What Dietary Vitamins and Minerals Might Be Protective against Parkinson’s Disease?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Dietary Intake
2.3. Assessment of Anthropometric Parameters
2.4. Severity of PD
2.5. Statistical Analysis
3. Results
3.1. Dietary Intake of Vitamins and Minerals
3.2. Dietary Intake of Vitamins and Minerals and Odds of PD
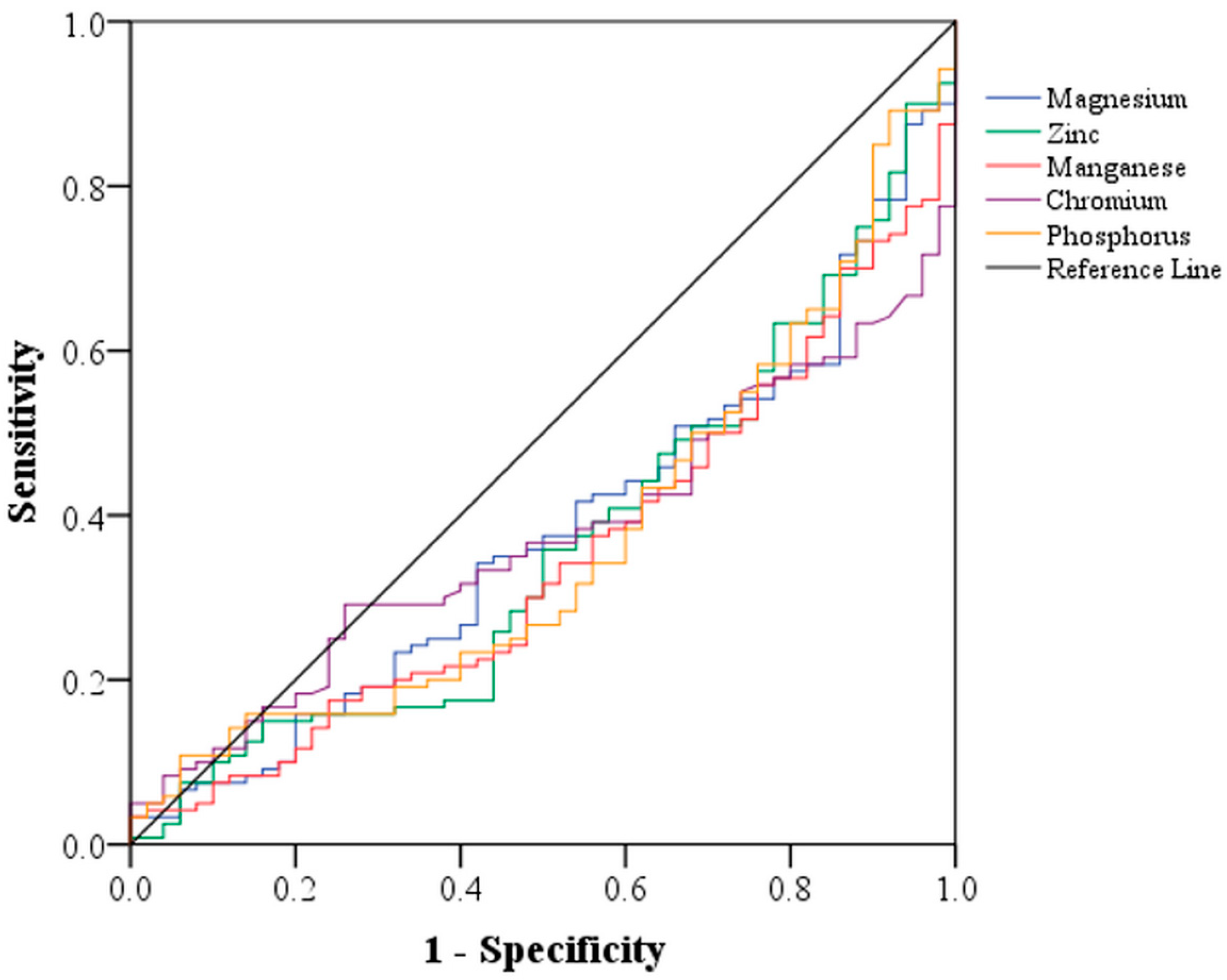
3.3. Area under the Curve (AUC) of Vitamins and Minerals in Predicting Parkinson’s Disease
3.4. Dietary Intake of Vitamins and Minerals and Severity of Parkinson’s Disease
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139 (Suppl. S1), 318–324. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Parkinson’s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Ali, N.; Banerjee, E.; Singh, R.; Naskar, A.; Paidi, R.K.; Mohanakumar, K.P. Low levels of prohibitin in substantia nigra makes dopaminergic neurons vulnerable in Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 804–821. [Google Scholar] [CrossRef] [PubMed]
- Madetko, N.; Migda, B.; Alster, P.; Turski, P.; Koziorowski, D.; Friedman, A. Platelet-to-lymphocyte ratio and neutrophil-tolymphocyte ratio may reflect differences in PD and MSA-P neuroinflammation patterns. Neurol. Neurochir. Pol. 2022, 56, 148–155. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; McGeer, E.G. History of innate immunity in neurodegenerative disorders. Front. Pharmacol. 2011, 2, 77. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Benito, M.; Granado, N.; García-Sanz, P.; Michel, A.; Dumoulin, M.; Moratalla, R. Modeling Parkinson’s Disease with the alpha-synuclein protein. Front. Pharmacol. 2020, 11, 356. [Google Scholar] [CrossRef]
- Dutta, D.; Paidi, R.K.; Raha, S.; Roy, A.; Chandra, S.; Pahan, K. Treadmill exercise reduces α-synuclein spreading via PPARα. Cell Rep. 2022, 40, 111058. [Google Scholar] [CrossRef]
- Rivero-Ríos, P.; Romo-Lozano, M.; Fasiczka, R.; Naaldijk, Y.; Hilfiker, S. LRRK2-related Parkinson’s disease due to altered endolysosomal biology with variable lewy body pathology: A Hypothesis. Front. Neurosci. 2020, 14, 556. [Google Scholar] [CrossRef]
- Goldman, S.M. Environmental toxins and Parkinson’s disease. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 141–164. [Google Scholar] [CrossRef]
- Mischley, L.K.; Lau, R.C.; Bennett, R.D. Role of diet and nutritional supplements in Parkinson’s disease progression. OxidativeMed. Cell Longev. 2017, 2017, 6405278. [Google Scholar] [CrossRef]
- Hughes, K.C.; Gao, X.; Kim, I.Y.; Wang, M.; Weisskopf, M.G.; Schwarzschild, M.A.; Ascherio, A. Intake of dairy foods and risk of Parkinson disease. Neurology 2017, 89, 46–52. [Google Scholar] [CrossRef]
- Agarwal, P.; Wang, Y.; Buchman, A.S.; Holland, T.M.; Bennett, D.A.; Morris, M.C. Dietary antioxidants associated with slower progression of parkinsonian signs in older adults. Nutr. Neurosci. 2022, 25, 550–557. [Google Scholar] [CrossRef]
- Bazán-Rodríguez, L.; Cruz-Vicioso, R.; Cervantes-Arriaga, A.; Alcocer-Salas, A.; Pinto-Solís, D.; Rodríguez-Violante, M. Malnutrition and associated motor and non-motor factors in people with Parkinson’s disease. Rev. Investig. Clin. 2020, 72, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Strikwerda, A.J.; Dommershuijsen, L.J.; Ikram, M.K.; Voortman, T. Diet quality and risk of Parkinson’s disease: The Rotterdam study. Nutrients 2021, 13, 3970. [Google Scholar] [CrossRef] [PubMed]
- Keramati, M.; Musazadeh, V.; Kheirouri, S. Association between Mediterranean diet and Parkinson’s disease in adults: A systematic review and meta-analysis of cohort studies. Mediterr. J. Nutr. Metab. 2022, 15, 1–10. [Google Scholar] [CrossRef]
- Balomenos, V.; Bounou, L.; Charisis, S.; Stamelou, M.; Ntanasi, E.; Georgiadi, K.; Mourtzinos, I.; Tzima, K.; Anastasiou, C.A.; Xiromerisiou, G.; et al. Dietary inflammatory index score and prodromal Parkinson’s disease incidence: The HELIAD study. J. Nutr. Biochem. 2022, 105, 108994. [Google Scholar] [CrossRef]
- Emre, M.; Aarsland, D.; Brown, R.; Burn, D.J.; Duyckaerts, C.; Mizuno, Y.; Broe, G.A.; Cummings, J.; Dickson, D.W.; Gauthier, S.; et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. 2007, 22, 1689–1707, quiz 1837. [Google Scholar] [CrossRef]
- Mirmiran, P.; Esfahani, F.H.; Mehrabi, Y.; Hedayati, M.; Azizi, F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 2010, 13, 654–662. [Google Scholar] [CrossRef]
- Hantikainen, E.; Lagerros, Y.T.; Ye, W.; Serafini, M.; Adami, H.O.; Bellocco, R.; Bonn, S. Dietary antioxidants and the risk of Parkinson disease: The Swedish national march cohort. Neurology 2021, 96, e895–e903. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, T.; Martella, G.; Imbriani, P.; Lazzaro, G.D.; Franco, D.; Colona, V.L.; Alwardat, M.; Salimei, P.S.; Mercuri, N.B.; Pierantozzi, M.; et al. Dietary vitamin E as a protective factor for Parkinson’s disease: Clinical and experimental evidence. Front. Neurol. 2019, 10, 148. [Google Scholar] [CrossRef]
- Miyake, Y.; Fukushima, W.; Tanaka, K.; Sasaki, S.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; Kawamura, N.; et al. Dietary intake of antioxidant vitamins and risk of Parkinson’s disease: A case-control study in Japan. Eur. J. Neurol. 2011, 18, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; LeWitt, P.A.; Xu, K.; Eberly, S.; Watts, A.; Matson, W.R.; Marras, C.; Kieburtz, K.; Rudolph, A.; Bogdanov, M.B.; et al. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch. Neurol. 2009, 66, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Abbott, R.D.; Ross, G.W.; White, L.R.; Sanderson, W.T.; Burchfiel, C.M.; Kashon, M.; Sharp, D.S.; Masaki, K.H.; Curb, J.D.; Petrovitch, H. Environmental, life-style, and physical precursors of clinical Parkinson’s disease: Recent findings from the Honolulu-Asia Aging Study. J. Neurol. 2003, 250 (Suppl. S3), III30-9. [Google Scholar] [CrossRef]
- Kim, J.H.; Hwang, J.; Shim, E.; Chung, E.J.; Jang, S.H.; Koh, S.B. Association of serum carotenoid, retinol, and tocopherol concentrations with the progression of Parkinson’s disease. Nutr. Res. Pract. 2017, 11, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Férnandez-Calle, P.; Molina, J.A.; Jiménez-Jiménez, F.J.; Vázquez, A.; Pondal, M.; García-Ruiz, P.J.; Urra, D.G.; Domingo, J.; Codoceo, R. Serum levels of alpha-tocopherol (vitamin E) in Parkinson’s disease. Neurology 1992, 42, 1064–1066. [Google Scholar] [CrossRef]
- Nicoletti, G.; Crescibene, L.; Scornaienchi, M.; Bastone, L.; Bagalà, A.; Napoli, I.D.; Caracciolo, M.; Quattrone, A. Plasma levels of vitamin E in Parkinson’s disease. Arch. Gerontol. Geriatr. 2001, 33, 7–12. [Google Scholar] [CrossRef]
- Scholefield, M.; Church, S.J.; Xu, J.; Patassini, S.; Hooper, N.M.; Unwin, R.D.; Cooper, G.J.S. Substantively lowered levels of pantothenic acid (vitamin B5) in several regions of the human brain in Parkinson’s disease dementia. Metabolites 2021, 11, 569. [Google Scholar] [CrossRef]
- Xu, J.; Patassini, S.; Begley, P.; Church, S.; Waldvogel, H.J.; Faull, R.L.M.; Unwin, R.D.; Cooper, G.J.S. Cerebral deficiency of vitamin B5 (d-pantothenic acid; pantothenate) as a potentially-reversible cause of neurodegeneration and dementia in sporadic Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2020, 527, 676–681. [Google Scholar] [CrossRef]
- Shao, Y.; Li, T.; Liu, Z.; Wang, X.; Xu, X.; Li, S.; Xu, G.; Le, W. Comprehensive metabolic profiling of Parkinson’s disease by liquid chromatography-mass spectrometry. Mol. Neurodegener. 2021, 16, 4. [Google Scholar] [CrossRef]
- Vascellari, S.; Palmas, V.; Melis, M.; Pisanu, S.; Cusano, R.; Uva, P.; Perra, D.; Madau, V.; Sarchioto, M.; Oppo, V.; et al. Gut microbiota and metabolome alterations associated with Parkinson’s disease. mSystems 2020, 5, e00561-20. [Google Scholar] [CrossRef]
- Baldini, F.; Hertel, J.; Sandt, E.; Thinnes, C.C.; Neuberger-Castillo, L.; Pavelka, L.; Betsou, F.; Krüger, R.; Thiele, I.; NCER-PD Consortium. Parkinson’s disease-associated alterations of the gut microbiome predict disease-relevant changes in metabolic functions. BMC Biol. 2020, 18, 62. [Google Scholar] [CrossRef]
- Semenovich, D.S.; Lukienko, E.P.; Kanunnikova, N.P. Modulating oxidative stress indices and thiol-disulfide balance in the brain structures by pantothenic acid derivatives in an experimental model of Parkinson’s disease. Neurochem. J. 2021, 15, 24–29. [Google Scholar] [CrossRef]
- Jamali, B.; Entezari, M.; Babaei, N.; Hashemi, M. β-carotene Has the Neuroprotective Effects in Parkinson’s Disease by Regulating Mitochondrial Apoptotic Pathway Genes. J. Human Gen. Genom. 2020, 4, e122531. [Google Scholar] [CrossRef]
- Wu, L.Y.; Chen, J.X.; Chen, G.S.; Gao, H.; Huo, J.H.; Pang, Y.F.; Gao, Q.H. Dietary β-carotene and vitamin A and risk of Parkinson disease: A protocol for systematic review and meta-analysis. Medicine 2022, 101, e31002. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.C.; Gao, X.; Kim, I.Y.; Rimm, E.B.; Wang, M.; Weisskopf, M.G.; Schwarzschild, M.A.; Ascherio, A. Intake of antioxidant vitamins and risk of Parkinson’s disease. Mov. Disord. 2016, 31, 1909–1914. [Google Scholar] [CrossRef]
- Murakami, K.; Miyake, Y.; Sasaki, S.; Tanaka, K.; Fukushima, W.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; et al. Fukuoka Kinki Parkinson’s Disease Study Group. Dietary intake of folate, vitamin B6, vitamin B12 and riboflavin and risk of Parkinson’s disease: A case-control study in Japan. Br. J. Nutr. 2010, 104, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Shen, L. Associations between B vitamins and Parkinson’s disease. Nutrients 2015, 7, 7197–7208. [Google Scholar] [CrossRef]
- Jiménez-Jimnénez, F.J.; Molina, J.A.; Hernánz, A.; Fernández-Vivancos, E.; de Bustos, F.; Barcenilla, B.; Gómez-Escalonilla, C.; Zurdo, M.; Berbel, A.; Villanueva, C. Cerebrospinal fluid levels of thiamine in patients with Parkinson’s disease. Neurosci. Lett. 1999, 271, 33–36. [Google Scholar] [CrossRef]
- Laforenza, U.; Patrini, C.; Poloni, M.; Mazzarello, P.; Ceroni, M.; Gajdusek, D.C.; Garruto, R.M. Thiamin mono- and pyrophosphatase activities from brain homogenate of Guamanian amyotrophic lateral sclerosis and parkinsonism-dementia patients. J. Neurol. Sci. 1992, 109, 156–161. [Google Scholar] [CrossRef]
- Håglin, L.; Domellöf, M.; Bäckman, L.; Forsgren, L. Low plasma thiamine and phosphate in male patients with Parkinson’s disease is associated with mild cognitive impairment. Clin. Nutr. ESPEN 2020, 37, 93–99. [Google Scholar] [CrossRef]
- Cowen, M.A.; Green, M.; Bertollo, D.N.; Abbott, K. A treatment for tardive dyskinesia and some other extrapyramidal symptoms. J. Clin. Psychopharmacol. 1997, 17, 190–193. [Google Scholar] [CrossRef]
- Saiki, M.; Matsui, T.; Soya, M.; Kashibe, T.; Shima, T.; Shimizu, T.; Naruto, T.; Kitayoshi, T.; Akimoto, K.; Ninomiya, S.; et al. Thiamine tetrahydrofurfuryl disulfide promotes voluntary activity through dopaminergic activation in the medial prefrontal cortex. Sci. Rep. 2018, 8, 10469. [Google Scholar] [CrossRef]
- Sjöquist, B.; Johnson, H.A.; Neri, A.; Lindén, S. The influence of thiamine deficiency and ethanol on rat brain catecholamines. Drug Alcohol Depend. 1988, 22, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Zhang, Y.X.; Nakamura, S. The effects of thiamine and its phosphate esters on dopamine release in the rat striatum. Neurosci. Lett. 1993, 158, 229–231. [Google Scholar] [CrossRef]
- Cha, J.H.; Kim, W.K.; Ha, A.W.; Kim, M.H.; Chang, M.J. Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr. Res. Pract. 2017, 11, 90–96. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Huang, C.; Chen, Z. A review for the pharmacological effect of lycopene in central nervous system disorders. Pharmacotherapy 2019, 111, 791–801. [Google Scholar] [CrossRef]
- Prema, A.; Janakiraman, U.; Manivasagam, T.; Thenmozhi, A.J. Neuroprotective effect of lycopene against MPTP-induced experimental Parkinson’s disease in mice. Neurosci. Lett. 2015, 599, 12–19. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Zhang, H.; Wang, L.; Wang, T.; Han, Z.; Wu, L.; Liu, G. Effect of plasma vitamin C levels on Parkinson’s disease and age at onset: A Mendelian randomization study. J. Transl. Med. 2021, 19, 221. [Google Scholar] [CrossRef]
- Ide, K.; Yamada, H.; Umegaki, K.; Mizuno, K.; Kawakami, N.; Hagiwara, Y.; Matsumoto, M.; Yoshida, H.; Kim, K.; Shiosaki, E.; et al. Lymphocyte vitamin C levels as potential biomarker for progression of Parkinson’s disease. Nutrition 2015, 31, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, M.; Li, C.; Jiang, X.; Su, Y.; Zhang, Y. Benefits of vitamins in the treatment of Parkinson’s disease. Oxidative Med. Cell. Longev. 2019, 2019, 9426867. [Google Scholar] [CrossRef] [PubMed]
- Palavra, N.C.; Lubomski, M.; Flood, V.M.; Davis, R.L.; Sue, C.M. Increased added sugar consumption is common in Parkinson’s disease. Front. Nutr. 2021, 8, 628845. [Google Scholar] [CrossRef]
- Scholefield, M.; Church, S.J.; Xu, J.; Patassini, S.; Roncaroli, F.; Hooper, N.M.; Unwin, R.D.; Cooper, G.J.S. Widespread decreases in cerebral copper are common to Parkinson’s disease dementia and Alzheimer’s disease dementia. Front. Aging Neurosci. 2021, 13, 641222. [Google Scholar] [CrossRef] [PubMed]
- Talebi, S.; Ghoreishy, S.M.; Jayedi, A.; Travica, N.; Mohammadi, H. Dietary antioxidants and risk of Parkinson’s disease: A systematic review and dose-response meta-analysis of observational studies. Adv. Nutr. 2022, 13, 1493–1504. [Google Scholar] [CrossRef]
- Adani, G.; Filippini, T.; Michalke, B.; Vinceti, M. Selenium and other trace elements in the etiology of Parkinson’s disease: A systematic review and meta-analysis of case-control studies. Neuroepidemiology 2020, 54, 1–23. [Google Scholar] [CrossRef]
- Qureshi, G.A.; Qureshi, A.A.; Memon, S.A.; Parvez, S.H. Impact of selenium, iron, copper and zinc in on/off Parkinson’s patients on L-dopa therapy. J. Neural. Transm. Suppl. 2006, 71, 229–236. [Google Scholar] [CrossRef]
- Oyanagi, K.; Kawakami, E.; Kikuchi-Horie, K.; Ohara, K.; Ogata, K.; Takahama, S.; Wada, M.; Kihira, T.; Yasui, M. Magnesium deficiency over generations in rats with special references to the pathogenesis of the Parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Neuropathology 2006, 26, 115–128. [Google Scholar] [CrossRef]
- Maier, J.A.M.; Locatelli, L.; Fedele, G.; Cazzaniga, A.; Mazur, A. Magnesium and the brain: A focus on neuroinflammation and neurodegeneration. Int. J. Mol. Sci. 2022, 24, 223. [Google Scholar] [CrossRef]
- Al-Harthi, S.; Kharchenko, V.; Mandal, P.; Gourdoupis, S.; Jaremko, T. Zinc ions prevent α-synuclein aggregation by enhancing chaperone function of human serum albumin. Int. J. Biol. Macromol. 2022, 222 Pt B, 2878–2887. [Google Scholar] [CrossRef]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.T.; Chen, K.Y.; Wang, W.; Chiu, J.Y.; Wu, D.; Chao, T.Y.; Hu, C.J.; Chau, K.Y.D.; Bamodu, O.A. Insulin resistance promotes Parkinson’s Disease through aberrant expression of α-Synuclein, mitochondrial dysfunction, and deregulation of the Polo-Like Kinase 2 signaling. Cells 2020, 9, 740. [Google Scholar] [CrossRef]
- Soares, N.M.; Pereira, G.M.; Altmann, V.; de Almeida, R.M.M.; Rieder, C.R.M. Cortisol levels, motor, cognitive and behavioral symptoms in Parkinson’s disease: A systematic review. J. Neural Transm. 2019, 126, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, S.S.; Sarfi, M.; Yousefi, T.; Ahangar, A.A.; Gholinia, H.; Ahangar, R.M.; Maniati, M.; Saadat, P. Comparison of the calcium-related factors in Parkinson’s disease patients with healthy individuals. Casp. J. Intern. Med. 2020, 11, 28–33. [Google Scholar] [CrossRef]
- Håglin, L.; Bäckman, L. Covariation between plasma phosphate and daytime cortisol in early Parkinson’s disease. Brain Behav. 2016, 6, e00556. [Google Scholar] [CrossRef] [PubMed]
- Dai, J. The relative deficiency of potassium ions in nerve cells causes abnormal functions and neurological and mental diseases. Nat. Sci. 2022, 14, 441–447. [Google Scholar] [CrossRef]
- Cisternas, P.; Lindsay, C.B.; Salazar, P.; Silva-Alvarez, C.; Retamales, R.M.; Serrano, F.G.; Vio, C.P.; Inestrosa, N.C. The increased potassium intake improves cognitive performance and attenuates histopathological markers in a model of Alzheimer’s disease. Biochim. Biophys. Acta 2015, 1852, 2630–2644. [Google Scholar] [CrossRef]
- Chen, X.; Xue, B.; Wang, J.; Liu, H.; Shi, L.; Xie, J. Potassium channels: A potential therapeutic target for Parkinson’s disease. Neurosci. Bull. 2018, 34, 341–348. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Y.; Xie, J.; Shi, L. Potassium channels and their emerging role in parkinson’s disease. Brain Res. Bull. 2020, 160, 1–7. [Google Scholar] [CrossRef]

| Variable | Participants | p-Value | |
|---|---|---|---|
| Patients (n = 120) | Healthy (n = 50) | ||
| Age * | 60.8 ± 9.8 | 60.4 ± 9.8 | 0.93 |
| Gender † | 0.98 | ||
| Men | 79 (65.8) | 33 (66) | |
| Women | 41 (34.2) | 17 (34) | |
| Body Mass Index * | 25.3 ± 4.3 | 26.0 ± 5.0 | 0.34 |
| Comorbidities † | |||
| Diabetes mellitus | 10 (8.3) | - | |
| Hypertension | 29 (24.2) | - | |
| Thyroid disorders | 14 (11.7) | - | |
| Cardiovascular disease | 15 (12.5) | - | |
| Smoking † (%) | 11 (9.2) | 12 (24.0) | 0.01 |
| Total UPDRS * | 46.2 ± 25.2 | - | |
| Symptoms of non-motor aspects of experiences of daily living * | 7 ± 5.4 | - | |
| Symptoms of motor aspects of experiences of daily living * | 12 ± 7.2 | - | |
| Symptoms of motor examination * | 26 ± 15.6 | - | |
| Parkinson’s Disease (n = 120) | Healthy Individuals (n = 50) | p | |
|---|---|---|---|
| Vitamin A (mg) * | 424.99 ± 173.87 | 391.64 ± 155.69 | 0.24 |
| β-Carotene (mg) † | 2260.01 (1220.86) | 2125.50 (1613.05) | 0.48 |
| α-Carotene (mg) † | 360.02 (320.82) | 242.01 (242.01) | 0.21 |
| Lutein (mg) † | 1058.08 (602.21) | 1234.79 (621.89) | 0.44 |
| β-Cryptoxanthin (mg) † | 127.71 (129.45) | 116.53 (87.74) | 0.14 |
| Lycopene (mg) * | 4113.21 ± 1749.60 | 4760.59 ± 1638.56 | 0.03 |
| Vitamin C (mg) * | 92.13 ± 41.84 | 80.92 ± 38.86 | 0.11 |
| Vitamin D (µg) † | 0.95 (1.20) | 0.87 (1.08) | 0.92 |
| Vitamin E (mg) * | 11.60 ± 3.22 | 11.08 ± 2.92 | 0.33 |
| α-Tocopherol (mg) * | 7.26 ± 2.10 | 6.59 ± 1.83 | 0.05 |
| Thiamin (mg) * | 1.72 ± 0.49 | 1.89 ± 0.39 | 0.03 |
| Riboflavin (mg) * | 1.58 ± 0.52 | 1.73 ± 0.48 | 0.10 |
| Niacin (mg) * | 19.34 ± 5.39 | 19.84 ± 4.50 | 0.56 |
| Vitamin B6 (mg) * | 1.53 ± 0.40 | 1.69 ± 0.35 | 0.02 |
| Folate (mg) * | 425.47 ± 107.25 | 456.79 ± 95.02 | 0.07 |
| Vitamin B12 (mg) † | 2.70 (2.09) | 3.56 (2.27) | 0.03 |
| Biotin * | 28.29 ± 9.56 | 31.27 ± 8.41 | 0.06 |
| Pantothenic acid (mg) * | 4.55 ± 1.30 | 5.18 ± 1.23 | 0.004 |
| Vitamin K (mg) † | 133.50 (76.58) | 139.63 (85.42) | 0.30 |
| Parkinson’s Disease (n = 120) | Healthy Individuals (n = 50) | p | |
|---|---|---|---|
| Iron (mg) * | 14.42 ± 3.84 | 15.20 ± 3.52 | 0.22 |
| Magnesium (mg) * | 360.68 ± 106.95 | 400.80 ± 90.50 | 0.02 |
| Zinc (mg) * | 10.09 ± 3.14 | 11.30 ± 2.73 | 0.02 |
| Copper (mg) * | 1.45 ± 0.39 | 1.55 ± 0.37 | 0.14 |
| Manganese (mg) * | 6.97 ± 2.66 | 8.19 ± 2.17 | 0.005 |
| Selenium (mg) * | 114.01 ± 40.88 | 129.27 ± 33.63 | 0.02 |
| Chromium * | 0.16 ± 0.12 | 0.20 ± 0.07 | 0.01 |
| Potassium (mg) * | 2754.88 ± 812.78 | 2935.23 ± 817.90 | 0.19 |
| Calcium (mg) * | 791.62 ± 353.43 | 888.02 ± 330.11 | 0.10 |
| Phosphorus (mg) * | 1248.62 ± 402.42 | 1403.77 ± 343.77 | 0.02 |
| Fluorine † | 1925.65 (1660.77) | 2624.42 (1912.97) | 0.25 |
| Q2 (n = 57) | p | Q3 (n = 57) | p | |
|---|---|---|---|---|
| Vitamin A (mg) | 1.52 (0.59, 3.89) | 0.38 | 3.57 (1.05, 12.10) | 0.04 |
| β-Carotene (mg) | 1.59 (0.64, 3.93) | 0.32 | 1.66 (0.61, 4.56) | 0.32 |
| α-Carotene (mg) | 1.92 (0.76, 4.84) | 0.16 | 3.27 (1.18, 9.04) | 0.02 |
| Lutein (Mg) | 1.57 (0.59, 4.16) | 0.36 | 0.96 (0.35, 2.60) | 0.94 |
| β-Cryptoxanthin (mg) | 2.10 (0.85, 5.21) | 0.11 | 3.41 (1.21, 9.60) | 0.02 |
| Lycopene (mg) | 0.53 (0.20, 1.46) | 0.22 | 0.24 (0.09, 0.67) | 0.006 |
| Vitamin C (mg) | 1.21 (0.51, 2.89) | 0.66 | 3.11 (1.11, 8.69) | 0.03 |
| Vitamin D (ug) | 0.87 (0.36, 2.08) | 0.75 | 1.15 (0.46, 2.86) | 0.77 |
| Vitamin E (mg) | 1.49 (0.62, 3.60) | 0.37 | 1.92 (0.69, 5.36) | 0.21 |
| α-Tocopherol (mg) | 2.72 (1.09, 6.78) | 0.03 | 4.31 (1.51, 12.28) | 0.006 |
| Thiamin (mg) | 0.29 (0.09, 0.94) | 0.04 | 0.14 (0.03, 0.64) | 0.01 |
| Riboflavin (mg) | 0.39 (0.14, 1.10) | 0.08 | 0.27 (0.07, 1.09) | 0.07 |
| Niacin (mg) | 1.23 (0.44, 3.43) | 0.70 | 0.75 (0.22, 2.52) | 0.64 |
| Vitamin B6 (mg) | 0.38 (0.13, 1.10) | 0.07 | 0.14 (0.03, 0.66) | 0.01 |
| Folate (Mg) | 0.70 (0.23, 2.11) | 0.52 | 0.28 (0.07, 1.14) | 0.07 |
| Vitamin B12 (mg) | 0.80 (0.29, 2.19) | 0.66 | 0.33 (0.11, 1.03) | 0.06 |
| Biotin | 0.53 (0.21, 1.38) | 0.19 | 0.65 (0.21, 1.99) | 0.45 |
| Pantothenic acid (mg) | 0.47 (0.15, 1.45) | 0.19 | 0.13 (0.03, 0.59) | 0.008 |
| Vitamin K (mg) | 1.18 (0.46, 3.04) | 0.73 | 0.66 (0.24, 1.77) | 0.41 |
| Iron (mg) | 1.14 (0.41, 3.18) | 0.80 | 0.46 (0.14, 1.54) | 0.21 |
| Magnesium (mg) | 0.14 (0.04, 0.46) | 0.001 | 0.11 (0.02, 0.50) | 0.004 |
| Zinc (mg) | 0.40 (0.12, 1.29) | 0.12 | 0.14 (0.03, 0.69) | 0.02 |
| Copper (mg) | 1.13 (0.41, 3.08) | 0.82 | 0.55 (0.17, 1.78) | 0.32 |
| Manganese (mg) | 0.29 (0.10, 0.85) | 0.02 | 0.15 (0.05, 0.49) | 0.002 |
| Selenium (mg) | 0.40 (0.14, 1.12) | 0.08 | 0.35 (0.11, 1.06) | 0.06 |
| Chromium | 0.15 (0.05, 0.45) | 0.001 | 0.20 (0.06, 0.61) | 0.005 |
| Potassium (mg) | 0.59 (0.21, 1.69) | 0.33 | 0.23 (0.05, 1.05) | 0.06 |
| Calcium (mg) | 0.68 (0.25, 1.83) | 0.44 | 0.37 (0.11, 1.26) | 0.11 |
| Phosphorus (mg) | 0.40 (0.13, 1.22) | 0.11 | 0.12 (0.03, 0.52) | 0.005 |
| Fluorine | 1.14 (0.47, 2.77) | 0.77 | 0.92 (0.39, 2.20) | 0.86 |
| Vitamins | AUC (95% CI) | p | Minerals | AUC (95% CI) | p |
|---|---|---|---|---|---|
| Vitamin A | 0.55 (0.45, 0.64) | 0.31 | Magnesium | 0.38 (0.29, 0.46) | 0.01 |
| α-Carotene | 0.56 (0.46, 0.66) | 0.21 | Zinc | 0.37 (0.28, 0.45) | 0.006 |
| β-Cryptoxanthin | 0.57 (0.48, 0.66) | 0.14 | Manganese | 0.35 (0.26, 0.43) | 0.002 |
| Lycopene | 0.37 (0.28, 0.46) | 0.007 | Chromium | 0.37 (0.29, 0.46) | 0.009 |
| Vitamin C | 0.58 (0.49, 0.68) | 0.09 | Phosphorus | 0.37 (0.28, 0.46) | 0.007 |
| α-Tocopherol | 0.59 (0.50, 0.68) | 0.06 | |||
| Thiamin | 0.38 (0.30, 0.47) | 0.02 | |||
| Vitamin B6 | 0.37 (0.28, 0.46) | 0.01 | |||
| Pantothenic acid | 0.35 (0.26, 0.44) | 0.003 |
| Total Score of UPDRS | Non-Motor Aspects of Experiences of Daily Living | Motor Aspects of Experiences of Daily Living | Motor Examination | Motor Complications | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Vitamin A (RAE)(Mg) | −0.12 (−0.05, 0.02) | 0.31 | −0.07 (−0.01, 0.005) | 0.53 | −0.050 (−0.012, 0.008) | 0.68 | −0.14 (−0.03, 0.01) | 0.25 | −0.07 (−0.005, 0.002) | 0.52 |
| β-Carotene (Mg) | −0.21 (−0.01, 0.00) | 0.049 | −0.08 (−0.001, 0.001) | 0.41 | −0.06 (−0.002, 0.001) | 0.55 | −0.27 (−0.01,−0.001) | 0.01 | −0.09 (−0.001, 0.00) | 0.37 |
| α-Carotene (mg) | −0.10 (−0.03, 0.01) | 0.32 | 0.002 (−0.004, 0.004) | 0.98 | 0.02 (−0.005, 0.01) | 0.87 | −0.15 (−0.02, 0.003) | 0.13 | −0.12 (−0.003, 0.001) | 0.21 |
| Lutein (mg) | −0.15 (−0.01, 0.002) | 0.14 | −0.09 (−0.002, 0.001) | 0.36 | −0.02 (−.0003, 0.002) | 0.85 | −0.20 (−0.01,−0.00004) | 0.048 | −0.03 (−0.001, 0.001) | 0.73 |
| β-Cryptoxanthin (mg) | −0.08 (−0.08, 0.03) | 0.45 | 0.14 (−0.003, 0.02) | 0.14 | 0.04 (−0.01, 0.02) | 0.71 | −0.18 (−0.06, 0.004) | 0.09 | −0.11 (−0.01, 0.002) | 0.24 |
| Lycopene (mg) | −0.12 (−0.005, 0.001) | 0.23 | −0.05 (−0.001, 0.0004) | 0.63 | 0.003 (−0.001, 0.001) | 0.97 | −0.16 (−0.003, 0.0003) | 0.12 | −0.14 (0.00, 0.00) | 0.14 |
| Vitamin C (mg) | −0.28 (−0.29,−0.04) | 0.01 | −0.08 (−0.04, 0.01) | 0.41 | −0.11 (−0.05, 0.02) | 0.31 | −0.35 (−0.20,−0.05) | 0.001 | −0.17 (−0.02, 0.002) | 0.08 |
| Vitamin D (µg) | −0.17 (−11.49, 0.98) | 0.10 | −0.14 (−2.15, 0.37) | 0.17 | −0.11 (−2.68, 0.82) | 0.29 | −0.16 (−6.83, 0.87) | 0.13 | −0.14 (−1.06, 0.15) | 0.14 |
| Vitamin E (mg) | −0.09 (−2.43, 1.02) | 0.42 | −0.05 (−0.43, 0.26) | 0.62 | −0.11 (−0.73, 0.22) | 0.30 | −0.08 (−1.44, 0.69) | 0.49 | 0.02 (−0.15, 0.18) | 0.88 |
| α-Tocopherol (mg) | −0.08 (−3.57, 1.73) | 0.49 | −0.05 (−0.66, 0.41) | 0.65 | −0.10 (−1.08, 0.40) | 0.36 | −0.07 (−2.14, 1.13) | 0.54 | 0.04 (−0.21, 0.31) | 0.72 |
| Thiamin (mg) | 0.003 (−17.07, 17.37) | 0.99 | −0.06 (−4.12, 2.81) | 0.71 | −0.04 (−5.44, 4.14) | 0.79 | 0.05 (−9.08, 12.13) | 0.78 | −0.01 (−1.75, 1.60) | 0.93 |
| Riboflavin (mg) | −0.32 (−30.86, 0.33) | 0.05 | −0.20 (−5.21, 1.14) | 0.21 | −0.35 (−9.19,−0.56) | 0.03 | −0.22 (−16.28, 3.11) | 0.18 | −0.35 (−3.27,−0.26) | 0.02 |
| Niacin (mg) | 0.09 (−0.86, 1.72) | 0.51 | 0.06 (−0.20, 0.32) | 0.63 | 0.24 (−0.04, 0.67) | 0.08 | 0.01 (−0.75, 0.84) | 0.92 | 0.01 (−0.12, 0.13) | 0.92 |
| Vitamin B6 (mg) | −0.40 (−47.26,−2.05) | 0.03 | 0.02 (−4.41, 4.90) | 0.92 | −0.10 (−8.26, 4.57) | 0.57 | −0.53 (−34.02,−6.66) | 0.004 | −0.42 (−4.90,−0.53) | 0.01 |
| Folate (mg) | 0.19 (−0.03, 0.12) | 0.24 | −0.03 (−0.02, 0.01) | 0.82 | 0.12 (−0.01, 0.03) | 0.43 | 0.23 (−0.01, 0.08) | 0.15 | 0.16 (−0.003, 0.01) | 0.30 |
| Vitamin B12 (mg) | 0.08 (−2.52, 5.00) | 0.52 | 0.07 (−0.54, 0.98) | 0.57 | 0.03 (−0.94, 1.16) | 0.83 | 0.10 (−1.38, 3.24) | 0.43 | −0.02 (−0.39, 0.34) | 0.88 |
| Biotin | −0.47 (−1.89,−0.61) | <0.001 | −0.20 (−0.25, 0.02) | 0.09 | −0.20 (−0.34, 0.04) | 0.12 | −0.54 (−1.27,−0.50) | <0.001 | −0.36 (−0.16,−0.04) | 0.002 |
| Pantothenic acid (mg) | −0.51 (−16.66,−3.23) | 0.004 | −0.14 (−1.99, 0.81) | 0.41 | −0.26 (−3.36, 0.49) | 0.14 | −0.60 (−11.27,−3.12) | 0.001 | −0.36 (−1.39,−0.06) | 0.03 |
| Vitamin K (mg) | −0.16 (−0.11, 0.01) | 0.10 | −0.08 (−0.02, 0.01) | 0.39 | −0.08 (−0.02, 0.01) | 0.40 | −0.19 (−0.07, 0.001) | 0.06 | −0.05 (−0.01, 0.004) | 0.59 |
| Total Score of UPDRS | Non-Motor Aspects of Experiences of Daily Living | Motor Aspects of Experiences of Daily Living | Motor Examination | Motor Complications | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Calcium (mg) | −0.25 (−0.04, 0.001) | 0.07 | −0.09 (−0.005, 0.002) | 0.46 | −0.38 (−0.01, −0.003) | 0.004 | −0.15 (−0.02, 0.005) | 0.26 | −0.24 (−0.004, 0.00) | 0.06 |
| Iron (mg) | −0.06 (−2.38, 1.58) | 0.69 | −0.10 (−0.55, 0.25) | 0.46 | 0.11 (−0.34, 0.75) | 0.46 | −0.10 (−1.63,0.81) | 0.50 | −0.07 (−0.24, 0.14) | 0.62 |
| Phosphorus (mg) | −0.40 (−0.05, −0.004) | 0.02 | −0.08 (−0.005, 0.003) | 0.63 | −0.30 (−0.01, 0.001) | 0.07 | −0.41 (−0.03, −0.003) | 0.02 | −0.43 (−0.005, −0.001) | 0.01 |
| Magnesium (mg) | −0.45 (−0.17, −0.04) | 0.002 | −0.24 (−0.03, 0.001) | 0.08 | −0.14 (−0.03, 0.01) | 0.32 | −0.52 (−0.12, −0.04) | <0.001 | −0.36 (−0.01, −0.002) | 0.01 |
| Zinc (mg) | −0.38 (−5.62, −0.45) | 0.02 | −0.10 (−0.71, 0.36) | 0.51 | −0.08 (−0.93, 0.54) | 0.60 | −0.48 (−3.97, −0.83) | 0.003 | −0.32 (−0.52, −0.01) | 0.04 |
| Copper (mg) | −0.05 (−22.57, 15.60) | 0.72 | −0.05 (−4.54, 3.15) | 0.72 | 0.15 (−2.52, 8.06) | 0.30 | −0.13 (−16.92, 6.52) | 0.38 | −0.05 (−2.21, 1.50) | 0.71 |
| Manganese (mg) | −0.21 (−4.05, 0.03) | 0.05 | −0.18 (−0.79, 0.04) | 0.07 | −0.04 (−0.68, 0.47) | 0.72 | −0.23 (−2.59, −0.08) | 0.04 | −0.20 (−0.39, 0.003) | 0.05 |
| Selenium (mg) | −0.16 (−0.24, 0.05) | 0.20 | −0.13 (−0.05, 0.01) | 0.25 | 0.05 (−0.03, 0.05) | 0.67 | −0.20 (−0.17, 0.01) | 0.09 | −0.16 (−0.02, 0.004) | 0.14 |
| Fluorine | −0.05 (−0.005, 0.003) | 0.59 | −0.18 (−0.001, 0.000) | 0.048 | −0.03 (−0.001, 0.001) | 0.78 | 0.01 (−0.002, 0.002) | 0.95 | −0.11 (−0.001, 0.00) | 0.26 |
| Chromium | −0.22 (−91.70, −5.61) | 0.03 | −0.13 (−14.93, 2.67) | 0.17 | −0.05 (−15.67, 8.82) | 0.58 | −0.26 (−61.06, −8.46) | 0.01 | −0.19 (−8.54, −0.14) | 0.04 |
| Potassium (mg) | −0.773 (−0.035, −0.013) | <0.001 | −0.34 (−0.005, 0.000) | 0.06 | −0.39 (−0.01, −0.0002) | 0.04 | −0.84 (−0.02, −0.01) | <0.001 | −0.66 (−0.003, −0.001) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alizadeh, M.; Kheirouri, S.; Keramati, M. What Dietary Vitamins and Minerals Might Be Protective against Parkinson’s Disease? Brain Sci. 2023, 13, 1119. https://doi.org/10.3390/brainsci13071119
Alizadeh M, Kheirouri S, Keramati M. What Dietary Vitamins and Minerals Might Be Protective against Parkinson’s Disease? Brain Sciences. 2023; 13(7):1119. https://doi.org/10.3390/brainsci13071119
Chicago/Turabian StyleAlizadeh, Mohammad, Sorayya Kheirouri, and Majid Keramati. 2023. "What Dietary Vitamins and Minerals Might Be Protective against Parkinson’s Disease?" Brain Sciences 13, no. 7: 1119. https://doi.org/10.3390/brainsci13071119
APA StyleAlizadeh, M., Kheirouri, S., & Keramati, M. (2023). What Dietary Vitamins and Minerals Might Be Protective against Parkinson’s Disease? Brain Sciences, 13(7), 1119. https://doi.org/10.3390/brainsci13071119





