Minimal Clinically Important Difference of Scales Reported in Stroke Trials: A Review
Abstract
1. Introduction
2. Literature Search
3. Statistical Significance of MCIDs in Clinical Trials
3.1. Trials Evaluating Superiority
3.2. Trials Evaluating Equivalence and Non-Inferiority
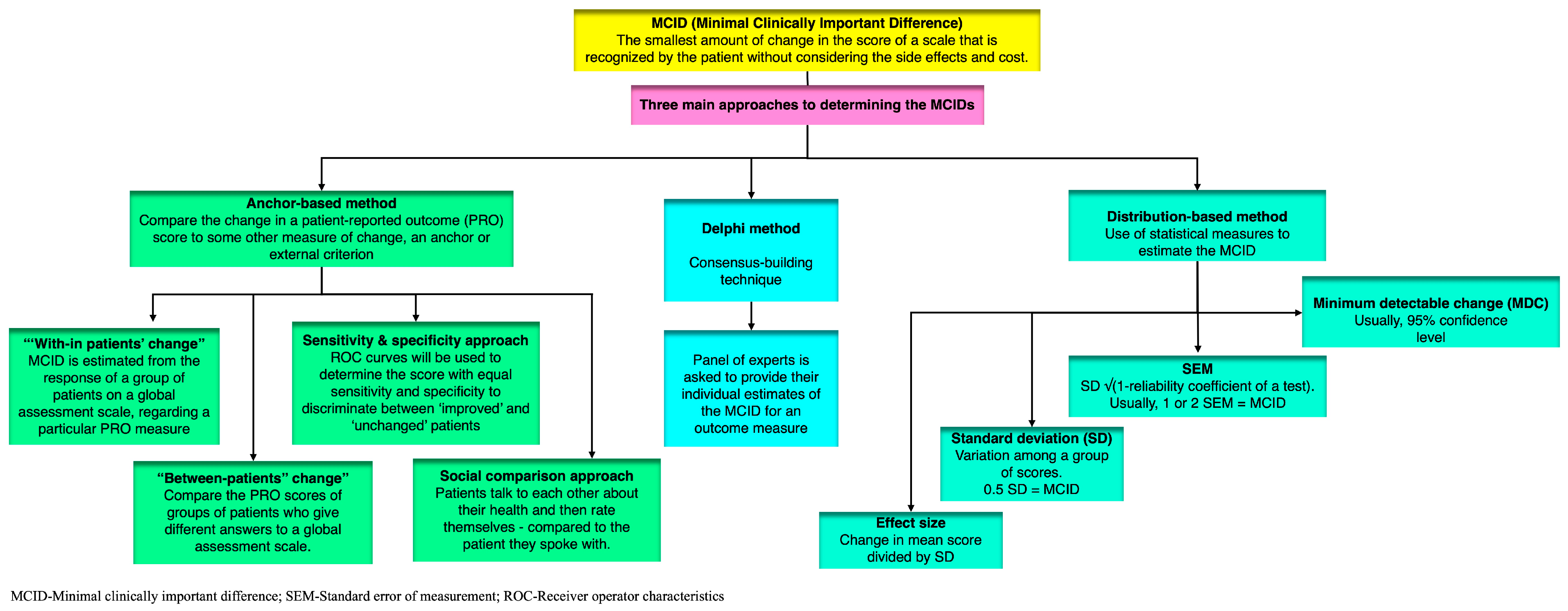
4. Definitions and Approaches to Estimate MCID
4.1. Anchor-Based Approach
- a.
- Objective anchors are based on physical measurements, such as the amount of pain medication a patient takes or the number of steps they can walk. These anchors are more reliable than subjective anchors, but they are not always available.
- b.
- Subjective anchors are based on the patient’s own assessment of their health, such as how much better or worse they feel. These anchors are less reliable than objective anchors, but they are more commonly used because they are easier to obtain.
- The amount of pain medication a patient takes;
- The number of steps a patient can walk;
- The patient’s functional status (e.g., their ability to bathe, dress, or walk);
- The patient’s quality of life.
- The patient’s global assessment of their health (e.g., “better”, “worse”, or “unchanged”) (e.g., patient global impression of change (PGIC) or global rating of change (GROC));
- The patient’s rating of their pain on a scale of 0 to 10;
- The patient’s rating of their overall health on a scale of 1 to 10;
- The clinician’s rating of the patient’s overall health on a scale of 1 to 10 (e.g., clinician global impression of change (CGIC)).
4.1.1. “With-in Patients” Score Change
4.1.2. “Between-Patients” Score Change
4.1.3. Sensitivity- and Specificity-Based Approach
4.1.4. Social Comparison Approach
4.2. Distribution-Based Approaches
4.2.1. SEM (Standard Error of Measurement)
4.2.2. MDC (Minimum Detectable Change)
4.2.3. SD (Standard Deviation)
4.2.4. Effect Size
4.3. The Delphi Method
4.4. Case Scenario: Hypothetical Illustration
- Method: Distribution-based method; estimated MCID: one-point change.
- Method: Anchor-based method.
- Method: Expert consensus/Delphi method.
4.5. Choice of Method Depending on Outcome Measure
4.6. Limitations of MCID
4.6.1. Multiplicity of MCID Determinations
4.6.2. Lack of Consideration for the Cost–Benefit Ratio
4.6.3. Challenges of Ordinal Scales in MCID Estimation
4.6.4. Changes in PRO Scores Are Linked to Baseline Scores
- a.
- Regression to the mean: extreme scores at baseline tend to move towards the average at follow-up.
- b.
- Floor and ceiling effects: scores near the ends of the scale cannot show large changes;
- c.
- a.
- Statistical control: this can eliminate the effect of baseline scores, but it may also mask true variation [37];
- b.
- Percent change: this can account for differences in baseline scores, but it can be affected by floor and ceiling effects [38];
- c.
- Range of MCID values: this can account for the fact that the meaning of change is not the same across all points on the scale.
- d.
- Percent change scores can correct for high baseline scores when high scores indicate a worse health status;
- e.
5. Overview of MCIDs in Scales Reported in Stroke Research
5.1. Modified Rankin Scale (mRS)
5.2. Barthel Index (BI)
5.3. Reperfusion Therapy (Substantial Reperfusion (TICI 2b-3))
5.4. Fugl-Meyer Assessment of the Upper Extremity (FMA-UE) and Fugl-Meyer Assessment of the Lower Extremity (FMA-LE)
5.5. Gait Speed
6. Applying the MCIDs Reported in this Review to Completed Clinical Trials
7. Discussion
- ▪
- Defining the MCID in animal experiments: identify animal outcome measures closely paralleling clinical MCIDs, focusing on functional outcomes relevant to human stroke, such as motor skills, cognition, and behavior;
- ▪
- Considering statistical approaches: employ statistical methods like mixed-effects models and within-subject designs to account for individual variability;
- ▪
- Increasing sample sizes: recruit larger animal cohorts to enhance statistical power and better represent population variability;
- ▪
- Utilizing homogeneous animal models: select animal models with reduced genetic and phenotypic diversity to minimize variability;
- ▪
- Prioritizing biological relevance: choose animal models that closely mimic human stroke pathophysiology and recovery processes;
- ▪
- Incorporating longitudinal assessments: track animal outcomes over time to capture meaningful changes and assess the durability of effects;
- ▪
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 10mWT | 10-Meter Walk Test |
| 5STS | Five-Repetition Sit-to-Stand Test |
| ADLs | Activities of Daily Living |
| AMAT | Arm Motor Ability Test |
| BBS | Berg Balance Scale |
| BI | Barthel Index |
| BMI | Body Mass Index |
| CGIC | Clinician Global Impression of Change |
| CGS | Comfortable Gait Speed |
| EBM | Evidence-Based Medicine |
| EVT | Endovascular Therapy |
| FAC | Functional Ambulation Categories |
| FIST | Function in Sitting Test |
| FMA-LE | Fugl-Meyer Assessment of the Lower Extremity |
| FMA-UE | Fugl-Meyer Assessment of the Upper Extremity |
| GROC | Global Rating of Change |
| GRPPC | Global Rating of Patient-Perceived Changes |
| HRQoL | Health-Related Quality of Life |
| MCID | Minimal Clinically Important Difference |
| MDC | Minimum Detectable Change |
| Mini-BESTest | Mini-Balance Evaluation Systems Test |
| mRS | Modified Rankin Scale |
| NEADL | Nottingham Extended Activities of Daily Living |
| PROM | Patient-Reported Outcome Measure |
| PRO | Patient-Reported Outcome |
| PROMs | Patient-Reported Outcome Measures |
| ROC | Receiver Operating Characteristic |
| SD | Standard Deviation |
| SEM | Standard Error of Measurement |
| SIS | Stroke Impact Scale |
| SIS-16 | Stroke Impact Scale-16 |
| STREAM | Stroke Rehabilitation Assessment of Movement |
| TICI | Thrombolysis in Cerebral Infarction |
| WGS | Wisconsin Gait Scale |
References
- Stang, A.; Poole, C.; Kuss, O. The ongoing tyranny of statistical significance testing in biomedical research. Eur. J. Epidemiol. 2010, 25, 225–230. [Google Scholar] [CrossRef]
- Vishnu, V.Y.; Vinny, P.W. Statistical Significance and Clinical Importance. Neurol. India 2021, 69, 1509. [Google Scholar]
- Copay, A.G.; Subach, B.R.; Glassman, S.D.; Polly, D.W.; Schuler, T.C. Understanding the minimum clinically important difference: A review of concepts and methods. Spine J. 2007, 7, 541–546. [Google Scholar] [CrossRef]
- Jaeschke, R.; Singer, J.; Guyatt, G.H. Measurement of health status: Ascertaining the minimal clinically important difference. Control. Clin. Trials 1989, 10, 407–415. [Google Scholar] [CrossRef]
- Kemmler, G.; Zabernigg, A.; Gattringer, K.; Rumpold, G.; Giesinger, J.; Sperner-Unterweger, B.; Holzner, B. A new approach to combining clinical relevance and statistical significance for evaluation of quality of life changes in the individual patient. J. Clin. Epidemiol. 2010, 63, 171–179. [Google Scholar] [CrossRef]
- Taylor-Rowan, M.; Wilson, A.; Dawson, J.; Quinn, T.J. Functional Assessment for Acute Stroke Trials: Properties, Analysis, and Application. Front. Neurol. 2018, 9, 191. Available online: https://www.frontiersin.org/articles/10.3389/fneur.2018.00191 (accessed on 26 February 2023). [CrossRef] [PubMed]
- Draak, T.H.P.; de Greef, B.T.A.; Faber, C.G.; Merkies, I.S.J.; PeriNomS study group. The minimum clinically important difference: Which direction to take. Eur. J. Neurol. 2019, 26, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; McDonough, R.; Fisher, M.; Ospel, J. The Challenge of Designing Stroke Trials That Change Practice: MCID vs. Sample Size and Pragmatism. J. Stroke 2022, 24, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Chuang-Stein, C.; Kirby, S.; Hirsch, I.; Atkinson, G. The role of the minimum clinically important difference and its impact on designing a trial. Pharm. Stat. 2011, 10, 250–256. [Google Scholar] [CrossRef]
- Lipscomb, J.; Gotay, C.C.; Snyder, C.F. Patient-reported Outcomes in Cancer: A Review of Recent Research and Policy Initiatives. CA A Cancer J. Clin. 2007, 57, 278–300. [Google Scholar] [CrossRef]
- Ousmen, A.; Touraine, C.; Deliu, N.; Cottone, F.; Bonnetain, F.; Efficace, F.; Brédart, A.; Mollevi, C.; Anota, A. Distribution- and anchor-based methods to determine the minimally important difference on patient-reported outcome questionnaires in oncology: A structured review. Health Qual. Life Outcomes 2018, 16, 228. [Google Scholar] [CrossRef] [PubMed]
- Stratford, P.W.; Binkley, J.M.; Riddle, D.L.; Guyatt, G.H. Sensitivity to change of the Roland-Morris Back Pain Questionnaire: Part 1. Phys. Ther. 1998, 78, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.L.; Hanscom, B.; Lurie, J.D.; Weinstein, J.N. Is a condition-specific instrument for patients with low back pain/leg symptoms really necessary? The responsiveness of the Oswestry Disability Index, MODEMS, and the SF-36. Spine 2003, 28, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Riddle, D.L.; Stratford, P.W.; Binkley, J.M. Sensitivity to change of the Roland-Morris Back Pain Questionnaire: Part 2. Phys. Ther. 1998, 78, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Osoba, D.; Wu, A.W.; Wyrwich, K.W.; Norman, G.R.; Clinical Significance Consensus Meeting Group. Methods to explain the clinical significance of health status measures. Mayo Clin. Proc. 2002, 77, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Norman, G.R.; Stratford, P.; Regehr, G. Methodological problems in the retrospective computation of responsiveness to change: The lesson of Cronbach. J. Clin. Epidemiol. 1997, 50, 869–879. [Google Scholar] [CrossRef]
- Hägg, O.; Fritzell, P.; Nordwall, A. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur. Spine J. 2003, 12, 12–20. [Google Scholar] [CrossRef]
- Juniper, E.F.; Guyatt, G.H.; Willan, A.; Griffith, L.E. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J. Clin. Epidemiol. 1994, 47, 81–87. [Google Scholar] [CrossRef]
- van der Roer, N.; Ostelo, R.W.J.G.; Bekkering, G.E.; van Tulder, M.W.; de Vet, H.C.W. Minimal clinically important change for pain intensity, functional status, and general health status in patients with nonspecific low back pain. Spine 2006, 31, 578–582. [Google Scholar] [CrossRef]
- Kulkarni, A.V. Distribution-based and anchor-based approaches provided different interpretability estimates for the Hydrocephalus Outcome Questionnaire. J. Clin. Epidemiol. 2006, 59, 176–184. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 1st ed.; Wiley: Hoboken, NJ, USA, 2000; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/0471722146 (accessed on 2 October 2023).
- Redelmeier, D.A.; Guyatt, G.H.; Goldstein, R.S. Assessing the minimal important difference in symptoms: A comparison of two techniques. J. Clin. Epidemiol. 1996, 49, 1215–1219. [Google Scholar] [CrossRef]
- Wyrwich, K.W.; Nienaber, N.A.; Tierney, W.M.; Wolinsky, F.D. Linking clinical relevance and statistical significance in evaluating intra-individual changes in health-related quality of life. Med. Care. 1999, 37, 469–478. [Google Scholar] [CrossRef]
- Wyrwich, K.W.; Tierney, W.M.; Wolinsky, F.D. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J. Clin. Epidemiol. 1999, 52, 861–873. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: Oxfordshire, UK, 2013; Available online: https://www.taylorfrancis.com/books/9781134742707 (accessed on 2 October 2023).
- Norman, G.R.; Sloan, J.A.; Wyrwich, K.W. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med. Care. 2003, 41, 582–592. [Google Scholar] [CrossRef]
- Samsa, G.; Edelman, D.; Rothman, M.L.; Williams, G.R.; Lipscomb, J.; Matchar, D. Determining clinically important differences in health status measures: A general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics 1999, 15, 141–155. [Google Scholar] [CrossRef]
- Watt, J.A.; Veroniki, A.A.; Tricco, A.C.; Straus, S.E. Using a distribution-based approach and systematic review methods to derive minimum clinically important differences. BMC Med. Res. Methodol. 2021, 21, 41. [Google Scholar] [CrossRef]
- Niederberger, M.; Spranger, J. Delphi Technique in Health Sciences: A Map. Front. Public Health 2020, 8, 457. Available online: https://www.frontiersin.org/articles/10.3389/fpubh.2020.00457 (accessed on 22 October 2023). [CrossRef]
- Mouelhi, Y.; Jouve, E.; Castelli, C.; Gentile, S. How is the minimal clinically important difference established in health-related quality of life instruments? Review of anchors and methods. Health Qual. Life Outcomes 2020, 18, 136. [Google Scholar] [CrossRef]
- Wang, Y.; Devji, T.; Carrasco-Labra, A.; King, M.T.; Terluin, B.; Terwee, C.B.; Walsh, M.; A Furukawa, T.; Guyatt, G.H. A step-by-step approach for selecting an optimal minimal important difference. BMJ 2023, 381, e073822. [Google Scholar] [CrossRef]
- Wright, A.; Hannon, J.; Hegedus, E.J.; Kavchak, A.E. Clinimetrics corner: A closer look at the minimal clinically important difference (MCID). J. Man. Manip. Ther. 2012, 20, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, M.; Boffa, A.; Pignotti, E.; Andriolo, L.; Zaffagnini, S.; Filardo, G. The Minimal Clinically Important Difference Changes Greatly Based on the Different Calculation Methods. Am. J. Sports Med. 2023, 51, 1067–1073. [Google Scholar] [CrossRef]
- Kirwan, J.R. Minimum clinically important difference: The crock of gold at the end of the rainbow? J. Rheumatol. 2001, 28, 439–444. [Google Scholar]
- Stevens, S.S. On the Theory of Scales of Measurement. Science 1946, 103, 677–680. [Google Scholar] [CrossRef]
- Farrar, J.T.; Portenoy, R.K.; Berlin, J.A.; Kinman, J.L.; Strom, B.L. Defining the clinically important difference in pain outcome measures. Pain 2000, 88, 287–294. [Google Scholar] [CrossRef]
- Beaton, D.E.; Boers, M.; Wells, G.A. Many faces of the minimal clinically important difference (MCID): A literature review and directions for future research. Curr. Opin. Rheumatol. 2002, 14, 109–114. [Google Scholar] [CrossRef]
- Bolton, J.E. Sensitivity and specificity of outcome measures in patients with neck pain: Detecting clinically significant improvement. Spine 2004, 29, 2410–2417, discussion 2418. [Google Scholar] [CrossRef]
- Tamura, S.; Miyata, K.; Kobayashi, S.; Takeda, R.; Iwamoto, H. The minimal clinically important difference in Berg Balance Scale scores among patients with early subacute stroke: A multicenter, retrospective, observational study. Top. Stroke Rehabil. 2021, 29, 423–429. [Google Scholar] [CrossRef]
- Beauchamp, M.K.; Niebuhr, R.; Roche, P.; Kirkwood, R.; Sibley, K.M. A prospective study to establish the minimal clinically important difference of the Mini-BESTest in individuals with stroke. Clin. Rehabil. 2021, 35, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Agustín, R.M.S.; Crisostomo, M.J.; Sánchez-Martínez, M.P.; Medina-Mirapeix, F. Responsiveness and Minimal Clinically Important Difference of the Five Times Sit-to-Stand Test in Patients with Stroke. Int. J. Environ. Res. Public Health 2021, 18, 2314. [Google Scholar] [CrossRef] [PubMed]
- Fu, V.; Weatherall, M.; McNaughton, H. Estimating the minimal clinically important difference for the Physical Component Summary of the Short Form 36 for patients with stroke. J. Int. Med. Res. 2021, 49, 3000605211067902. [Google Scholar] [CrossRef] [PubMed]
- Guzik, A.; Drużbicki, M.; Wolan-Nieroda, A.; Turolla, A.; Kiper, P. Estimating Minimal Clinically Important Differences for Knee Range of Motion after Stroke. J. Clin. Med. 2020, 9, 3305. [Google Scholar] [CrossRef] [PubMed]
- Alzyoud, J.; Medley, A.; Thompson, M.; Csiza, L. Responsiveness, minimal detectable change, and minimal clinically important difference of the sitting balance scale and function in sitting test in people with stroke. Physiother. Theory Pract. 2020, 38, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Everton, L.F.; Benfield, J.K.; Hedstrom, A.; Wilkinson, G.; Michou, E.; England, T.J.; Dziewas, R.; Bath, P.M.; Hamdy, S. Psychometric assessment and validation of the dysphagia severity rating scale in stroke patients. Sci. Rep. 2020, 10, 7268. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Saver, J.L. The Minimal Clinically Important Difference for Achievement of Substantial Reperfusion with Endovascular Thrombectomy Devices in Acute Ischemic Stroke Treatment. Front. Neurol. 2020, 11, 524220. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chen, C.-Y.; Chen, H.-C.; Wu, C.-Y.; Lin, K.-C.; Hsieh, Y.-W.; Shen, I.-H. Responsiveness and minimal clinically important difference of Modified Ashworth Scale in patients with stroke. Eur. J. Phys. Rehabil. Med. 2019, 55, 754–760. [Google Scholar] [CrossRef]
- Hiragami, S.; Inoue, Y.; Harada, K. Minimal clinically important difference for the Fugl-Meyer assessment of the upper extremity in convalescent stroke patients with moderate to severe hemiparesis. J. Phys. Ther. Sci. 2019, 31, 917–921. [Google Scholar] [CrossRef]
- Guzik, A.; Drużbicki, M.; Wolan-Nieroda, A.; Przysada, G.; Kwolek, A. The Wisconsin gait scale—The minimal clinically important difference. Gait Posture 2019, 68, 453–457. [Google Scholar] [CrossRef]
- Wu, C.Y.; Hung, S.J.; Lin, K.C.; Chen, K.H.; Chen, P.; Tsay, P.K. Responsiveness, Minimal Clinically Important Difference, and Validity of the MoCA in Stroke Rehabilitation. Occup. Ther. Int. 2019, 2019, 2517658. [Google Scholar] [CrossRef]
- Fulk, G.D.; He, Y. Minimal Clinically Important Difference of the 6-Minute Walk Test in People with Stroke. J. Neurol. Phys. Ther. 2018, 42, 235–240. [Google Scholar] [CrossRef]
- Chen, H.L.; Lin, K.C.; Hsieh, Y.W.; Wu, C.Y.; Liing, R.J.; Chen, C.L. A study of predictive validity, responsiveness, and minimal clinically important difference of arm accelerometer in real-world activity of patients with chronic stroke. Clin. Rehabil. 2018, 32, 75–83. [Google Scholar] [CrossRef]
- Song, M.J.; Lee, J.H.; Shin, W.S. Minimal Clinically Important Difference of Berg Balance Scale scores in people with acute stroke. Phys. Ther. Rehabil. Sci. 2018, 7, 102–108. [Google Scholar] [CrossRef]
- Cranston, J.S.; Kaplan, B.D.; Saver, J.L. Minimal Clinically Important Difference for Safe and Simple Novel Acute Ischemic Stroke Therapies. Stroke 2017, 48, 2946–2951. [Google Scholar] [CrossRef]
- New, P.W.; Scroggie, G.D.; Williams, C.M. The validity, reliability, responsiveness and minimal clinically important difference of the de Morton mobility index in rehabilitation. Disabil. Rehabil. 2017, 39, 1039–1043. [Google Scholar] [CrossRef]
- Lundquist, C.B.; Maribo, T. The Fugl-Meyer assessment of the upper extremity: Reliability, responsiveness and validity of the Danish version. Disabil. Rehabil. 2017, 39, 934–939. [Google Scholar] [CrossRef]
- Fulk, G.; Martin, R.; Page, S.J. Clinically Important Difference of the Arm Motor Ability Test in Stroke Survivors. Neurorehabil. Neural Repair. 2017, 31, 272–279. [Google Scholar] [CrossRef]
- Correa, K.P.; Devetak, G.F.; Martello, S.K.; de Almeida, J.C.; Pauleto, A.C.; Manffra, E.F. Reliability and Minimum Detectable Change of the Gait Deviation Index (GDI) in post-stroke patients. Gait Posture 2017, 53, 29–34. [Google Scholar] [CrossRef]
- Pandian, S.; Arya, K.N.; Kumar, D. Minimal clinically important difference of the lower-extremity fugl-meyer assessment in chronic-stroke. Top. Stroke Rehabil. 2016, 23, 233–239. [Google Scholar] [CrossRef]
- Chen, P.; Lin, K.C.; Liing, R.J.; Wu, C.Y.; Chen, C.L.; Chang, K.C. Validity, responsiveness, and minimal clinically important difference of EQ-5D-5L in stroke patients undergoing rehabilitation. Qual. Life Res. 2016, 25, 1585–1596. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, S.H.; Jo, M.W.; Lee, S.I. Estimation of minimally important differences in the EQ-5D and SF-6D indices and their utility in stroke. Health Qual. Life Outcomes 2015, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Andrews, A.W.; Glenney, S.S. Minimal clinically important difference for comfortable speed as a measure of gait performance in patients undergoing inpatient rehabilitation after stroke. J. Phys. Ther. Sci. 2013, 25, 1223–1225. [Google Scholar] [CrossRef] [PubMed]
- Page, S.J.; Fulk, G.D.; Boyne, P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys. Ther. 2012, 92, 791–798. [Google Scholar] [CrossRef]
- Arya, K.N.; Verma, R.; Garg, R.K. Estimating the minimal clinically important difference of an upper extremity recovery measure in subacute stroke patients. Top. Stroke Rehabil. 2011, 18 (Suppl. S1), 599–610. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Chuang, L.L.; Lin, K.C.; Lee, S.D.L.; Hong, W.H. Responsiveness, minimal detectable change, and minimal clinically important difference of the Nottingham Extended Activities of Daily Living Scale in patients with improved performance after stroke rehabilitation. Arch. Phys. Med. Rehabil. 2011, 92, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.N.; Lin, K.C.; Wu, C.Y.; Chung, C.Y.; Pei, Y.C.; Teng, Y.K. Validity, responsiveness, and clinically important difference of the ABILHAND questionnaire in patients with stroke. Arch. Phys. Med. Rehabil. 2011, 92, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Fulk, G.D.; Ludwig, M.; Dunning, K.; Golden, S.; Boyne, P.; West, T. Estimating clinically important change in gait speed in people with stroke undergoing outpatient rehabilitation. J. Neurol. Phys. Ther. 2011, 35, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-C.; Fu, T.; Wu, C.-Y.; Hsieh, C.-J. Assessing the stroke-specific quality of life for outcome measurement in stroke rehabilitation: Minimal detectable change and clinically important difference. Health Qual. Life Outcomes 2011, 9, 5. [Google Scholar] [CrossRef]
- Fulk, G.D.; Ludwig, M.; Dunning, K.; Golden, S.; Boyne, P.; West, T. How much change in the stroke impact scale-16 is important to people who have experienced a stroke? Top. Stroke Rehabil. 2010, 17, 477–483. [Google Scholar] [CrossRef]
- Tilson, J.K.; Sullivan, K.J.; Cen, S.Y.; Rose, D.K.; Koradia, C.H.; Azen, S.P.; Duncan, P.W.; Locomotor Experience Applied Post Stroke (LEAPS) Investigative Team. Meaningful gait speed improvement during the first 60 days poststroke: Minimal clinically important difference. Phys. Ther. 2010, 90, 196–208. [Google Scholar] [CrossRef]
- Hsieh, Y.W.; Wang, C.H.; Sheu, C.F.; Hsueh, I.P.; Hsieh, C.L. Estimating the minimal clinically important difference of the Stroke Rehabilitation Assessment of Movement measure. Neurorehabilit. Neural Repair. 2008, 22, 723–727. [Google Scholar] [CrossRef]
- Lang, C.E.; Edwards, D.F.; Birkenmeier, R.L.; Dromerick, A.W. Estimating minimal clinically important differences of upper-extremity measures early after stroke. Arch. Phys. Med. Rehabil. 2008, 89, 1693–1700. [Google Scholar] [CrossRef]
- Hsieh, Y.W.; Wang, C.H.; Wu, S.C.; Chen, P.C.; Sheu, C.F.; Hsieh, C.L. Establishing the minimal clinically important difference of the Barthel Index in stroke patients. Neurorehabilit. Neural Repair. 2007, 21, 233–238. [Google Scholar] [CrossRef]
- Beninato, M.; Gill-Body, K.M.; Salles, S.; Stark, P.C.; Black-Schaffer, R.M.; Stein, J. Determination of the minimal clinically important difference in the FIM instrument in patients with stroke. Arch. Phys. Med. Rehabil. 2006, 87, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L.; Chaisinanunkul, N.; Campbell, B.C.V.; Grotta, J.C.; Hill, M.D.; Khatri, P.; Landen, J.; Lansberg, M.G.; Venkatasubramanian, C.; Albers, G.W.; et al. Standardized Nomenclature for Modified Rankin Scale Global Disability Outcomes: Consensus Recommendations From Stroke Therapy Academic Industry Roundtable XI. Stroke 2021, 52, 3054–3062. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.; Tilley, B.C. 63—Conduct of Stroke-Related Clinical Trials. In Stroke, 6th ed.; Grotta, J.C., Albers, G.W., Broderick, J.P., Kasner, S.E., Lo, E.H., Mendelow, A.D., Sacco, R.L., Wong, L.K.S., Eds.; Elsevier: London, UK, 2016; pp. 1030–1041. Available online: https://www.sciencedirect.com/science/article/pii/B9780323295444000633 (accessed on 5 May 2023).
- Ganesh, A.; Luengo-Fernandez, R.; Wharton, R.M.; Rothwell, P.M. Ordinal vs dichotomous analyses of modified Rankin Scale, 5-year outcome, and cost of stroke. Neurology 2018, 91, e1951–e1960. [Google Scholar] [CrossRef]
- Banks, J.L.; Marotta, C.A. Outcomes Validity and Reliability of the Modified Rankin Scale: Implications for Stroke Clinical Trials. Stroke 2007, 38, 1091–1096. [Google Scholar] [CrossRef]
- Quinn, T.J.; Dawson, J.; Walters, M.R.; Lees, K.R. Reliability of the Modified Rankin Scale. Stroke 2009, 40, 3393–3395. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Widimsky, P.; Snyder, K.; Sulzenko, J.; Hopkins, L.N.; Stetkarova, I. Acute ischaemic stroke: Recent advances in reperfusion treatment. Eur. Heart J. 2022, 44, 1205–1215. [Google Scholar] [CrossRef]
- Ansari, J.; Triay, R.; Kandregula, S.; Adeeb, N.; Cuellar, H.; Sharma, P. Endovascular Intervention in Acute Ischemic Stroke: History and Evolution. Biomedicines 2022, 10, 418. [Google Scholar] [CrossRef]
- Michaelsen, S.M.; Rocha, A.S.; Knabben, R.J.; Rodrigues, L.P.; Fernandes, C.G.C. Translation, adaptation and inter-rater reliability of the administration manual for the Fugl-Meyer assessment. Rev. Bras. Fisioter. 2011, 15, 80–88. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The Fugl-Meyer Assessment of Motor Recovery after Stroke: A Critical Review of Its Measurement Properties. Neurorehabilit. Neural Repair. 2002, 16, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Woytowicz, E.J.; Rietschel, J.C.; Goodman, R.N.; Conroy, S.S.; Sorkin, J.D.; Whitall, J.; Waller, S.M. Determining Levels of Upper Extremity Movement Impairment by Applying a Cluster Analysis to the Fugl-Meyer Assessment of the Upper Extremity in Chronic Stroke. Arch. Phys. Med. Rehabil. 2017, 98, 456–462. [Google Scholar] [CrossRef]
- Hernández, E.D.; Forero, S.M.; Galeano, C.P.; Barbosa, N.E.; Sunnerhagen, K.S.; Alt Murphy, M. Intra- and inter-rater reliability of Fugl-Meyer Assessment of Lower Extremity early after stroke. Braz. J. Phys. Ther. 2021, 25, 709–718. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Scrivener, K.; Schurr, K.; Sherrington, C. Responsiveness of the ten-metre walk test, Step Test and Motor Assessment Scale in inpatient care after stroke. BMC Neurol. 2014, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Stokic, D.S.; Horn, T.S.; Ramshur, J.M.; Chow, J.W. Agreement between temporospatial gait parameters of an electronic walkway and a motion capture system in healthy and chronic stroke populations. Am. J. Phys. Med. Rehabil. 2009, 88, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Marques-Sule, E.; Arnal-Gómez, A.; Buitrago-Jiménez, G.; Suso-Martí, L.; Cuenca-Martínez, F.; Espí-López, G.V. Effectiveness of Nintendo Wii and Physical Therapy in Functionality, Balance, and Daily Activities in Chronic Stroke Patients. J. Am. Med. Dir. Assoc. 2021, 22, 1073–1080. [Google Scholar] [CrossRef]
- Kim, K.H.; Jang, S.H. Effects of Task-Specific Training after Cognitive Sensorimotor Exercise on Proprioception, Spasticity, and Gait Speed in Stroke Patients: A Randomized Controlled Study. Medicina 2021, 57, 1098. [Google Scholar] [CrossRef]
- Lee, K.; Lee, D.; Hong, S.; Shin, D.; Jeong, S.; Shin, H.; Choi, W.; An, S.; Lee, G. The relationship between sitting balance, trunk control and mobility with predictive for current mobility level in survivors of sub-acute stroke. PLoS ONE 2021, 16, e0251977. [Google Scholar] [CrossRef]
- Bath, P.M.; Woodhouse, L.J.; Suntrup-Krueger, S.; Likar, R.; Koestenberger, M.; Warusevitane, A.; Herzog, J.; Schuttler, M.; Ragab, S.; Everton, L.; et al. Pharyngeal electrical stimulation for neurogenic dysphagia following stroke, traumatic brain injury or other causes: Main results from the PHADER cohort study. EClinicalMedicine 2020, 28, 100608. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.G.; Frei, D.; Kirmani, J.F.; Zaidat, O.; Lopes, D.; Turk, A.S.; Heck, D.; Mason, B.; Haussen, D.C.; Levy, E.I.; et al. Safety and Efficacy of a 3-Dimensional Stent Retriever with Aspiration-Based Thrombectomy vs. Aspiration-Based Thrombectomy Alone in Acute Ischemic Stroke Intervention: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 304–311. [Google Scholar] [CrossRef] [PubMed]
- de Gooijer-van de Groep, K.L.; de Vlugt, E.; van der Krogt, H.J.; Helgadóttir, Á.; Arendzen, J.H.; Meskers, C.G.M.; de Groot, J.H. Estimation of tissue stiffness, reflex activity, optimal muscle length and slack length in stroke patients using an electromyography driven antagonistic wrist model. Clin. Biomech. 2016, 35, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Li, L.; Li, X.; Zha, H.; Liu, Z.; Peng, Y.; Liu, X.; Liu, H.; Yang, Q.; Wang, J. Therapeutic Role of Additional Mirror Therapy on the Recovery of Upper Extremity Motor Function after Stroke: A Single-Blind, Randomized Controlled Trial. Neural Plast. 2022, 2022, 8966920. [Google Scholar] [CrossRef] [PubMed]
- Kwong, P.W.H.; Ng, S.S.M. Cutoff Score of the Lower-Extremity Motor Subscale of Fugl-Meyer Assessment in Chronic Stroke Survivors: A Cross-Sectional Study. Arch. Phys. Med. Rehabil. 2019, 100, 1782–1787. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A.; Carlucci, G.; Falsini, C.; Lunghi, F.; Verdesca, S.; Grippo, A. Gait in hemiplegia: Evaluation of clinical features with the Wisconsin Gait Scale. J. Rehabil. Med. 2007, 39, 170–174. [Google Scholar] [CrossRef]
- Busk, H.; Holm, P.; Skou, S.T.; Seitner, S.; Siemsen, T.; Wienecke, T. Inter-rater reliability and agreement of 6 Minute Walk Test and 10 Meter Walk Test at comfortable walk speed in patients with acute stroke. Physiother. Theory Pract. 2022, 39, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Berkhemer, O.A.; Fransen, P.S.S.; Beumer, D.; Berg, L.A.V.D.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.H.; et al. A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef]
- Campbell, B.C.; Mitchell, P.J.; Churilov, L.; Yassi, N.; Kleinig, T.J.; Yan, B.; Dowling, R.J.; Bush, S.J.; Dewey, H.M.; Thijs, V.; et al. Tenecteplase versus alteplase before endovascular thrombectomy (EXTEND-IA TNK): A multicenter, randomized, controlled study. Int. J. Stroke 2018, 13, 328–334. [Google Scholar] [CrossRef]
- Samsa, G.P.; Matchar, D.B. Have Randomized Controlled Trials of Neuroprotective Drugs Been Underpowered? Stroke 2001, 32, 669–674. [Google Scholar] [CrossRef]
- Braun, T.; Marks, D.; Thiel, C.; Grüneberg, C. A generic outcome assessment of mobility capacity in neurorehabilitation: Measurement properties of the de Morton Mobility Index. BMC Neurol. 2021, 21, 298. [Google Scholar] [CrossRef]
- Kunkel, A.; Kopp, B.; Müller, G.; Villringer, K.; Villringer, A.; Taub, E.; Flor, H. Constraint-induced movement therapy for motor recovery in chronic stroke patients. Arch. Phys. Med. Rehabil. 1999, 80, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Golicki, D.; Niewada, M.; Karlińska, A.; Buczek, J.; Kobayashi, A.; Janssen, M.F.; Pickard, A.S. Comparing responsiveness of the EQ-5D-5L, EQ-5D-3L and EQ VAS in stroke patients. Qual. Life Res. 2015, 24, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Logan, P.; Ahern, J.; Gladman, J.; Lincoln, N. A randomized controlled trial of enhanced Social Service occupational therapy for stroke patients. Clin. Rehabil. 1997, 11, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Ekstrand, E.; Alt Murphy, M.; Sunnerhagen, K.S. Clinical interpretation and cutoff scores for manual ability measured by the ABILHAND questionnaire in people with stroke. Top. Stroke Rehabil. 2023, 30, 21–31. [Google Scholar] [CrossRef]
- Wu, C.Y.; Chuang, L.L.; Lin, K.C.; Horng, Y.S. Responsiveness and validity of two outcome measures of instrumental activities of daily living in stroke survivors receiving rehabilitative therapies. Clin. Rehabil. 2011, 25, 175–183. [Google Scholar] [CrossRef]
- Ahmed, S.; Mayo, N.E.; Higgins, J.; Salbach, N.M.; Finch, L.; Wood-Dauphinée, S.L. The Stroke Rehabilitation Assessment of Movement (STREAM): A Comparison with Other Measures Used to Evaluate Effects of Stroke and Rehabilitation. Phys. Ther. 2003, 83, 617–630. [Google Scholar] [CrossRef]
- Sunderland, A.; Tinson, D.; Bradley, L.; Hewer, R.L. Arm function after stroke. An evaluation of grip strength as a measure of recovery and a prognostic indicator. J. Neurol. Neurosurg. Psychiatry 1989, 52, 1267–1272. [Google Scholar] [CrossRef]
- Rabadi, M.H.; Rabadi, F.M. Comparison of the action research arm test and the Fugl-Meyer assessment as measures of upper-extremity motor weakness after stroke. Arch. Phys. Med. Rehabil. 2006, 87, 962–966. [Google Scholar] [CrossRef]
- Page, S.J.; Sisto, S.; Levine, P.; McGrath, R.E. Efficacy of modified constraint-induced movement therapy in chronic stroke: A single-blinded randomized controlled trial 11No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors(s) or upon any organization with which the author(s) is/are associated. Arch. Phys. Med. Rehabil. 2004, 85, 14–18. [Google Scholar]
- Lindenberg, R.; Renga, V.; Zhu, L.L.; Nair, D.; Schlaug, G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology 2010, 75, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Hammer, A.M.; Lindmark, B. Responsiveness and validity of the Motor Activity Log in patients during the subacute phase after stroke. Disabil. Rehabil. 2010, 32, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Nasb, M.; Li, Z.; S A Youssef, A.; Dayoub, L.; Chen, H. Comparison of the effects of modified constraint-induced movement therapy and intensive conventional therapy with a botulinum-a toxin injection on upper limb motor function recovery in patients with stroke. Libyan J. Med. 2019, 14, 1609304. [Google Scholar] [CrossRef] [PubMed]
- van der Putten, J.J.; Hobart, J.C.; Freeman, J.A.; Thompson, A.J. Measuring change in disability after inpatient rehabilitation: Comparison of the responsiveness of the Barthel index and the Functional Independence Measure. J. Neurol. Neurosurg. Psychiatry 1999, 66, 480–484. [Google Scholar] [CrossRef]
- Laffont, I.; Froger, J.; Jourdan, C.; Bakhti, K.; van Dokkum, L.E.; Gouaich, A.; Bonnin, H.Y.; Armingaud, P.; Jaussent, A.; Picot, M.C.; et al. Rehabilitation of the upper arm early after stroke: Video games versus conventional rehabilitation. A randomized controlled trial. Ann. Phys. Rehabil. Med. 2020, 63, 173–180. [Google Scholar] [CrossRef]
- Fisher, M. Enhancing the Development and Approval of Acute Stroke Therapies. Stroke 2005, 36, 1808–1813. [Google Scholar] [CrossRef]
- Broderick, J.P.; Adeoye, O.; Elm, J. The Evolution of the Modified Rankin Scale and Its Use in Future Stroke Trials. Stroke 2017, 48, 2007–2012. [Google Scholar] [CrossRef]
- Harrison, J.K.; McArthur, K.S.; Quinn, T.J. Assessment scales in stroke: Clinimetric and clinical considerations. Clin. Interv. Aging 2013, 8, 201–211. [Google Scholar]
- Roydhouse, J.K.; Wilson, I.B. Systematic review of caregiver responses for patient health-related quality of life in adult cancer care. Qual. Life Res. 2017, 26, 1925–1954. [Google Scholar] [CrossRef]
- Pickard, A.S.; Lin, H.W.; Knight, S.J.; Sharifi, R.; Wu, Z.; Hung, S.Y.; Witt, W.P.; Chang, C.H.; Bennett, C.L. Proxy Assessment of Health-Related Quality of Life in African American and White Respondents with Prostate Cancer: Perspective Matters. Med. Care 2009, 47, 176. [Google Scholar] [CrossRef]
- Yperzeele, L.; Shoamanesh, A.; Venugopalan, Y.V.; Chapman, S.; Mazya, M.V.; Charalambous, M.; Caso, V.; Hacke, W.; Bath, P.M.; Koltsov, I. Key design elements of successful acute ischemic stroke treatment trials. Neurol. Res. Pract. 2023, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.J.; Bailey, J.; Cassotta, M.; Herrmann, K.; Pistollato, F. Poor Translatability of Biomedical Research Using Animals—A Narrative Review. Altern. Lab. Anim. 2023, 51, 102–135. [Google Scholar] [CrossRef] [PubMed]
- Lyden, P.D.; Bosetti, F.; Diniz, M.A.; Rogatko, A.; Koenig, J.I.; Lamb, J.; Nagarkatti, K.A.; Cabeen, R.P.; Hess, D.C.; Kamat, P.K.; et al. The Stroke Preclinical Assessment Network: Rationale, Design, Feasibility, and Stage 1 Results. Stroke 2022, 53, 1802–1812. [Google Scholar] [CrossRef]
- Spanagel, R. Ten Points to Improve Reproducibility and Translation of Animal Research. Front. Behav. Neurosci. 2022, 16, 869511. Available online: https://www.frontiersin.org/articles/10.3389/fnbeh.2022.869511 (accessed on 7 January 2024). [CrossRef] [PubMed]
- Jickling, G.C.; Sharp, F.R. Improving the translation of animal ischemic stroke studies to humans. Metab. Brain Dis. 2015, 30, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Pound, P.; Ritskes-Hoitinga, M. Can prospective systematic reviews of animal studies improve clinical translation? J. Transl. Med. 2020, 18, 15. [Google Scholar] [CrossRef]
- Hackam, D.G. Translating animal research into clinical benefit. BMJ 2007, 334, 163–164. [Google Scholar] [CrossRef]
- Read “Improving the Utility and Translation of Animal Models for Nervous System Disorders: Workshop Summary” at NAP.edu [Internet]. Available online: https://nap.nationalacademies.org/read/13530/chapter/4 (accessed on 7 January 2024).
- de Vet, H.C.W.; Mokkink, L.B.; Terwee, C.B. Minimal Clinically Important Difference (MCID). In Encyclopedia of Quality of Life and Well-Being Research; Michalos, A.C., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 4071–4072. [Google Scholar] [CrossRef]
- Bland, M.D.; Sturmoski, A.; Whitson, M.; Connor, L.T.; Fucetola, R.; Huskey, T.; Corbetta, M.; Lang, C.E. Prediction of discharge walking ability from initial assessment in a stroke inpatient rehabilitation facility population. Arch. Phys. Med. Rehabil. 2012, 93, 1441–1447. [Google Scholar] [CrossRef]
- Blum, L.; Korner-Bitensky, N. Usefulness of the Berg Balance Scale in stroke rehabilitation: A systematic review. Phys. Ther. 2008, 88, 559–566. [Google Scholar] [CrossRef]
- Berg Balance Scale—An overview|ScienceDirect Topics [Internet]. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/berg-balance-scale (accessed on 21 April 2023).
- Di Carlo, S.; Bravini, E.; Vercelli, S.; Massazza, G.; Ferriero, G. The Mini-BESTest: A review of psychometric properties. Int. J. Rehabil. Res. 2016, 39, 97–105. [Google Scholar] [CrossRef]
- Tsang, C.S.L.; Liao, L.R.; Chung, R.C.K.; Pang, M.Y.C. Psychometric properties of the Mini-Balance Evaluation Systems Test (Mini-BESTest) in community-dwelling individuals with chronic stroke. Phys. Ther. 2013, 93, 1102–1115. [Google Scholar] [CrossRef] [PubMed]
- Franchignoni, F.; Horak, F.; Godi, M.; Nardone, A.; Giordano, A. Using psychometric techniques to improve the Balance Evaluation Systems Test: The mini-BESTest. J. Rehabil. Med. 2010, 42, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Medley, A.; Teran, S. Validity of the Sitting Balance Scale in older adults who are non-ambulatory or have limited functional mobility. Clin. Rehabil. 2013, 27, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Sandin, K.J.; Smith, B.S. The measure of balance in sitting in stroke rehabilitation prognosis. Stroke 1990, 21, 82–86. [Google Scholar] [CrossRef]
- Gorman, S.L.; Radtka, S.; Melnick, M.E.; Abrams, G.M.; Byl, N.N. Development and Validation of the Function in Sitting Test in Adults with Acute Stroke. J. Neurol. Phys. Ther. 2010, 34, 150. [Google Scholar] [CrossRef]
- Whitney, S.L.; Wrisley, D.M.; Marchetti, G.F.; Gee, M.A.; Redfern, M.S.; Furman, J.M. Clinical measurement of sit-to-stand performance in people with balance disorders: Validity of data for the Five-Times-Sit-to-Stand Test. Phys. Ther. 2005, 85, 1034–1045. [Google Scholar] [CrossRef]
- Jones, S.E.; Kon, S.S.C.; Canavan, J.L.; Patel, M.S.; Clark, A.L.; Nolan, C.M.; I Polkey, M.; Man, W.D.-C. The five-repetition sit-to-stand test as a functional outcome measure in COPD. Thorax 2013, 68, 1015–1020. [Google Scholar] [CrossRef]
- Bernabeu-Mora, R.; Giménez-Giménez, L.M.; Montilla-Herrador, J.; García-Guillamón, G.; García-Vidal, J.A.; Medina-Mirapeix, F. Determinants of each domain of the Short Physical Performance Battery in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 2539–2544. [Google Scholar] [CrossRef]
- Cooper, A.; Alghamdi, G.A.; Alghamdi, M.A.; Altowaijri, A.; Richardson, S. The Relationship of Lower Limb Muscle Strength and Knee Joint Hyperextension during the Stance Phase of Gait in Hemiparetic Stroke Patients. Physiother. Res. Int. 2012, 17, 150–156. [Google Scholar] [CrossRef]
- Beyaert, C.; Vasa, R.; Frykberg, G.E. Gait post-stroke: Pathophysiology and rehabilitation strategies. Neurophysiol. Clin. 2015, 45, 335–355. [Google Scholar] [CrossRef]
- Wellmon, R.; Degano, A.; Rubertone, J.A.; Campbell, S.; Russo, K.A. Interrater and intrarater reliability and minimal detectable change of the Wisconsin Gait Scale when used to examine videotaped gait in individuals post-stroke. Arch. Physiother. 2015, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, K.H.; Purdy, M.; Falk, J.; Gallo, L. The Dysphagia Outcome and Severity Scale. Dysphagia 1999, 14, 139–145. [Google Scholar] [CrossRef]
- Kopp, B.; Kunkel, A.; Flor, H.; Platz, T.; Rose, U.; Mauritz, K.-H.; Gresser, K.; McCulloch, K.L.; Taub, E. The Arm Motor Ability Test: Reliability, validity, and sensitivity to change of an instrument for assessing disabilities in activities of daily living. Arch. Phys. Med. Rehabil. 1997, 78, 615–620. [Google Scholar] [CrossRef]
- Daley, K.; Mayo, N.; Wood-Dauphinée, S. Reliability of Scores on the Stroke Rehabilitation Assessment of Movement (STREAM) Measure. Phys. Ther. 1999, 79, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Sullivan, K.J.; Behrman, A.L.; Azen, S.P.; Wu, S.S.; Nadeau, S.E.; Dobkin, B.H.; Rose, D.K.; Tilson, J.K.; Cen, S.; et al. Body-weight-supported treadmill rehabilitation after stroke. N. Engl. J. Med. 2011, 364, 2026–2036. [Google Scholar] [CrossRef]
- Lai, S.M.; Perera, S.; Duncan, P.W.; Bode, R. Physical and social functioning after stroke: Comparison of the Stroke Impact Scale and Short Form-36. Stroke 2003, 34, 488–493. [Google Scholar] [CrossRef]
- Nouri, F.; Lincoln, N. An extended activities of daily living scale for stroke patients. Clin. Rehabil. 1987, 1, 301–305. [Google Scholar] [CrossRef]
- Chong, D.K. Measurement of instrumental activities of daily living in stroke. Stroke 1995, 26, 1119–1122. [Google Scholar] [CrossRef]
- Salter, K.L.; Teasell, R.W.; Foley, N.C.; Jutai, J.W. Outcome assessment in randomized controlled trials of stroke rehabilitation. Am. J. Phys. Med. Rehabil. 2007, 86, 1007–1012. [Google Scholar] [CrossRef]
- Urbin, M.A.; Waddell, K.J.; Lang, C.E. Acceleration metrics are responsive to change in upper extremity function of stroke survivors. Arch. Phys. Med. Rehabil. 2015, 96, 854–861. [Google Scholar] [CrossRef]
- Accelerometer—An Overview|ScienceDirect Topics [Internet]. Available online: https://www.sciencedirect.com/topics/materials-science/accelerometer (accessed on 23 April 2023).
- de Morton, N.A.; Davidson, M.; Keating, J.L. The de Morton Mobility Index (DEMMI): An essential health index for an ageing world. Health Qual. Life Outcomes 2008, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Cimolin, V.; Galli, M.; Vimercati, S.L.; Albertini, G. Use of the Gait Deviation Index for the assessment of gastrocnemius fascia lengthening in children with Cerebral Palsy. Res. Dev. Disabil. 2011, 32, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, A.M.; Mercier, C.; Bourbonnais, D.; Desrosiers, J.; Gravel, D. Reliability of maximal static strength measurements of the arms in subjects with hemiparesis. Clin. Rehabil. 2007, 21, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.T.; Toews, J.V. Grip strength as measured by the Jamar dynamometer. Arch. Phys. Med. Rehabil. 1970, 51, 321–327. [Google Scholar]
- Andrews, A.W.; Thomas, M.W.; Bohannon, R.W. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Phys. Ther. 1996, 76, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.M.; Lang, C.E.; Sahrmann, S.A.; Edwards, D.F.; Dromerick, A.W. Sensorimotor impairments and reaching performance in subjects with poststroke hemiparesis during the first few months of recovery. Phys. Ther. 2007, 87, 751–765. [Google Scholar] [CrossRef]
- Platz, T.; Pinkowski, C.; van Wijck, F.; Kim, I.H.; di Bella, P.; Johnson, G. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: A multicentre study. Clin. Rehabil. 2005, 19, 404–411. [Google Scholar] [CrossRef]
- Van der Lee, J.H.; De Groot, V.; Beckerman, H.; Wagenaar, R.C.; Lankhorst, G.J.; Bouter, L.M. The intra- and interrater reliability of the action research arm test: A practical test of upper extremity function in patients with stroke. Arch. Phys. Med. Rehabil. 2001, 82, 14–19. [Google Scholar] [CrossRef]
- Wolf, S.L.; Catlin, P.A.; Ellis, M.; Archer, A.L.; Morgan, B.; Piacentino, A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke 2001, 32, 1635–1639. [Google Scholar] [CrossRef]
- Morris, D.M.; Uswatte, G.; Crago, J.E.; Cook, E.W.; Taub, E. The reliability of the wolf motor function test for assessing upper extremity function after stroke. Arch. Phys. Med. Rehabil. 2001, 82, 750–755. [Google Scholar] [CrossRef]
- van der Lee, J.H.; Beckerman, H.; Knol, D.L.; de Vet, H.C.W.; Bouter, L.M. Clinimetric properties of the motor activity log for the assessment of arm use in hemiparetic patients. Stroke 2004, 35, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Uswatte, G.; Taub, E.; Morris, D.; Vignolo, M.; McCulloch, K. Reliability and validity of the upper-extremity Motor Activity Log-14 for measuring real-world arm use. Stroke 2005, 36, 2493–2496. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.E.; Wagner, J.M.; Edwards, D.F.; Dromerick, A.W. Upper extremity use in people with hemiparesis in the first few weeks after stroke. J. Neurol. Phys. Ther. 2007, 31, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Uswatte, G.; Miltner, W.H.; Foo, B.; Varma, M.; Moran, S.; Taub, E. Objective measurement of functional upper-extremity movement using accelerometer recordings transformed with a threshold filter. Stroke 2000, 31, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Penta, M.; Thonnard, J.L.; Tesio, L. ABILHAND: A Rasch-built measure of manual ability. Arch. Phys. Med. Rehabil. 1998, 79, 1038–1042. [Google Scholar] [CrossRef]
- EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef] [PubMed]
- User’s Manual for the SF36v2 Health Survey—ScienceOpen [Internet]. Available online: https://www.scienceopen.com/document?vid=0a250605-f5f8-489a-a73c-329de570f424 (accessed on 7 February 2023).
- Anderson, C.; Laubscher, S.; Burns, R. Validation of the Short Form 36 (SF-36) health survey questionnaire among stroke patients. Stroke 1996, 27, 1812–1816. [Google Scholar] [CrossRef]
- Ware, J.E.; Snow, K.K.; Kosinski, M.; Gandek, B. SF36 Health Survey: Manual and Interpretation Guide; Quality Metric, Inc.: Johnston, RI, USA, 1993; p. 30. [Google Scholar]
- Duncan, P.W.; Wallace, D.; Lai, S.M.; Johnson, D.; Embretson, S.; Laster, L.J. The Stroke Impact Scale Version 2.0. Stroke 1999, 30, 2131–2140. [Google Scholar] [CrossRef]
- Moore, J.L.; Potter, K.; Blankshain, K.; Kaplan, S.L.; O’Dwyer, L.C.; Sullivan, J.E. A Core Set of Outcome Measures for Adults with Neurologic Conditions Undergoing Rehabilitation: A Clinical Practice Guideline. J. Neurol. Phys. Ther. 2018, 42, 174–220. [Google Scholar] [CrossRef]
- Blackburn, M.; van Vliet, P.; Mockett, S.P. Reliability of measurements obtained with the modified Ashworth scale in the lower extremities of people with stroke. Phys. Ther. 2002, 82, 25–34. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.V.; Hamilton, B.B.; Keith, R.A.; Zielezny, M.; Sherwin, F.S. Advances in functional assessment for medical rehabilitation. Top. Geriatr. Rehabil. 1986, 1, 59. [Google Scholar] [CrossRef]
- Gresham, G.E.; Duncan, P.W.; Stason, W.B.; Adams, H.P.; Adelman, A.M.; Alexander, D.N.; Bishop, D.S.; Diller, L.; Donaldson, N.E.; Granger, C.V. Post-Stroke Rehabilitation. Clinical Practice Guideline Number 16; Post-Stroke Rehabilitation: Assessment, Referral, and Patient Management. Quick Reference Guide for Clinicians Number 16; Recovering after a Stroke, Consumer Version. Patient and Family Guide. [Internet]. Agency for Health Care Policy and Research, Rockville, MD. Center for Research Dissemination and Liaison. 1995. Report No.: PB95226890. Available online: https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/PB95226890.xhtml (accessed on 24 April 2023).
- Ottenbacher, K.J.; Hsu, Y.; Granger, C.V.; Fiedler, R.C. The reliability of the functional independence measure: A quantitative review. Arch. Phys. Med. Rehabil. 1996, 77, 1226–1232. [Google Scholar] [CrossRef]
- Dodds, T.A.; Martin, D.P.; Stolov, W.C.; Deyo, R.A. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch. Phys. Med. Rehabil. 1993, 74, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment: MOCA: A brief screening tool for mci. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- MoCA Cognition [Internet]. MoCA Cognition. Available online: https://mocacognition.com/ (accessed on 13 February 2023).
- Park, J.H.; Kim, B.J.; Bae, H.-J.; Lee, J.; Lee, J.; Han, M.-K.; Park, S.H.; Kang, Y.; Yu, K.-H.; Lee, B.C.; et al. Impact of post-stroke cognitive impairment with no dementia on health-related quality of life. J. Stroke 2013, 15, 49–56. [Google Scholar] [CrossRef]
- Pedersen, S.G.; Friborg, O.; Heiberg, G.A.; Arntzen, C.; Stabel, H.H.; Thrane, G.; Nielsen, J.F.; Anke, A. Stroke-Specific Quality of Life one-year post-stroke in two Scandinavian country-regions with different organisation of rehabilitation services: A prospective study. Disabil. Rehabil. 2021, 43, 3810–3820. [Google Scholar] [CrossRef]

| Serial no. | Author, Year | Design | Patients by Anchor/ Distribution/ Delphi (Total) | Mean Age | Disease | Specific Treatment | Scale | Subscale/ Dimension | Type of Scale (Clinician- or Patient-Reported). Were Proxies Permitted to Respond on Behalf of Patients? | Anchor Based | In the Anchor Method, What No. of Participants Who Provided Perspectives on Likert-Type Scales Were Patients? | Distribution-Based | Delphi Method | Delphi Method: How Many in the “Expert” Groups Were Patients with Illness? | MCID Thresholds |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tamura et al., 2021 [39] | Multi-centric, retrospective study | 80/-/- (80) | - | Subacute stroke | - | Berg Balance Scale (BBS) | x |
| Yes | Likely that all 80 participants who provided perspectives on the Likert-type scale (GROC) were patients. | x | - | N/A | Assisted walking group: 5 points; unassisted walking group: 4 points |
| 2 | Beauchamp et al., 2021 [40] | Prospective cohort | 50/50/- (50) | 60.8 (9.4) | Stroke | Rehabilitation | Mini-Balance Evaluation Systems Test (Mini-BESTest) | x |
| Yes | Likely that all 50 participants who provided perspectives on the Likert-type scale (GROC) were patients. | Yes | - | N/A | 4 points |
| 3 | Agustin et al., 2021 [41] | Prospective cohort | 111/-/- (111) | 68.3 (12.1) | Stroke | Rehabilitation | Five-Repetition Sit-to-Stand test (5STS) in seconds, Gait speed in m/s | x |
| Yes | Likely that all 50 participants who provided perspectives on the Likert-type scale (GROC) were patients. | x | - | N/A | 5STS at 8 weeks: all patients: 0.76, household limited: 0.72, limited community: 3.09; at 4 weeks: all patients: 1.18, household limited: 1.9, limited community: 2.92. Gait speed at 8 weeks: all patients: 0.09, household limited: 0.04, limited community: 0.11; at 4 weeks: all patients: 0.19, household limited: 0.19, limited community: 0.21 |
| 4 | Fu et al. [42] | Secondary analysis of RCT | 400/400/- (400) | 72.0 (12.5) | Stroke | - | Physical Component Summary (PCS) score of the Short Form 36 (SF-36) | x |
| Yes | Likely that all 400 participants who provided perspectives on the PCS SF scale (Likert-type scale) were patients. | Yes | - | N/A | 1.8 to 3.0 units |
| 5 | Guzik et al., 2020 [43] | Prospective cohort | 50/50/- (50) | 60.9 (11.2) | Stroke | Rehabilitation | Knee range of motion (ROM) | x |
| Yes | Likely that all 50 participants who provided perspectives on the Likert-type scale (patient perception of improvement) were patients. | Yes | - | N/A | Affected side: 8.48 degrees, unaffected side: 6.81 degrees |
| 6 | Alzyoud et al., 2020 [44] | Prospective cohort | 43/43/- (43) | 71.6 (11.4) | Stroke | Rehabilitation | Sitting Balance Scale (SBS), Function in Sitting Test (FIST) | x |
| Yes | Likely that all 50 participants who provided perspectives on the Barthel Index were patients. | Yes | - | N/A | SBS: 5, FIST: 4 |
| 7 | Everton et al., 2020 [45] | Post hoc analysis of previous RCT (STEPS); Survey | 154/154/84 (238) | - | Stroke | Rehabilitation | Dysphagia Severity Rating Scale (DSRS) | x |
| Yes | No information on the anchor used. | Yes | Yes | No explicit information on how many were patients but all were probably SLTs. | Anchors: aspiration at week 2: 2.5 points; oral vs. non-oral feeding at week 2: 1.0; 0.5 SD: 1.9, SEM 0.3. Survey: 1 point |
| 8 | Lin et al., 2020 [46] | Survey | -/-/58 (58) | - | Stroke | Endovascular thrombectomy | Substantial reperfusion (TICI 2b-3) | x |
| x | Not applicable. | x | Yes | None were patients. | Median: 3.1–5% |
| 9 | Chen et al., 2019 [47] | Prospective observational study | -/115/- (115) | 54.2 (11.1) | Stroke | Rehabilitation | Modified Ashworth Scale (MAS) | x |
| x | Not applicable. | Yes | - | N/A | 0.5 SD: upper extremity: 0.48, lower extremity: 0.45. 0.8 SD: upper extremity: 0.45, lower extremity: 0.73 |
| 10 | Hiragami et al., 2019 [48] | Post hoc analysis of a previous RCT | 12/-/- (12) | 67.8 (10.5) | Stroke (moderate to severe hemiparesis) | Rehabilitation | Fugl-Meyer assessment of the upper extremity (FMA-UE) | Motor |
| Yes | Likely that all 12 participants who provided perspectives on the Likert-type scale. (GROC) were patients. | x | - | N/A | Upper extremity: 12.4; upper arm: 5.6; wrist/hand: 4.9 |
| 11 | Guzik et al., 2019 [49] | Prospective observational study | 50/50/- (50) | 60.9 (11.2) | Stroke | Rehabilitation | Wisconsin Gait Scale (WGS) | x |
| Yes | Likely that all 12 participants who provided perspectives on the Likert-type scale (GROC) were patients. | Yes | - | N/A | 2.25 |
| 12 | Wu et al., 2019 [50] | Prospective observational study | 65/65/- (65) | 53.5 (11.7) | Stroke | Rehabilitation | Montreal Cognitive Assessment (MoCA) | x |
| Yes | Likely that all 65 participants who provided perspectives on the Stroke Impact Scale 3.0 (Likert-type scale) were patients. | Yes | - | N/A | Anchor-based: 1.22 points; distribution-based: 2.15 points |
| 13 | Fulk et al., 2018 [51] | RCT | 265/-/- (265) | 61.3 (12.8) | Stroke | Rehabilitation | 6-min walk test (6MWT) | x |
| Yes | Likely that all 265 participants who provided perspectives on the Stroke Impact Scale 3.0 (Likert-type scale) were patients. | x | - | N/A | 71 m |
| 14 | Chen et al., 2018 [52] | Pooled data from three clinical trials | 82/82/- (82) | 55.3 (10.7) | Stroke | Rehabilitation | Arm accelerometer, tools used: clinical measurement tools: Motor Activity Log (MAL), Stroke Impact Scale (SIS), and Nottingham Extended Activities of Daily Living (NEADL). | x |
| Yes | Likely that all 82 participants who provided perspectives on Likert-type scale (SIS) were patients. | Yes | - | N/A | 547–751 mean counts |
| 15 | Song et al.,2018 [53] | Prospective cohort | 73/-/- (73) | 63.94 (12.78) | Stroke | - | Berg Balance Scale (BBS) | x |
| Yes | Likely that all 73 participants who provided perspectives on the 15 GROC (15-point Likert scale) were patients. | x | - | N/A | 12.5 points |
| 16 | Cranston et al., 2017 [54] | Survey | -/-/122 (122) | Stroke | Novel safe neuroprotective agent | Modified Rankin Scale (mRS), safe acute ischemic stroke treatment | x |
| x | Not applicable. | x | Yes | None were patients. | Modified Rankin Scale (mRS): 1 point; safe acute ischemic stroke treatment: 1.1–1.5% | |
| 17 | New et al., 2016 [55] | Prospective cohort | 366/366/- (366) | - | Stroke | Rehabilitation | de Morton Mobility Index (DEMMI) | x |
| Yes | Likely that all 366 participants who provided perspectives on the Likert-type scale (GROC) were patients | Yes | - | N/A | Anchor-based: 8.0; distribution-based: 2.9 |
| 18 | Lundquist et al., 2017 [56] | Prospective observational study | 50/-/- (50) | 70.2 (10.1) | Stroke | - | Fugl-Meyer assessment of the upper extremity (FMA-UE) | Danish version |
| Yes | Likely that all 366 participants who provided perspectives on the Likert-type scale (GROC) were patients. | x | - | N/A | ≥4 |
| 19 | Fulk et al., 2017 [57] | Secondary analysis of data from the Everest RCT | 146/146/- (146) | 57.1 (11) | Stroke | Rehabilitation | Arm Motor Ability Test (AMAT) | x |
| Yes | Likely that all 146 participants who provided perspectives on the Likert-type scale (GROC) were patients. | Yes | - | N/A | ≥0.44 points |
| 20 | Correa et al., 2017 [58] | Prospective cohort | -/20/- (20) | 55.2 (9.9) | Stroke | Rehabilitation | Gait Deviation Index (GDI) | x |
| x | Not applicable; only distribution-based method used. | Yes | - | N/A | Non-paretic limb: 9.4; paretic limb: 7.5 |
| 21 | Pandian et al., 2016 [59] | Prospective observational study | 65/-/- | 44.2 (12.8) | Stroke | Rehabilitation | Fugl-Meyer assessment: Lower extremity (FMA-LE) | x |
| Yes | Likely that all 65 participants who provided perspectives on the Likert-type scale (GRPPC) were patients. | x | - | N/A | 6 |
| 22 | Chen et al., 2016 [60] | Prospective cohort | 65/65/- | 52.8 (11.6) | Stroke | Rehabilitation | EuroQoL 5-Dimensions Questionnaire (EQ-5D-5L); Visual analog scale (EQ-VAS) | x |
| Yes | Likely that all 65 participants who provided perspectives on the Likert-type scale (FAC and GRPPC) were patients. | Yes | - | N/A | EQ-Index: 0.1; EQ-VAS: 8.61–10.82 |
| 23 | Kim et al., 2015 [61] | Prospective observational study | 487/-/- | 68.3 (8.1) | Stroke | - | EQ-5D, SF-36 v2 | x |
| Yes | Likely that all 487 participants who provided perspectives on the 5-point Likert-type scale were patients. | x | - | N/A | EQ-5D: 0.08–0.12; SF-6D 0.04–0.14 |
| 24 | Bohannon et al., 2013 [62] | Retrospective cohort | 35/-/- | - | Stroke | Rehabilitation | Comfortable gait speed | x |
| Yes | Likely that all 35 participants who provided perspectives on the 5-point Likert-type scale were patients. | x | - | N/A | Change in walking speed of 0.13 m/s |
| 25 | Page et al., 2012 [63] | RCT | 146/-/- (146) | 57.1 (11) | Stroke | Rehabilitation | Fugl-Meyer assessment of the upper extremity (UE-FM) | x |
| Yes | Likely that all 146 participants on whom clinician rated 5-point Likert- scale (GROC) were patients. | x | - | N/A | 4.25–7.25 |
| 26 | Arya et al., 2011 [64] | RCT | 71/-/- (71) | 52.4 (9.5) | Stroke | Rehabilitation | Fugl-Meyer assessment of the upper extremity (UE-FM) | x |
| Yes | Likely that all 71 participants who provided perspectives on the Likert-type scale (GRPPC) were patients. | x | - | N/A | 9 to 10 |
| 27 | Wu et al., 2011 [65] | RCT | 78/78/- (78) | 54.3 (11.9) | Stroke | Rehabilitation | Nottingham Extended Activities of Daily Living (NEADL) scale | x |
| Yes | Likely that all 78 participants who provided perspectives on the Likert-type scale (SIS 3.0) were patients. | Yes | - | N/A | 6.1 points |
| 28 | Wang et al., 2011 [66] | Pooled data from three clinical studies | 51/51/- (51) | 55.3 (10.3) | Stroke | Robot-assisted training | ABILHAND questionnaire | x |
| Yes | Likely that all 51 participants who provided perspectives on the Likert-type scale (SIS 3.0) were patients. | Yes | - | N/A | 0.26 to 0.35 |
| 29 | Fulk et al., 2011 [67] | Prospective cohort | 44/-/- (44) | 61.8 (14.7) | Stroke | Rehabilitation | Change in gait speed | x |
| Yes | Likely that all 44 participants who provided perspectives on the 7-point Likert-type scale (GROC) were patients. | x | - | N/A | 0.175 to 0.19 m/s |
| 30 | Lin et al., 2011 [68] | RCT | 74/74/- (74) | 57.1 (11.7) | Stroke | Rehabilitation | Stroke-Specific Quality of Life Scale (SS-QOL) | Mobility, self-care, UE function |
| Yes | Likely that all 74 participants who provided perspectives on the 5-point Likert-scale were patients. | Yes | - | N/A | Mobility: 1.5–2.4; self-care: 1.2–1.9; UE function: 1.2–1.8 |
| 31 | Fulk et al., 2010 [69] | Prospective cohort | 36/36/- (36) | 60.9 (15.6) | Stroke | Rehabilitation | Stroke Impact Scale-16 (SIS-16) | x |
| Yes | Likely that all 36 participants who provided perspectives on GROC scale were patients. | x | - | N/A | 9.4–14.1 |
| 32 | Tilson et al., 2010 [70] | Secondary analysis of the LEAPS RCT | 283/-/- (283) | 63.5 (12.5) | Stroke | Rehabilitation | Comfortable gait speed | x |
| Yes | Likely that all 283 participants who were assessed on mRS were patients. | x | - | N/A | 0.16 m/s |
| 33 | Hsieh et al., 2008 [71] | Prospective cohort | 81/81/- (81) | 55.9 (13.3) | Stroke | Rehabilitation | Stroke Rehabilitation Assessment of Movement (STREAM) measure | Lower extremity, upper extremity, mobility |
| Yes | Likely all 81 participants who provided perspectives on GROC (Likert-type scale) scale were patients. | x | - | N/A | UE: 2.2, LE: 1.9, mobility: 4.8 |
| 34 | Lang et al., 2008 [72] | RCT | 52/52/- (52) | 64 (14) | Stroke | Constraint induced movement therapy | Grip strength, composite upper extremity strength, Action Research Arm Test (ARAT), Wolf Motor Function Test (WMFT), Motor Activity Log (MAL), duration of upper extremity use | Dominant and non-dominant hand |
| Yes | Likely that all 81 participants who provided perspectives on GRPPC (5-point Likert scale) were patients. | x | - | N/A | Dominant hand: grip strength: 5.0 Kg, ARAT: 12 points, WMFT: 1.0 points, MAL score: 1.0. Non-dominant hand: grip strength: 6.2 Kg, ARAT: 17 points, WMFT: 1.2 points, MAL: 1.1 points |
| 35 | Hsieh et al., 2007 [73] | Prospective cohort | 81/-/- (81) | 55.9 (13.3) | Stroke | - | Barthel Index (BI) | x |
| Yes | Likely that all 81 participants who provided perspectives on 15-point Likert scale were patients. | - | - | N/A | 1.85 |
| 36 | Beninato et al., 2006 [74] | Case series | 113/-/- (113) | - | Stroke | - | FIM instrument | Total, motor, cognitive |
| Yes | Likely that all 113 participants who provided perspectives on GROC on a 15-point Likert scale were patients. | x | - | N/A | Total: 22, motor: 17, cognitive: 3 |
| Serial No. | Author | Year | Anchor-Based Methods | Distribution-Based Methods | Delphi Method/From Surveys | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of Anchors | Anchor(s) | Viewpoint | Cutoffs Used | Statistical Methods | Number of Distribution Criteria Used | Distribution Criteria | ||||
| 1 | Tamura et al., [39] | 2021 | 1 | Functional Ambulation Categories (FACs) | Clinician | FAS change ≥ 1 point | ROC | x | x | x |
| 2 | Beauchamp et al., [40] | 2021 | 1 | Global rating of change | Clinician | Response on a scale | Mean change approach, ROC | 1 | SEM | x |
| 3 | Agustin et al., [41] | 2021 | 1 | Global rating of change | Patient | Response on a scale (15-point Likert scale) | Median change (within patient change), ROC | x | x | x |
| 4 | Fu et al. [42] | 2021 | 2 | Perceived Health Change | Patient | Response on a scale (Physical Component Summary (PCS) score of the Short Form 36 (SF-36), range 0 to 100) | Linear regression analysis, root mean square error (RMSE) ANOVA | 1 | 0.2 SD | x |
| 5 | Guzik et al., [43] | 2020 | 1 | Patients’ perception of improvement | Patient | No change vs. improvement vs. worsening | Mean change, regression analysis, ROC | 1 | SEM | x |
| 6 | Alzyoud et al., [44] | 2020 | 1 | Barthel Index (BI) | Clinician | BI ≥ 2 vs. <2 | ROC | 1 | Effect size, SEM | x |
| 7 | Everton et al., [45] | 2020 | x | Aspiration at week 2 and oral vs. non-oral feeding at week 2 | Clinician | Penetration Aspiration Score (PAS) ≥ 3 | Mean change | 2 | 0.5 SD, SEM | 1 |
| 8 | Lin et al., [46] | 2020 | x | x | x | x | x | x | x | Median: 3.1–5% |
| 9 | Chen et al., [47] | 2019 | x | x | x | x | x | 2 | 0.5 SD, 0.8 SD | x |
| 10 | Hiragami et al., [48] | 2019 | 1 | Global rating of change | Patient | Response on a scale (7-point Likert scale) | Mean change | x | x | x |
| 11 | Guzik et al., [49] | 2019 | 1 | Patients’ perception of change in gait | Patient | Positive change vs. no change vs. worse | Mean change, regression analysis, ROC | 1 | SEM | x |
| 12 | Wu et al., [50] | 2019 | 1 | Perceived recovery score of the SIS 3.0 | Patient | 10–15% change | Mean change | 1 | 0.5 SD | x |
| 13 | Fulk et al., [51] | 2018 | 2 | mRS, SIS | Patient, clinician | Improvement in mRS ≥ 1; increase in SIS by 10% | ROC | x | x | x |
| 14 | Chen et al., [52] | 2018 | 3 | Motor Activity Log, Stroke Impact Scale, and Nottingham Extended Activities of Daily Living | Patient, clinician | 10–20% increase | Mean change | 1 | 0.5 SD | x |
| 15 | Song et al., [53] | 2018 | 1 | GROC | Patient | Response on a scale (15-point GROC (global rating of change) scale) | ROC | x | x | x |
| 16 | Cranston et al., [54] | 2017 | x | x | x | x | x | x | x | 1 points; 1.1–1.5% |
| 17 | New et al., [55] | 2017 | 1 | Global rating of change | Patient, clinician | Response on a scale (7-point Likert scale) | Mean change | 1 | Effect size | x |
| 18 | Lundquist et al., [56] | 2017 | 1 | Global rating of change | Patient | Response on a scale (7-point Likert scale) | ROC | x | x | x |
| 19 | Fulk et al., [57] | 2017 | 1 | Global rating of change | Patient, clinician | Response on a scale (5-point Likert scale) | ROC | 1 | SEM | x |
| 20 | Correa et al., [58] | 2017 | x | x | x | x | x | 1 | SEM | x |
| 21 | Pandian et al., [59] | 2016 | 2 | Global rating of patient-perceived changes (GRPPC); Functional Ambulation Classification (FAC) | Patient | Improvement in score ≤2 on GRPPC or ≥1 on FAC | ROC | x | x | x |
| 22 | Chen et al., [60] | 2016 | 1 | SIS | Patient | 10–15% change | Mean change | 1 | 0.5 SD | x |
| 23 | Kim et al., [61] | 2015 | 2 | mRS, Barthel Index (BI) | Patient | mRS: response on a 5-point Likert scale; BI: difference of at least 4 points | Mean change | x | x | x |
| 24 | Bohannon et al., [62] | 2013 | 1 | Decrease in assistance required | Patient | Decrease or not | ROC | x | x | x |
| 25 | Page et al., [63] | 2012 | 1 | Global rating of change | Clinician | Response on a scale (5-point Likert scale) | ROC | x | x | x |
| 26 | Arya et al., [64] | 2011 | 2 | mRS, GRPPC | Patient, clinician | mRS ≥ 1; GRPPC ≥ 2 | ROC | x | x | x |
| 27 | Wu et al., [65] | 2011 | 1 | SIS | Patient | 5 to 7.5 points (10–15% of the total scale score range) on the ADL/IADL domain of the SIS | Mean change | 1 | 0.2 SD | x |
| 28 | Wang et al., [66] | 2011 | 1 | SIS | Patient | 10–15% change | Mean change | 1 | 0.2 SD | x |
| 29 | Fulk et al., [67] | 2011 | 2 | GROC (patient and clinician) | Patient, clinician | Response on a scale (15-point Likert scale) | ROC | x | x | x |
| 30 | Lin et al., [68] | 2011 | 1 | SIS | Patient | 10–15% change | Mean change | 1 | 0.5 SD | x |
| 31 | Fulk et al., [69] | 2010 | 2 | GROC (patient and clinician) | Patient, clinician | Response on a scale (15-point Likert scale) | ROC | x | x | x |
| 32 | Tilson et al., [70] | 2010 | 1 | mRS | Clinician | Improvement in mRS ≥ 1 | ROC, regression analysis | x | x | x |
| 33 | Hsieh et al., [71] | 2008 | 1 | GROC | Patient | Response on a scale (15-point Likert scale) | Mean change | x | x | x |
| 34 | Lang et al., [72] | 2008 | 1 | Global rating of patient-perceived changes in their affected upper extremity | Patient | Response on a scale (7-point Likert scale) | Mean change | x | x | x |
| 35 | Hsieh et al., [73] | 2007 | 1 | Global ratings of ADL function | Patient | Response on a scale (15-point Likert scale) | Mean change | 1 | SEM | x |
| 36 | Beninato et al., [74] | 2006 | 1 | Global rate of overall clinical change in function | Clinician | Response on a scale (15-point Likert scale) | ROC | x | x | x |
| Sl No. | Scale | Studies Reporting MCID Thresholds and Included in This Review | MCID Thresholds Reported | Example of Study Evaluating the Same Scale in Stroke Patients | Title of Study | Change in Score Considered Significant | Interpretation as Per Reported MCID | Interpretation of Clinical and Statistical Relevance |
|---|---|---|---|---|---|---|---|---|
| 1 | Berg Balance Scale (BBS) | Tamura et al., 2021 [39] | 4 to 5 points | Marques-Sule et al., 2021 [91] | Effectiveness of Nintendo Wii and Physical Therapy in Functionality, Balance, and Daily Activities in Chronic Stroke Patients | Mean SBS difference between VRWiiG group (intervention) and CPTG (conventional physical therapy group) = 6.4 (95% CI 0.2 to 12.6) | Results achieved MCID threshold | Statistically significant and also clinically meaningful  |
| Song et al. et al., 2018 [53] | 12.5 points | -do- [91] | -do- | -do- | Results did not achieve MCID threshold | Statistically significant but not clinically meaningful | ||
| 2 | MiniBESTest | Beauchamp et al., 2021 [40] | 4 points | No suitable study found for comparison | - | - | - | - |
| 3 | Five-Repetition Sit-to-Stand test (5STS) in seconds | Augustin et al., 2021 [41] | 0.76 to 1.18 s: all patients | No suitable study found for comparison | - | - | - | - |
| 4 | Knee range of motion (ROM) | Guzik et al., 2020 [43] | 8.48 degrees: affected side | No suitable study found for comparison | - | - | - | - |
| 5 | Gait speed | Agustin et al., 2021 [41] | 0.09 m/s: all patients | Kim et al., 2021 [92] | Effects of Task-Specific Training after Cognitive Sensorimotor Exercise on Proprioception, Spasticity, and Gait Speed in Stroke Patients: A Randomized Controlled Study | Mean difference between intervention and control group: 0.21 m/s | Results achieved MCID threshold | Statistically significant and also clinically meaningful  |
| Bohannon et al., 2013 [62] | 0.13 m/s | -do- [92] | -do- | -do- | Results achieved MCID threshold | Statistically significant and also clinically meaningful  | ||
| Fulk et al., 2011 [67] | 0.17 m/s | -do- [92] | -do- | -do- | Results achieved MCID threshold | Statistically significant and also clinically meaningful  | ||
| Tilson et al., 2010 [70] | 0.16 m/s | -do- [92] | -do- | -do- | Results achieved MCID threshold | Statistically significant and also clinically meaningful  | ||
| 6 | Sitting Balance Scale (SBS) | Alzyoud et al., 2020 [44] | 5 points | Lee et al., 2021 [93] | The relationship between sitting balance, trunk control and mobility with predictive for current mobility level in survivors of subacute stroke | Cutoff score for SBS using ROC was calculated as 28.5 points for predicting mobility of subacute stroke survivors | Results achieved MCID threshold | Statistically significant and also clinically meaningful  |
| 7 | Function in Sitting Test (FIST) | Alzyoud et al., 2020 [44] | 4 points | No suitable study found for comparison | - | - | - | |
| 8 | Dysphagia Severity Rating Scale (DSRS) | Everton et al., 2020 [45] | 1 to 2.5 points | Bath et al., 2020 * [94] | Pharyngeal electrical stimulation for neurogenic dysphagia following stroke, traumatic brain injury or other causes: Main results from the PHADER cohort study | DSRS improved significantly in all; dysphagia: 6.5 to 6.7 points | Results achieved MCID threshold | Statistically significant and also clinically meaningful  |
| 9 | Substantial reperfusion (TICI 2b-3) | Lin et al., 2020 [46] | 3.1–5% | Nogueira et al., 2018 [95] | Safety and Efficacy of a 3-Dimensional Stent Retriever With Aspiration-Based Thrombectomy vs. Aspiration-Based Thrombectomy Alone in Acute Ischemic Stroke Intervention: A Randomized Clinical Trial | 15% | Results achieved MCID threshold | Statistically significant and also clinically meaningful  |
| 10 | Modified Ashworth Scale (MAS) | Chen et al., 2019 [47] | UE: 0.48, LE: 0.45 points | de Gooijer-van de et al., 2016 [96] | Estimation of tissue stiffness, reflex activity, optimal muscle length and slack length in stroke patients using an electromyography driven antagonistic wrist model | 1 point | Results achieved MCID threshold | Statistically significant and also clinically meaningful  |
| 11 | Fugl-Meyer assessment of the upper extremity (FMA-UE) | Hiragami et al., 2019 [48] | UE: 12.9 points | Wen et al., 2022 [97] | Therapeutic Role of Additional Mirror Therapy on the Recovery of Upper Extremity Motor Function after Stroke: A Single-Blind, Randomized Controlled Trial | Mean difference between intervention and control group: 6.32 points | Results did not achieve MCID threshold | Statistically significant but not clinically meaningful |
| Lundquist et al., 2017 [56] | ≥4 points | -do- [97] | -do- | -do- | Results achieved MCID threshold | Statistically significant and also clinically meaningful  | ||
| Arya et al., 2011 [64] | 10 points | -do- [97] | -do- | -do- | Results did not achieve MCID threshold | Statistically significant but not clinically meaningful  | ||
| Page et al., 2012 [63] | 4.25 to 7.25 points | -do- [97] | -do- | -do- | Results achieved MCID threshold | Statistically significant and also clinically meaningful  | ||
| 12 | Fugl-Meyer assessment of the lower extremity (FMA-LE) | Pandian et al., 2016 [59] | 6 points | Kwong et al., 2019 * [98] | Cutoff Score of the Lower-Extremity Motor Subscale of Fugl-Meyer Assessment in Chronic Stroke Survivors: A Cross-Sectional Study | ≥21 points | Results achieved MCID threshold | Statistically significant and also clinically meaningful  |
| 13 | Wisconsin Gait Scale (WGS) | Guzik et al., 2019 [49] | 2.5 points | Pizzi et al., 2007 [99] | Gait In Hemiplegia: Evaluation Of Clinical Features With The Wisconsin Gait Scale | 1.5 points | Results did not achieve MCID threshold | Statistically significant but not clinically meaningful  |
| 14 | Montreal Cognitive Assessment (MoCA) | Wu et al., 2019 [50] | 1.22 to 2.15 points | No suitable study found for comparison | - | - | - | - |
| 15 | 6-min walk test (6MWT) | Fulk et al., 2018 [51] | 71 m | Busk et al., 2022 * [100] | Inter-rater reliability and agreement of 6 Minute Walk Test and 10 Meter Walk Test at comfortable walk speed in patients with acute stroke. | 75.4 m | Results achieved MCID threshold | Statistically significant and also clinically meaningful  |
| 16 | Arm Accelerometer | Chen et al., 2018 [52] | 547–751 counts | No suitable study found for comparison | - | - | - | |
| 17 | Modified Rankin Scale (mRS) | Cranston et al., 2017 [54] | 1 level change | Berkhemer et al., 2015 [101] | A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke (MR CLEAN trial) | 1 point | Results achieved MCID threshold | Statistically significant and also clinically meaningful  |
| -do- [54] | -do- | Campbell et al., 2017 [102] | Tenecteplase versus alteplase before endovascular thrombectomy (EXTEND-IA TNK): A multicenter, randomized, controlled study | 1 point | Results achieved MCID threshold | Statistically significant and also clinically meaningful  | ||
| 18 | Safe Acute Ischaemic stroke treatment | Cranston et al., 2017 [54] | 1.1–1.5% | Samsa et al., 2001 * [103] | Have Randomized Controlled Trials of Neuroprotective Drugs Been Underpowered? | 2 to 3% | Results achieved MCID threshold | Statistically significant and also clinically meaningful  |
| 19 | de Morton Mobility Index (DEMMI) | New et al., 2017 [55] | 2.9 to 8 points | Braun et al., 2021 * [104] | A generic outcome assessment of mobility capacity in neurorehabilitation: measurement properties of the de Morton Mobility Index | 15 points | Results achieved MCID threshold | Statistically significant and also clinically meaningful  |
| 20 | Arm Motor Ability Test (AMAT) | Fulk et al., 2017 [57] | ≥0.44 points | Kunkel et al., 1999 * [105] | Constraint-Induced Movement Therapy for Motor Recovery in Chronic Stroke Patients | 2.04 points | Results achieved MCID threshold | Statistically significant and also clinically meaningful  |
| 21 | Gait Deviation Index (GDI) | Correa et al., 2017 [58] | 9.4 points: non-paretic limb, 7.5 points: paretic limb | No suitable study found for comparison | - | - | - | - |
| 22 | EuroQoL 5-Dimensions Questionnaire (EQ-5D-5L); Visual analog scale (EQ-VAS) | Chen et al., 2016 [60] | EQ-Index: 0.1 points, EQ-VAS: 8.61–10.82 points | Golicki et al., 2015 * [106] | Comparing responsiveness of the EQ-5D-5L, EQ-5D-3L and EQ VAS in stroke patients | EQ-5D-5L: 0.11 points, EQ-VAS: 6.4 points | EQ-5D-5L results achieved MCID threshold but EQ-VAS results did not achieve MCID threshold | EQ-5D-5L: statistically significant and also clinically meaningful  EQ-VAS: statistically significant but not clinically meaningful  |
| Kim et al., 2015 [61] | EQ-5D: 0.08–0.12 | No suitable study found for comparison | - | - | - | - | ||
| 23 | Nottingham Extended Activities of Daily Living (NEADL) scale | Wu et al., 2011 [65] | 6.1 points | Logan et al., 1997 [107] | A randomized controlled trial of enhanced Social Service occupational therapy for stroke patients | At 3 months: intervention vs. control group = 5 points At 6 months: intervention vs. control group = 2 points | Results did not achieve MCID threshold | Statistically significant but not clinically meaningful  |
| 24 | ABILHAND questionnaire | Wang et al., 2011 [66] | 0.26 to 0.35 points | Ekstrand et al., 2023 * [108] | Clinical interpretation and cutoff scores for manual ability measured by the ABILHAND questionnaire in people with stroke | 1.78 points | Results achieved MCID threshold | Statistically significant and also clinically meaningful  |
| 25 | Stroke-Specific Quality of Life Scale (SS-QOL) | Lin et al., 2011 [68] | Mobility: 1.5–2.4, self-care: 1.2–1.9, UE function: 1.2–1.8 | No suitable study found for comparison | - | - | - | - |
| 26 | Stroke Impact Scale-16 (SIS-16) | Fulk et al., 2010 [69] | 9.4–14.1 points | Wu et al., 2012 * [109] | Effect of Therapist-Based Versus Robot-Assisted Bilateral Arm Training on Motor Control, Functional Performance, and Quality of Life After Chronic Stroke: A Clinical Trial | Change pre–post intervention: RBAT: 5.3 points, TBAT: 3.4 points | Results did not achieve MCID threshold | Statistically significant but not clinically meaningful  |
| 27 | Stroke Rehabilitation Assessment of Movement (STREAM) measure | Hsieh et al., 2008 [71] | UE: 2.2, LE: 1.9, mobility: 4.8 points | Ahmed et al., 2003 * [110] | The Stroke Rehabilitation Assessment of Movement (STREAM): A Comparison With Other Measures Used to Evaluate Effects of Stroke and Rehabilitation | UE:15, LE: 15, mobility: 17 points | Results achieved MCID threshold | Statistically significant and also clinically meaningful |
| 28 | Grip Strength | Lang et al., 2008 [72] | 5.0 kg | Sunderland et al., 1989 * [111] | Arm function after stroke. An evaluation of grip strength as a measure of recovery and a prognostic indicator | Pre–post intervention change: 1 kg | Results did not achieve MCID threshold | Statistically significant but not clinically meaningful  |
| 29 | Action Research Arm Test (ARAT) | Lang et al., 2008 [72] | 12 points | Rabadi et al., 2006 * [112] | Comparison of the action research arm test and the Fugl-Meyer assessment as measures of upper-extremity motor weakness after stroke | 13 points | Results achieved MCID threshold | Statistically significant and also clinically meaningful  |
| -do- [72] | -do- | Page et al., 2004 [113] | Efficacy of modified constraint-induced movement therapy in chronic stroke: a single-blinded randomized controlled trial | 11.4 points | Results did not achieve MCID threshold | Statistically significant but not clinically meaningful  | ||
| 30 | Wolf Motor Function Test (WMFT) | Lang et al., 2008 [72] | 1.0 points | Lindenberg et al., 2010 [114] | Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients | Mean difference between intervention and control group: 0.5 points | Results did not achieve MCID threshold | Statistically significant but not clinically meaningful  |
| 31 | Motor Activity Log (MAL) | Lang et al., 2008 [72] | 1.0 points | Hammer et al., 2010 * [115] | Responsiveness and validity of the Motor Activity Log in patients during the subacute phase after stroke | 1.0 points | Results achieved MCID threshold | Statistically significant and also clinically meaningful |
| 32 | Barthel Index (BI) | Hsieh et al., 2007 [73] | 1.85 points | Nasb et al., 2019 * [116] | Comparison of the effects of modified constraint-induced movement therapy and intensive conventional therapy with a botulinum-a toxin injection on upper limb motor function recovery in patients with stroke | Change between groups (BTX-mCIMT vs. BTX-ICT groups) at 4 weeks: 9.6 points | Results achieved MCID threshold | Statistically significant and also clinically meaningful |
| 33 | FIM instrument | Beninato et al., 2006 [74] | Total: 22, motor: 17, cognitive: 3 | Putten et al., 1999 * [117] | Measuring change in disability after inpatient rehabilitation: comparison of the responsiveness of the Barthel Index and the Functional Independence Measure | Pre–post intervention mean change in score: FIM total 21.9 ± 19.0; FIM motor 19.1 ± 16.1; FIM cognitive 2.8 ± 4.8 | FIM motor achieved MCID threshold FIM total and FIM cognition did not achieve MCID threshold | FIM motor: statistically significant and also clinically meaningful  FIM total and FIM cognition: statistically significant but not clinically meaningful  |
| 34 | Physical Component Summary (PCS) score of the Short Form 36 (SF-36) | Fu et al., 2021 [42] | 1.8 to 3.0 units | Laffont et al., 2020 * [118] | Rehabilitation of the upper arm early after stroke: Video games versus conventional rehabilitation. A randomized controlled trial | Baseline to 6 month mean change in intervention group: 2.8 ± 6.5 points | Results achieved MCID threshold | Statistically significant and also clinically meaningful |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, B.; Sudheer, P.; Agarwal, A.; Nilima, N.; Srivastava, M.V.P.; Vishnu, V.Y. Minimal Clinically Important Difference of Scales Reported in Stroke Trials: A Review. Brain Sci. 2024, 14, 80. https://doi.org/10.3390/brainsci14010080
Mishra B, Sudheer P, Agarwal A, Nilima N, Srivastava MVP, Vishnu VY. Minimal Clinically Important Difference of Scales Reported in Stroke Trials: A Review. Brain Sciences. 2024; 14(1):80. https://doi.org/10.3390/brainsci14010080
Chicago/Turabian StyleMishra, Biswamohan, Pachipala Sudheer, Ayush Agarwal, Nilima Nilima, Madakasira Vasantha Padma Srivastava, and Venugopalan Y. Vishnu. 2024. "Minimal Clinically Important Difference of Scales Reported in Stroke Trials: A Review" Brain Sciences 14, no. 1: 80. https://doi.org/10.3390/brainsci14010080
APA StyleMishra, B., Sudheer, P., Agarwal, A., Nilima, N., Srivastava, M. V. P., & Vishnu, V. Y. (2024). Minimal Clinically Important Difference of Scales Reported in Stroke Trials: A Review. Brain Sciences, 14(1), 80. https://doi.org/10.3390/brainsci14010080





