275. [Google Scholar] [CrossRef]
- Lengenfelder, J.; Bryant, D.; Diamond, B.J.; Kalmar, J.H.; Moore, N.B.; DeLuca, J. Processing speed interacts with working memory efficiency in multiple sclerosis. Arch. Clin. Neuropsychol. 2006, 21, 229–238. [Google Scholar] [CrossRef]
- Ojeda, N.; Peña, J.; Sánchez, P.; Elizagárate, E.; Ezcurra, J. Processing speed mediates the relationship between verbal memory, verbal fluency, and functional outcome in chronic schizophrenia. Schizophr. Res. 2008, 101, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-H.; Deng, C.; Hamilton, J.; Lee, C.S.-C.; Wei, W.; Georgiou, G.K. The role of rapid naming in reading development and dyslexia in Chinese. J. Exp. Child Psychol. 2015, 130, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; O’rourke, A.G.; Gidney, C.; Lovett, M.; Cirino, P.; Morris, R. The second deficit: An investigation of the independence of phonological and naming-speed deficits in developmental dyslexia. Read. Writ. 2002, 15, 43–72. [Google Scholar] [CrossRef]
- Elgamal, S.A.; Roy, E.A.; Sharratt, M.T. Age and verbal fluency: The mediating effect of speed of processing. Can. Geriatr. J. CGJ 2011, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Kirby, J.R.; Georgiou, G.K.; Martinussen, R.; Parrila, R. Naming speed and reading: From prediction to instruction. Read. Res. Q. 2010, 45, 341–362. [Google Scholar] [CrossRef]
- Johnston, T.C.; Kirby, J.R. The contribution of naming speed to the simple view of reading. Read. Writ. 2006, 19, 339–361. [Google Scholar] [CrossRef]
- Shareef, Z.; Östberg, P.; Hedenius, M. Verbal fluency in relation to reading ability in students with and without dyslexia. Appl. Psycholinguist. 2019, 40, 445–472. [Google Scholar] [CrossRef]
- Wolf, M. Naming speed and reading: The contribution of the cognitive neurosciences. Read. Res. Q. 1991, 26, 123–141. [Google Scholar] [CrossRef]
- Pellegrino, M.; Ben-Soussan, T.D.; Paoletti, P. A Scoping Review on Movement, Neurobiology and Functional Deficits in Dyslexia: Suggestions for a Three-Fold Integrated Perspective. Int. J. Environ. Res. Public Health 2023, 20, 3315. [Google Scholar] [CrossRef]
- Basharpoor, S.; Seif, E.; Narimani, M. Systematic review of studies related to executive functions in children with dyslexia in the Iranian studies (2001–2018). J. Learn. Disabil. 2022, 11, 33–46. [Google Scholar]
- Ben-Soussan, T.D.; Glicksohn, J.; Berkovich-Ohana, A. From cerebellar activation and connectivity to cognition: A review of the Quadrato Motor Training. BioMed Res. Int. 2015, 2015, 954901. [Google Scholar] [CrossRef] [PubMed]
- Goulème, N.; Gérard, C.-L.; Bucci, M.P. The effect of training on postural control in dyslexic children. PLoS ONE 2015, 10, e0130196. [Google Scholar] [CrossRef] [PubMed]
- Abbasi Fashami, N.; Akbari, B.; Hosseinkhanzadeh, A.A. Comparison of the effectiveness of cognitive rehabilitation and neurofeedback on improving the executive functions in children with dyslexia. Q. J. Child Ment. Health 2020, 7, 294–311. [Google Scholar] [CrossRef]
- Akyurek, G.; Efe, A.; Kilic, B.G.; Bumin, G. The effect of cognitive therapy on executive functions and occupational routines in children with dyslexia. Arch. Phys. Med. Rehabil. 2018, 99, e19. [Google Scholar] [CrossRef]
- Afshari, A.; Rezaei, R. The effectiveness of Sand Smart software on executive functions (focused attention, the ability to organize and plan, and auditory and visual work memory) in students with dyslexia. J. Learn. Disabil. 2019, 8, 26–48. [Google Scholar]
- Ramezani, M.; Behzadipour, S.; Fawcett, A.J.; Joghataei, M.T. Verbal Working Memory-Balance program training alters the left fusiform gyrus resting-state functional connectivity: A randomized clinical trial study on children with dyslexia. Dyslexia 2023, 29, 264–285. [Google Scholar] [CrossRef]
- Erickson, K.I.; Colcombe, S.J.; Wadhwa, R.; Bherer, L.; Peterson, M.S.; Scalf, P.E.; Kim, J.S.; Alvarado, M.; Kramer, A.F. Training-induced functional activation changes in dual-task processing: An FMRI study. Cereb. Cortex 2007, 17, 192–204. [Google Scholar] [CrossRef]
- Hiyamizu, M.; Morioka, S.; Shomoto, K.; Shimada, T. Effects of dual task balance training on dual task performance in elderly people: A randomized controlled trial. Clin. Rehabil. 2012, 26, 58–67. [Google Scholar] [CrossRef]
- Pantoja-Cardoso, A.; Aragão-Santos, J.C.; Santos, P.d.J.; Dos-Santos, A.C.; Silva, S.R.; Lima, N.B.C.; Vasconcelos, A.B.S.; Fortes, L.D.S.; Da Silva-Grigoletto, M.E. Functional Training and Dual-Task Training Improve the Executive Function of Older Women. Geriatrics 2023, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shin, W. How to do random allocation (randomization). Clin. Orthop. Surg. 2014, 6, 103–109. [Google Scholar] [CrossRef]
- Rostami, R.; Sadeghi, V.; Zarei, J.; Haddadi, P.; Mohazzab-Torabi, S.; Salamati, P. Concurrent validity of persian version of wechsler intelligence scale for children-and cognitive assessment system in patients with learning disorder. Iran. J. Pediatr. 2013, 23, 183. [Google Scholar] [PubMed]
- Mohamadesmaiel, E.; Alipour, A. A preliminary study on the reliability, validity and cut off points of the disorders of Children Symptom Inventory-4 (CSI-4). J. Except. Child. 2002, 2, 239–254. [Google Scholar]
- Moradi, A.R.; Hosaini, M.; Kormi-Nouri, R.; Hassani, J.; Parhoon, H. Reliability and validity of reading and dyslexia test (NEMA). Adv. Cogn. Sci. 2016, 18, 22–34. [Google Scholar]
- Veale, J.F. Edinburgh handedness inventory–short form: A revised version based on confirmatory factor analysis. Laterality Asymmetries Body Brain Cogn. 2014, 19, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Conners, F.A. Reading skills and cognitive abilities of individuals with mental retardation. Int. Rev. Res. Ment. Retard. 2003, 27, 191–229. [Google Scholar]
- Germanò, E.; Gagliano, A.; Curatolo, P. Comorbidity of ADHD and dyslexia. Dev. Neuropsychol. 2010, 35, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Duncan, L.G.; Johnston, R.S. How does phonological awareness relate to nonword reading skill amongst poor readers? Read. Writ. 1999, 11, 405–439. [Google Scholar]
- Li, R.; Qin, W.; Zhang, Y.; Jiang, T.; Yu, C. The neuronal correlates of digits backward are revealed by voxel-based morphometry and resting-state functional connectivity analyses. PLoS ONE 2012, 7, e31877. [Google Scholar] [CrossRef]
- Lefebvre, C.D.; Marchand, Y.; Eskes, G.A.; Connolly, J.F. Assessment of working memory abilities using an event-related brain potential (ERP)-compatible digit span backward task. Clin. Neurophysiol. 2005, 116, 1665–1680. [Google Scholar] [CrossRef]
- Llinàs-Reglà, J.; Vilalta-Franch, J.; López-Pousa, S.; Calvó-Perxas, L.; Torrents Rodas, D.; Garre-Olmo, J. The Trail Making Test: Association with other neuropsychological measures and normative values for adults aged 55 years and older from a Spanish-speaking population-based sample. Assessment 2017, 24, 183–196. [Google Scholar] [CrossRef]
- Sánchez-Cubillo, I.; Periáñez, J.A.; Adrover-Roig, D.; Rodríguez-Sánchez, J.M.; Ríos-Lago, M.; Tirapu, J.; Barceló, F. Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J. Int. Neuropsychol. Soc. 2009, 15, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Gustavson, D.E.; Panizzon, M.S.; Franz, C.E.; Reynolds, C.A.; Corley, R.P.; Hewitt, J.K.; Lyons, M.J.; Kremen, W.S.; Friedman, N.P. Integrating verbal fluency with executive functions: Evidence from twin studies in adolescence and middle age. J. Exp. Psychol. Gen. 2019, 148, 2104. [Google Scholar] [CrossRef]
- Shao, Z.; Janse, E.; Visser, K.; Meyer, A.S. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front. Psychol. 2014, 5, 772. [Google Scholar] [CrossRef] [PubMed]
- Henry, L.A.; Messer, D.J.; Nash, G. Executive functioning and verbal fluency in children with language difficulties. Learn. Instr. 2015, 39, 137–147. [Google Scholar] [CrossRef]
- Kraan, C.; Stolwyk, R.J.; Testa, R. The abilities associated with verbal fluency performance in a young, healthy population are multifactorial and differ across fluency variants. Appl. Neuropsychol. Adult 2013, 20, 159–168. [Google Scholar] [CrossRef]
- Johann, V.; Könen, T.; Karbach, J. The unique contribution of working memory, inhibition, cognitive flexibility, and intelligence to reading comprehension and reading speed. Child Neuropsychol. 2020, 26, 324–344. [Google Scholar] [CrossRef]
- De Rom, M.; Szmalec, A.; Van Reybroeck, M. The involvement of inhibition in word and sentence reading. Read. Writ. 2023, 36, 1283–1318. [Google Scholar] [CrossRef]
- Borella, E.; Ghisletta, P.; de Ribaupierre, A. Age differences in text processing: The role of working memory, inhibition, and processing speed. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2011, 66, 311–320. [Google Scholar] [CrossRef]
- Lazarus, P.J.; Ludwig, R.P.; Aberson, B. Stroop color-word test: A screening measure of selective attention to differentiate LD from non LD children. Psychol. Sch. 1984, 21, 53–60. [Google Scholar] [CrossRef]
- Scarpina, F.; Tagini, S. The stroop color and word test. Front. Psychol. 2017, 8, 557. [Google Scholar] [CrossRef]
- Pourghayoomi, E.; Behzadipour, S.; Ramezani, M.; Joghataei, M.T.; Shahidi, G.A. A new postural stability-indicator to predict the level of fear of falling in Parkinson’s disease patients. Biomed. Eng. Online 2020, 19, 64. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; SAGE Publications: Thousand Oaks, CA, USA, 2013. [Google Scholar]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed]
- Allen, M. The SAGE Encyclopedia of Communication Research Methods; SAGE Publications: Thousand Oaks, CA, USA, 2017. [Google Scholar]
- Ramezani, M.; Pourghayoomi, E.; Taghizadeh, G. Job requirements and physical demands (JRPD) questionnaire: Cross-cultural adaptation and psychometric evaluation in Iranian Army personnel with chronic low back pain. BMC Musculoskelet. Disord. 2022, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Kang, H. Sample size determination and power analysis using the G* Power software. J. Educ. Eval. Health Prof. 2021, 18, 1149215. [Google Scholar] [CrossRef] [PubMed]
- IBM Corporation. IBM SPSS Statistics for Windows; IBM Corporation: Armonk, NY, USA, 2012. [Google Scholar]
- Waters, G.S.; Caplan, D. The reliability and stability of verbal working memory measures. Behav. Res. Methods Instrum. Comput. 2003, 35, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A. Exploring the central executive. Q. J. Exp. Psychol. Sect. A 1996, 49, 5–28. [Google Scholar] [CrossRef]
- Case, R.; Kurland, D.M.; Goldberg, J. Operational efficiency and the growth of short-term memory span. J. Exp. Child Psychol. 1982, 33, 386–404. [Google Scholar] [CrossRef]
- Rohl, M.; Pratt, C. Phonological awareness, verbal working memory and the acquisition of literacy. Read. Writ. 1995, 7, 327–360. [Google Scholar] [CrossRef]
- Gathercole, S.E.; Alloway, T.P.; Willis, C.; Adams, A.-M. Working memory in children with reading disabilities. J. Exp. Child Psychol. 2006, 93, 265–281. [Google Scholar] [CrossRef]
- Periáñez, J.A.; Lubrini, G.; García-Gutiérrez, A.; Ríos-Lago, M. Construct validity of the stroop color-word test: Influence of speed of visual search, verbal fluency, working memory, cognitive flexibility, and conflict monitoring. Arch. Clin. Neuropsychol. 2021, 36, 99–111. [Google Scholar] [CrossRef]
- Demakis, G.J. Frontal lobe damage and tests of executive processing: A meta-analysis of the category test, stroop test, and trail-making test. J. Clin. Exp. Neuropsychol. 2004, 26, 441–450. [Google Scholar] [CrossRef]
- Tam, N.D. Improvement of processing speed in executive function immediately following an increase in cardiovascular activity. Cardiovasc. Psychiatry Neurol. 2013, 2013, 212767. [Google Scholar] [CrossRef]
- Lynch, S.G.; Dickerson, K.J.; Denney, D.R. Evaluating processing speed in multiple sclerosis: A comparison of two rapid serial processing measures. Clin. Neuropsychol. 2010, 24, 963–976. [Google Scholar] [CrossRef]
- Golden, C.; Freshwater, S.M.; Golden, Z. Stroop Color and Word Test; American Psychological Association: Washington, DC, USA, 1978. [Google Scholar]
- Cartwright, K.B. The role of cognitive flexibility in reading comprehension. In Handbook of Research on Reading Comprehension; Routledge: London, UK, 2009; pp. 115–139. [Google Scholar]
- Borella, E.; de Ribaupierre, A. The role of working memory, inhibition, and processing speed in text comprehension in children. Learn. Individ. Differ. 2014, 34, 86–92. [Google Scholar] [CrossRef]
- Kieffer, M.J.; Vukovic, R.K.; Berry, D. Roles of attention shifting and inhibitory control in fourth-grade reading comprehension. Read. Res. Q. 2013, 48, 333–348. [Google Scholar] [CrossRef]
- Palladino, P.; Cornoldi, C.; De Beni, R.; Pazzaglia, F. Working memory and updating processes in reading comprehension. Mem. Cogn. 2001, 29, 344–354. [Google Scholar] [CrossRef]
- Carretti, B.; Borella, E.; Cornoldi, C.; De Beni, R. Role of working memory in explaining the performance of individuals with specific reading comprehension difficulties: A meta-analysis. Learn. Individ. Differ. 2009, 19, 246–251. [Google Scholar] [CrossRef]
- Mella, N.; Fagot, D.; Lecerf, T.; de Ribaupierre, A. Working memory and intraindividual variability in processing speed: A lifespan developmental and individual-differences study. Mem. Cogn. 2015, 43, 340–356. [Google Scholar] [CrossRef] [PubMed]
- Levelt, W.J.; Roelofs, A.; Meyer, A.S. A theory of lexical access in speech production. Behav. Brain Sci. 1999, 22, 1–38. [Google Scholar] [CrossRef]
- Schmidt, M.; Mavilidi, M.F.; Singh, A.; Englert, C. Combining physical and cognitive training to improve kindergarten children’s executive functions: A cluster randomized controlled trial. Contemp. Educ. Psychol. 2020, 63, 101908. [Google Scholar] [CrossRef]
- Laatar, R.; Kachouri, H.; Borji, R.; Rebai, H.; Sahli, S. Combined physical-cognitive training enhances postural performances during daily life tasks in older adults. Exp. Gerontol. 2018, 107, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Shatil, E. Does combined cognitive training and physical activity training enhance cognitive abilities more than either alone? A four-condition randomized controlled trial among healthy older adults. Front. Aging Neurosci. 2013, 5, 8. [Google Scholar] [CrossRef]
- Fraizer, E.V.; Mitra, S. Methodological and interpretive issues in posture-cognition dual-tasking in upright stance. Gait Posture 2008, 27, 271–279. [Google Scholar] [CrossRef]
- Nicolson, R.I.; Fawcett, A.J.; Dean, P. Developmental dyslexia: The cerebellar deficit hypothesis. Trends Neurosci. 2001, 24, 508–511. [Google Scholar] [CrossRef]
- Biesbroek, J.M.; Lim, J.-S.; Weaver, N.A.; Arikan, G.; Kang, Y.; Kim, B.J.; Kuijf, H.J.; Postma, A.; Lee, B.-C.; Lee, K.-J. Anatomy of phonemic and semantic fluency: A lesion and disconnectome study in 1231 stroke patients. Cortex 2021, 143, 148–163. [Google Scholar] [CrossRef]
- Minamoto, T.; Osaka, M.; Osaka, N. Individual differences in working memory capacity and distractor processing: Possible contribution of top–down inhibitory control. Brain Res. 2010, 1335, 63–73. [Google Scholar] [CrossRef]
- Mangun, G.R.; Hopfinger, J.B.; Kussmaul, C.L.; Fletcher, E.M.; Heinze, H.J. Covariations in ERP and PET measures of spatial selective attention in human extrastriate visual cortex. Hum. Brain Mapp. 1997, 5, 273–279. [Google Scholar] [CrossRef]
- Heinze, H.; Mangun, G.R.; Burchert, W.; Hinrichs, H.; Scholz, M.; Münte, T.; Gös, A.; Scherg, M.; Johannes, S.; Hundeshagen, H. Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature 1994, 372, 543–546. [Google Scholar] [CrossRef]
- Küper, M.; Kaschani, P.; Thürling, M.; Stefanescu, M.; Burciu, R.; Göricke, S.; Maderwald, S.; Ladd, M.; Hautzel, H.; Timmann, D. Cerebellar fMRI activation increases with increasing working memory demands. Cerebellum 2016, 15, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, H.; Ma, L.; Wang, Y.; Chen, R.; Liu, N.; Men, W.; Tan, S.; Gao, J.-H.; Qin, S. Reduced volume of the left cerebellar lobule VIIb and its increased connectivity within the cerebellum predict more general psychopathology one year later via worse cognitive flexibility in children. Dev. Cogn. Neurosci. 2023, 63, 101296. [Google Scholar] [CrossRef]
- Richter, S.; Gerwig, M.; Aslan, B.; Wilhelm, H.; Schoch, B.; Dimitrova, A.; Gizewski, E.R.; Ziegler, W.; Karnath, H.-O.; Timmann, D. Cognitive functions in patients with MR-defined chronic focal cerebellar lesions. J. Neurol. 2007, 254, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Moroso, A.; Ruet, A.; Lamargue-Hamel, D.; Munsch, F.; Deloire, M.; Coupé, P.; Ouallet, J.-C.; Planche, V.; Moscufo, N.; Meier, D.S. Posterior lobules of the cerebellum and information processing speed at various stages of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2017, 88, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Tunmer, W.E.; Fletcher, C.M. The relationship between conceptual tempo, phonological awareness, and word recognition in beginning readers. J. Read. Behav. 1981, 13, 173–185. [Google Scholar] [CrossRef]
- McBride-Chang, C.; Cho, J.-R.; Liu, H.; Wagner, R.K.; Shu, H.; Zhou, A.; Cheuk, C.S.; Muse, A. Changing models across cultures: Associations of phonological awareness and morphological structure awareness with vocabulary and word recognition in second graders from Beijing, Hong Kong, Korea, and the United States. J. Exp. Child Psychol. 2005, 92, 140–160. [Google Scholar] [CrossRef]
- Aaron, P.; Joshi, R.; Ayotollah, M.; Ellsberry, A.; Henderson, J.; Lindsey, K. Decoding and sight-word naming: Are they independent components of word recognition skill? Read. Writ. 1999, 11, 89–127. [Google Scholar]
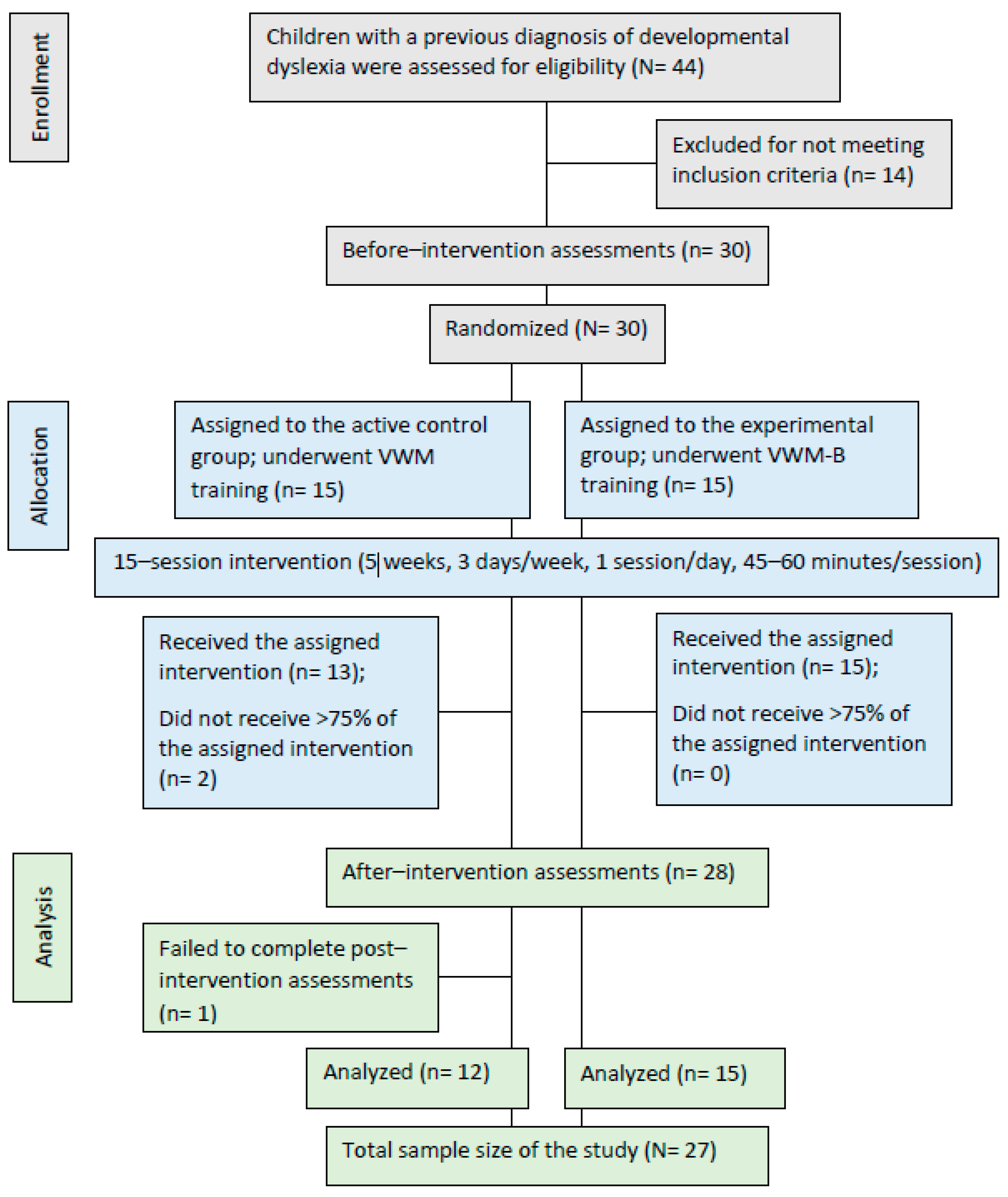

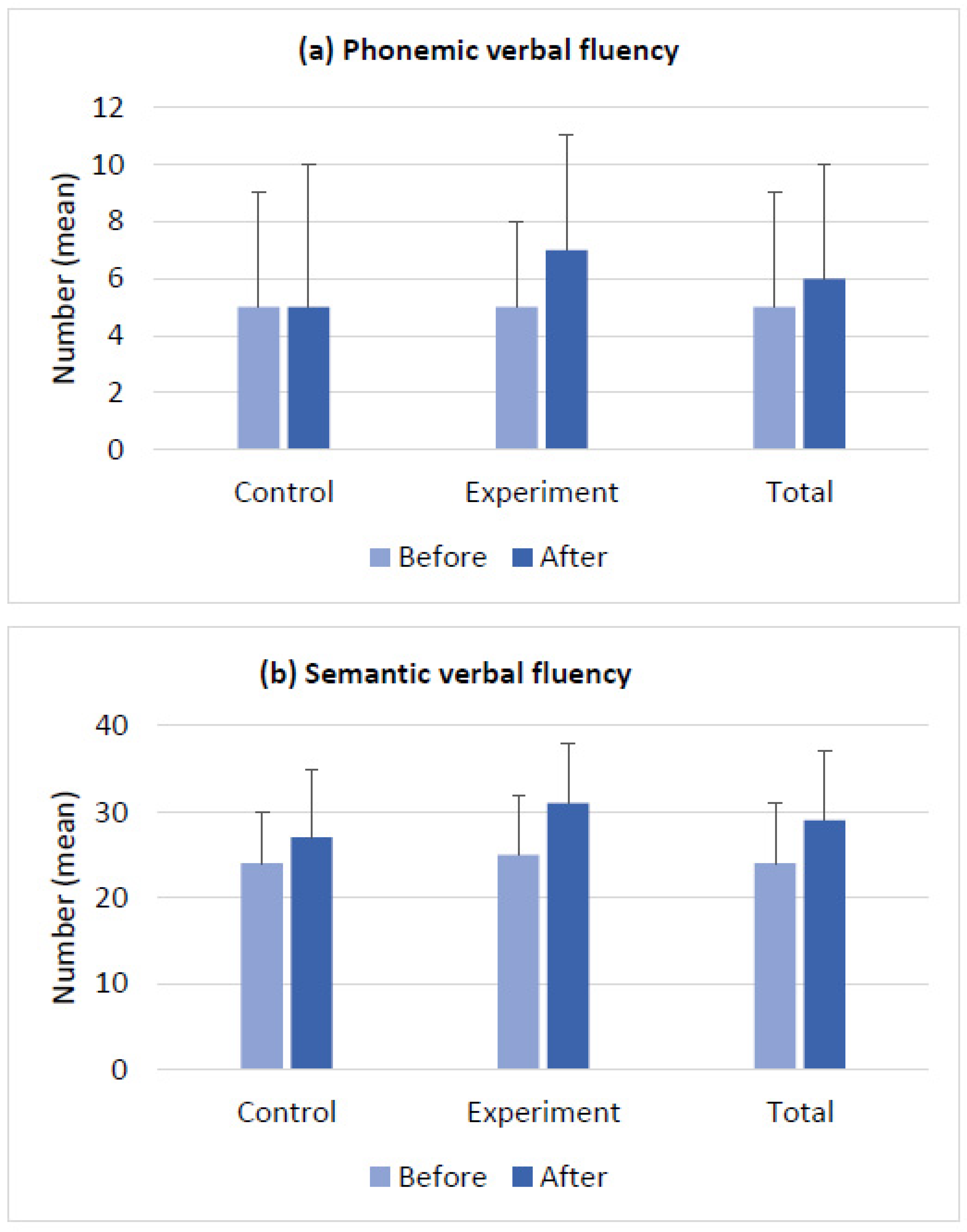

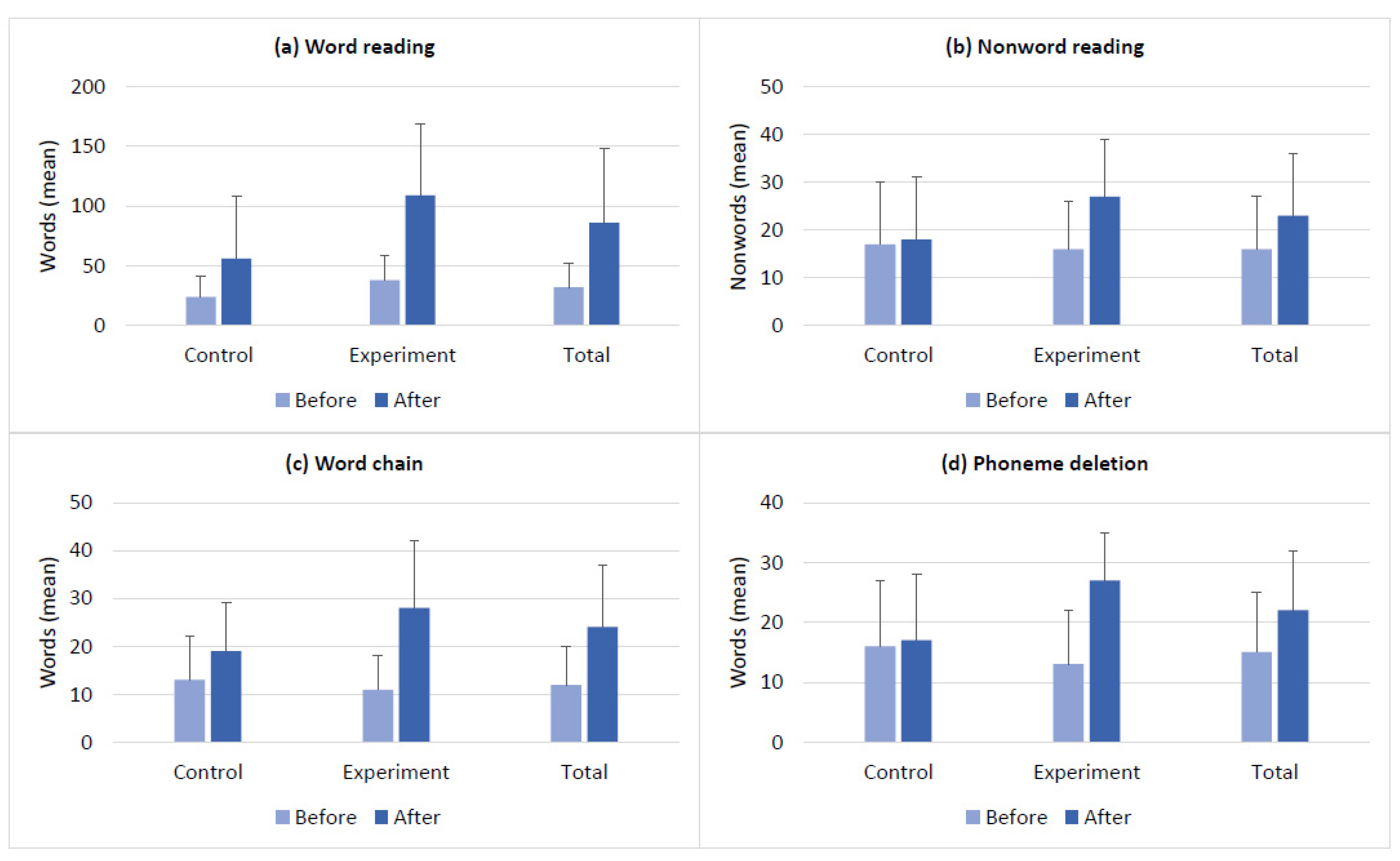
| Demography | Control (n = 12) | Experiment (n = 15) | Total (N = 27) | Group Differences (p-Value) | |
|---|---|---|---|---|---|
| Mean (SD) | |||||
| Age (y) | 9 (0.90) | 8 (0.74) | 9 (0.86) | u = 55.50 (0.065) | |
| IQ (WISC-IV total score) | 95 (6.89) | 94 (7.99) | 95 (1.42) | u = 82.00 (0.69) | |
| Attention (CSI-4, total scores of 1 to 18 items) | 5 (1.78) | 3 (2.46) | 4 (2.27) | u = 61.00 (0.146) | |
| Frequency (%) | |||||
| Gender | Boy | 4 (33.3) | 3 (20.0) | 7 (25.9) | χ2 = 0.617 (0.432) |
| Girl | 8 (66.7) | 12 (80.0) | 20 (74.1) | ||
| Disability | Reading | 3 (25.0) | 2 (13.3) | 5 (18.5) | χ2 = 4.78 (0.188) |
| Reading and writing | 4 (33.3) | 10 (66.7) | 14 (51.9) | ||
| Reading and math | 0 (0) | 1 (6.7) | 1 (3.7) | ||
| Reading, writing, and math | 5 (41.7) | 2 (13.3) | 7 (25.9) | ||
| School grade | Second | 4 (33.3) | 11 (73.3) | 15 (55.6) | χ2 = 4.47 (0.107) |
| Third | 3 (25.0) | 2 (13.3) | 5 (18.5) | ||
| Fourth | 5 (41.7) | 2 (13.3) | 7 (25.9) | ||
| Eyes condition | Normal | 11 (91.7) | 13 (86.7) | 24 (88.9) | χ2 = 0.169 (0.681) |
| Corrected | 1 (8.3) | 2 (13.3) | 3 (11.1) | ||
| Ears condition | Normal | 12 (100) | 14 (93.3) | 26 (96.3) | χ2 = 0.831 (0.362) |
| Corrected | 0 (0) | 1 (6.7) | 1 (3.7) | ||
| Outcomes | Time Effects | Group Effects | Time × Group Interaction | ||||
|---|---|---|---|---|---|---|---|
| f (1–25) | p-Value | f (1-25) | p-Value | f (1-25) | p-Value | Effect Size ηp2 | |
| BDS | 17.36 | <0.001 | 1.90 | 0.180 | 9.25 | 0.005 | 0.27 |
| TMT-A | 21.43 | <0.001 | 0.54 | 0.470 | 1.51 | 0.231 | 0.06 |
| TCT | 157.55 | <0.001 | 1.81 | 0.191 | 14.40 | 0.001 | 0.37 |
| PVFT | 10.13 | 0.004 | 0.55 | 0.466 | 5.02 | 0.034 | 0.18 |
| SVFT | 18.07 | <0.001 | 0.61 | 0.443 | 3.00 | 0.096 | 0.11 |
| SCT | 1.15 | 0.229 | 3.22 | 0.085 | 1.26 | 0.273 | 0.05 |
| SWT | 16.67 | <0.001 | 0.08 | 0.785 | 0.00 | 0.991 | 0.00 |
| SCWT | 17.70 | <0.001 | 1.20 | 0.283 | 9.15 | 0.006 | 0.27 |
| SCWI | 4.16 | 0.052 | 2.52 | 0.125 | 10.42 | 0.003 | 0.29 |
| WRT | 36.92 | <0.001 | 6.04 | 0.021 | 5.11 | 0.033 | 0.17 |
| NWRT | 26.57 | <0.001 | 1.28 | 0.268 | 8.76 | 0.007 | 0.26 |
| CWT | 52.85 | <0.001 | 0.99 | 0.328 | 12.90 | 0.002 | 0.33 |
| PDT | 54.50 | <0.001 | 3.25 | 0.083 | 12.82 | 0.001 | 0.34 |
| Outcomes | WRT | NWRT | CWT | PDT |
|---|---|---|---|---|
| BDS | 0.30 (0.129) | 0.31 (0.115) | 0.21 (0.304) | 0.56 ** (0.002) |
| TMT-A | 0.23 (0.242) | 0.30 (0.130) | 0.09 (0.650) | 0.11 (0.575) |
| TCT | 0.37 (0.057) | 0.42 * (0.029) | 0.63 ** (<0.001) | 0.47 * (0.015) |
| PVFT | 0.55 ** (0.003) | 0.12 (0.552) | 0.65 ** (<0.001) | 0.51 ** (0.007) |
| SVFT | 0.48 * (0.011) | 0.17 (0.394) | 0.39 * (0.042) | 0.25 (0.208) |
| SCT | −0.03 (0.865) | 0.11 (0.585) | −0.10 (0.639) | −0.01 (0.965) |
| SWT | 0.25 (0.218) | 0.11 (0.568) | 0.21 (0.290) | −0.07 (0.721) |
| SCWT | −0.15 (0.467) | −0.07 (0.733) | −0.40 * (0.037) | −0.28 (0.159) |
| SCWI | −0.28 (0.164) | −0.13 (0.520) | −0.53 ** (0.005) | −0.26 (0.199) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramezani, M.; Fawcett, A.J. Cognitive-Motor Training Improves Reading-Related Executive Functions: A Randomized Clinical Trial Study in Dyslexia. Brain Sci. 2024, 14, 127. https://doi.org/10.3390/brainsci14020127
Ramezani M, Fawcett AJ. Cognitive-Motor Training Improves Reading-Related Executive Functions: A Randomized Clinical Trial Study in Dyslexia. Brain Sciences. 2024; 14(2):127. https://doi.org/10.3390/brainsci14020127
Chicago/Turabian StyleRamezani, Mehdi, and Angela J. Fawcett. 2024. "Cognitive-Motor Training Improves Reading-Related Executive Functions: A Randomized Clinical Trial Study in Dyslexia" Brain Sciences 14, no. 2: 127. https://doi.org/10.3390/brainsci14020127
APA StyleRamezani, M., & Fawcett, A. J. (2024). Cognitive-Motor Training Improves Reading-Related Executive Functions: A Randomized Clinical Trial Study in Dyslexia. Brain Sciences, 14(2), 127. https://doi.org/10.3390/brainsci14020127






