Abstract
Background and Purpose: Little is known about the effect of soluble adhesion molecules on malignant brain edema (MBE) after endovascular thrombectomy (EVT). This study aimed to explore the association between serum concentrations of E-selectin and the risk of MBE in patients who received EVT. Methods: Patients with a large vessel occlusion stroke in the anterior circulation who underwent EVT were prospectively recruited. Serum soluble E-selectin concentrations were measured after admission for all patients. MBE was defined as a midline shift of ≥5 mm on follow-up imaging within 72 h after surgery. Multivariate logistic regression analyses were performed to determine the association between E-selectin levels and the risk of MBE. Results: Among the 261 included patients (mean age, 69.7 ± 12.3 years; 166 males), 59 (22.6%) developed MBE. Increasing circulating E-selectin levels were associated with an increased risk of MBE after multivariable adjustment (odds ratios [OR], highest vs. lowest quartile: 3.593; 95% confidence interval [CI], 1.178−10.956; p = 0.025). We further observed a significantly positive association between E-selectin and MBE (per 1-standard deviation increase; OR, 1.988; 95% CI, 1.379−2.866, p = 0.001) when the E-selectin levels were analyzed as a continuous variable. Furthermore, the restricted cubic spline demonstrated a linear correlation between serum E-selectin levels and the risk of MBE (p < 0.001 for linearity). Conclusions: In this prospective study, circulating levels of E-selectin were associated with an increased risk of MBE after EVT. Further mechanistic studies are warranted to elucidate the pathophysiology underlying this association.
1. Introduction
Stroke is a leading cause of death and disability globally, particularly in low- and middle-income countries, and this burden is increasing [1]. Endovascular thrombectomy (EVT) has been recognized as the standard treatment for large vessel occlusion strokes in the anterior cerebral circulation [2,3,4]. The common complication after EVT is malignant brain edema (MBE), which increases the risk of functional dependence and reduces the beneficial effect of EVT treatment [5,6,7,8]. Hence, determining the predictors of MBE may help in understanding the underlying mechanisms and prompting early identification of patients at risk for brain edema after EVT.
E-selectin, also known as CD62 antigen-like family member E, is an inducible endothelial cell surface molecule of 115 kDa that is specifically expressed on endothelial cells activated by various proinflammatory substances [9]. E-Selectin mediates leukocyte rolling on the endothelium and is thus involved in the recruitment of neutrophils, monocytes, and T cells to inflammatory foci. This process might partly induce endothelial dysfunction and an inflammatory response [10,11]. In the murine stroke model, upregulation of E-selectin expression was observed in the ischemic cerebral vasculature after reperfusion and persisted for 24 h. Blocking E-selectin with a monoclonal antibody increased ischemic cortical cerebral blood flow up to 2.6-fold [12]. Clinical studies indicated that several inflammatory factors, including E-selectin, are significantly higher in stroke patients compared with controls, suggesting a possible predictive role for these markers [13,14]. Furthermore, in patients with ischemic stroke, serum sE-selectin levels were significantly associated with the presence of cerebral microbleeds, which further confirmed that E-selectin is involved in endothelial injury after stroke [15]. However, few studies have investigated the relationship between E-selectin levels and MBE in patients treated with EVT.
We therefore conducted this prospective study to determine whether circulating E-selectin concentrations may predict the presence of MBE in large vessel occlusion stroke patients after EVT treatment.
2. Materials and Methods
2.1. Study Population
Between September 2019 and July 2021, patients with large vessel occlusion strokes receiving EVT treatment at Nanjing First Hospital were prospectively included. This study included patients who (1) were ≥18 years old; (2) had occlusion of the internal carotid artery or middle cerebral artery confirmed by preoperative imaging; (3) received EVT treatment with a stent-like retriever and/or aspiration system. Patients were excluded if they were diagnosed with a concomitant aneurysm, arteriovenous malformation, moyamoya disease, or leukemia. The local institutional review board approved the study, and all subjects provided informed consent before entering this study.
2.2. Baseline Data Collection
Baseline data, including demographic characteristics, clinical data, procedural parameters, and laboratory data, were included in this analysis. Admission neurological deficits were evaluated using the National Institutes of Health Stroke Scale (NIHSS) score [16]. The pre-treatment ischemic change was measured by the Alberta Stroke Program Early Computerized Tomography Score (ASPECTS) [17]. Stroke etiology was classified according to the criteria of the Trial of Org 10172 in Acute Stroke Treatment [18]. The American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology grading system was utilized to assess the collateral status [19] and was defined as poor (grades 0–1) or good (grades 2–4). Additionally, successful reperfusion was defined as a modified Thrombolysis in Cerebral Infarction score of 2b–3 [20,21]. Whether patients received intravenous thrombolysis before EVT was also recorded in this study.
2.3. Blood Sampling and Laboratory Methods
Venous blood was drawn within 24 h of admission. Serum E-selectin levels were measured by the enzyme-linked immunosorbent assay kit (Abcam, Cambridge, UK, Cat#: ab100512). The sensitivity was 30 pg/mL, and the range was 24.69–18,000 pg/mL. The intra- and inter-assay coefficients of variation for E-selectin were <10.0% and <10.0%, respectively. All procedures were performed in strict accordance with the manufacturer’s instructions by a laboratory technician who was blinded to the clinical information.
2.4. Diagnosis of MBE
A computed tomography scan was conducted 24–72 h after EVT treatment. According to previous studies [5,22,23], MBE was diagnosed according to the following criteria: (1) parenchymal hypodensity of ≥50% middle cerebral artery territory and signs of local brain swelling such as sulcal effacement and compression of the lateral ventricle; (2) midline shift of ≥5 mm at the septum pellucidum or pineal gland with obliteration of the basal cisterns. The imaging parameters were evaluated by 2 neurologists who were blinded to the clinical data. Disagreements about imaging analysis were resolved through a consensus conference. If disagreements between the reviewers were identified, they were discussed until a consensus was reached.
2.5. Statistical Analysis
Categorical variables were expressed as percentages and analyzed using the χ2 test or Fisher’s exact test. Continuous variables were demonstrated as mean ± standard deviation or medians (interquartile), analyzed using the Mann–Whitney U test, t-test, Kruskal–Wallis test, and one-way analysis of variance, when appropriate. We used 2 multiple-adjusted logistic regression models to explore the relationship between E-selectin levels and MBE. Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, and covariates with a p value < 0.1 in the univariate analysis, including hypertension, baseline NIHSS score, poor collateral status, successful reperfusion, and baseline blood glucose. Furthermore, restricted cubic splines with 4 knots (at the 5th, 35th, 65th, and 95th percentiles) were conducted to explore the pattern and magnitude of the association between serum E-selectin concentrations and the risk of MBE [24].
A p value < 0.05 was considered significant. Statistical analyses were performed with SPSS version 24.0 (IBM, New York, NY, USA) and R statistical software version 4.0.0 (R Foundation, Vienna, Austria).
3. Results
3.1. Baseline Characteristics of the Study Population
In this study, 283 patients with large artery occlusions receiving EVT treatment were screened for analysis. We excluded 14 patients treated without a stent-like retriever or aspiration system; 5 patients diagnosed with a concomitant aneurysm, arteriovenous malformation, moyamoya disease, or leukemia; and 3 patients without follow-up imaging data for evaluating the MBE. Finally, a total of 261 patients (mean age, 69.7 ± 12.3 years; 166 male) were included in this study. Table 1 demonstrates the baseline data of the study population stratified by the quartiles of serum E-selectin levels. Among these patients, the median time from onset to recanalization was 360.0 min, the median pre-treatment ASPECT score was 9.0, and the baseline NIHSS score was 13.0. Successful recanalization was achieved in 226 (86.6%) patients. Poor collateral circulation status was observed in 143 (54.8%) patients. Additionally, based on the TOAST criteria, 46.0% of patients were diagnosed with large-artery atherosclerosis, and 43.7% of patients were diagnosed with cardio-embolism.

Table 1.
Baseline data of the study population stratified by the quartile of E-selectin levels.
3.2. Incidence and Factors Associated with MBE
During hospitalization, 59 (22.6%) developed MBE. The clinical characteristics comparing patients with and without MBE are shown in Table 2. On univariate analysis, hypertension was more common in patients with MBE than in patients without (83.1% versus 68.3%; p = 0.027). Patients with MBE had a higher baseline NIHSS score (median, 12.0 versus 12.0; p = 0.001), blood glucose levels (mean, 8.0 versus 6.9 mmol/L, p = 0.002), and E-selectin levels (median, 8.1 versus 6.8 ng/mL, p = 0.001; Figure 1). Furthermore, poor collateral circulation status (79.7% versus 47.5%; p = 0.001) and unsuccessful recanalization (35.6% versus 6.9%; p = 0.001) were higher in patients with MBE than in patients without MBE.

Table 2.
Comparison of the baseline data in patients with and without malignant brain edema.
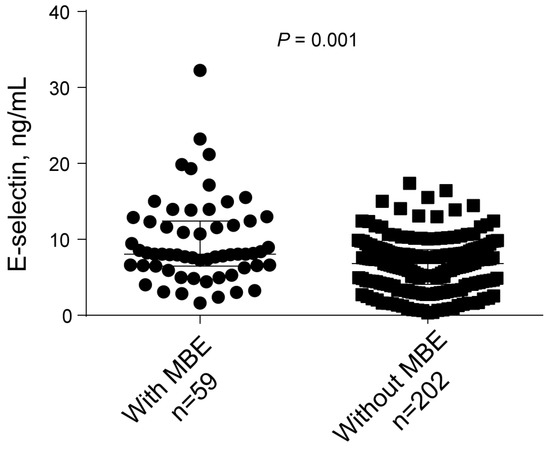
Figure 1.
Univariate analysis of the E-selectin levels with MBE.
3.3. Correlations between E-Selectin Levels and MBE
In multivariate analysis after adjusting for potential confounders, E-selectin levels were associated with an increased risk of MBE after multivariable adjustment (odds ratios [OR], highest vs. lowest quartile: 3.593; 95% confidence interval [CI], 1.178−10.956; p = 0.025) (Table 3). We further observed a significantly positive association between E-selectin and MBE (per 1-standard deviation increase; OR, 1.988; 95% CI, 1.379−2.866, p = 0.001) when the E-selectin levels were analyzed as a continuous variable. Furthermore, the restricted cubic spline demonstrated a linear correlation between serum E-selectin levels and the risk of MBE (p < 0.001 for linearity; Figure 2).

Table 3.
Binary regression analysis for the associations of serum E-selectin levels with malignant brain edema.
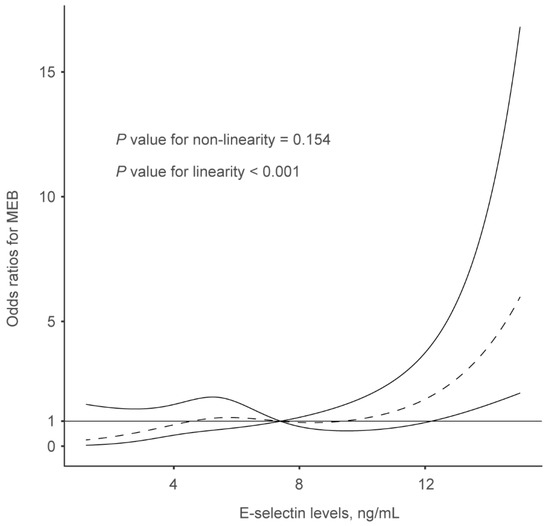
Figure 2.
Restricted cubic spline was utilized to explore the relationship between serum E-selectin levels and malignant brain edema, which fitted with four knots (at the 5th, 35th, 65th, and 95th percentiles), adjusting for covariates with p values < 0.1 in the univariate analysis, including hypertension, baseline NIHSS score, poor collateral status, successful reperfusion, and baseline blood glucose. The median serum E-selectin levels are set as the reference point.
4. Discussion
In this prospective study, higher serum levels of E-selectin were statistically significantly associated with an increased risk of MBE in ischemic stroke patients receiving EVT treatment. This association remained significant after adjusting for confounders including age, sex, hypertension, baseline NIHSS score, poor collateral status, successful reperfusion, and baseline blood glucose.
As one of the serious complications in acute ischemic stroke patients after EVT treatment, MBE might lead to a higher rate of functional dependence mortality at 90 days [5,6,7,8]. Davoli et al. analyzed patients from a prospective single-center database and found that in patients with large intracranial vessel occlusions undergoing EVT, 35.7% of patients developed malignant middle cerebral artery infarction [25]. Then, another study demonstrated that 43.7% of patients experienced midline shifts after EVT [6]. However, our prospective study showed that 22.6% of patients developed MBE, which is similar to data from a retrospective analysis of 130 patients (25.6% of patients with MBE) [5]. Furthermore, previous clinical studies have found several predictive factors for the presence of MBE in patients following EVT [5,6,25,26]. These factors included advanced age, fasting blood glucose, hypertension, baseline NIHSS score, ASPECT score, collateral score, and unsuccessful recanalization. However, this study did not find a significant association between MBE and age or hypertension. Previous researchers investigating the MBE used inconsistent definitions, leading to discrepancies in incidence rates and associated factors. These data indicated the need for an internationally agreed-upon definition of MBE.
E-selectin is a glycoprotein adhesion molecule specifically expressed on activated endothelial cells after ischemic stimulation [27]. As described previously, E-selectin is an independent predictor of clinical outcomes in a population of patients with cerebrovascular diseases [28]. Our study extended the current knowledge about the adverse effect of E-selectin in ischemic stroke as it demonstrated a negative association between E-selectin concentrations and MBE in ischemic stroke patients after EVT. The mechanisms by which circulating E-selectin affects MBE after mechanical recanalization are unclear, but several potential pathophysiological pathways have been suggested. First, increased E-selectin might indicate dysfunction of the vascular endothelium, leading to a subsequent breakdown of blood–brain barrier function after reperfusion [29,30]. Second, as described previously, immunomodulatory and anti-inflammatory cytokine release is targeted to activate blood vessels by the E-selectin antigen specificity of regulatory T cells, which are believed to be associated with brain tissue damage after stroke [29,30,31]. Thirdly, it has been reported that vascular endothelium expression of E-selectin occurs in the first few minutes to hours after ischemic stroke [12,32,33]. This process might induce leukocyte or neutrophil migration into brain tissue, which further contributes to cytokine release and free radical-mediated damage. Finally, hyperglycemia is likely to represent an important link between these two conditions. Our results demonstrated that increased E-selectin levels were associated with baseline blood glucose, and blood glucose has been confirmed as a significant predictor of MBE [5]. Further studies are needed to clarify this potential mechanism.
This study should be approached with caution due to its limitations. First, the E-selectin levels used in this study were only measured from one blood test after admission. Determining the longitudinal changes in circulating E-selectin levels could provide meaningful insights into the presence of MBE. Second, although we utilized a multivariate model to predict MBE with a high degree of predictive accuracy, there may be other confounders and unmeasured markers of MBE. Finally, this was a single-center, small-scale study, which might lead to a selective bias. Therefore, our results need to be confirmed in an external cohort before they can be generalized to different populations. Also, experimental studies are warranted to elucidate the pathophysiologic mechanisms that may underlie the described relationship between E-selectin and MBE risk.
In summary, our data demonstrated that elevated circulating E-selectin levels were significantly associated with the risk of MBE after EVT. Whether treatment of blood–brain barrier dysfunction in patients with large vessel occlusion would improve outcomes is uncertain, but measurement of serum E-selectin may serve as a predictor for the risk of blood–brain barrier breakdown. Further investigations to determine whether E-selectin is a modifiable target to reduce MBE are needed.
Author Contributions
M.D. and Y.Z. designed the study. F.Z., M.D. and Y.E. interpreted the data and wrote the manuscript. F.Z., M.D., Y.E., W.W. and S.C. undertook statistical analyses. H.S., J.Z., M.D. and Y.Z. revised the manuscript. M.D. and Y.Z. supervised the study. All authors have made an intellectual contribution to the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Nanjing First Hospital (2019-695).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Krishnamurthi, R.V.; Ikeda, T.; Feigin, V.L. Global, Regional and Country-Specific Burden of Ischaemic Stroke, Intracerebral Haemorrhage and Subarachnoid Haemorrhage: A Systematic Analysis of the Global Burden of Disease Study 2017. Neuroepidemiology 2020, 54, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Menon, B.K.; Van Zwam, W.H.; Dippel, D.W.J.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.L.M.; Van Der Lugt, A.; De Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Román, L.; Menon, B.; Blasco, J.; Hernández-Pérez, M.; Dávalos, A.; Majoie, C.; Campbell, B.; Guillemin, F.; Lingsma, H.; Anxionnat, R.; et al. Imaging features and safety and efficacy of endo-vascular stroke treatment: A meta-analysis of individual patient-level data. Lancet Neurol. 2018, 17, 895–904. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, Y.; Wang, H.; Yang, D.; Jiang, T.; Yuan, K.; Gong, P.; Xu, P.; Li, Y.; Chen, J.; et al. Symptomatic Intracranial Hemorrhage After Mechanical Throm-bectomy in Chinese Ischemic Stroke Patients: The ASIAN Score. Stroke 2020, 51, 2690–2696. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, Q.; Shi, X.; Xu, X.; Ge, L.; Ding, X.; Zhou, Z. Predictors of malignant brain edema after mechanical thrombectomy for acute ischemic stroke. J. NeuroInterventional Surg. 2019, 11, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, Q.; Deng, Q.; Shen, R.; Liu, Y.; Lu, M.; Shi, H.; Zhou, J. A prediction model of brain edema after endovascular treatment in patients with acute ischemic stroke. J. Neurol. Sci. 2019, 407, 116507. [Google Scholar] [CrossRef] [PubMed]
- Pu, M.; Chen, J.; Chen, Z.; Li, Z.; Li, Z.; Tang, Y.; Li, Q. Predictors and outcome of malignant cerebral edema after successful reperfusion in anterior circulation stroke. J. Stroke Cerebrovasc. Dis. 2023, 32, 107139. [Google Scholar] [CrossRef]
- Cannarsa, G.J.; Wessell, A.P.; Chryssikos, T.; Stokum, J.A.; Kim, K.; Carvalho, H.D.P.; Miller, T.R.; Morris, N.; Badjatia, N.; Chaturvedi, S.; et al. Initial Stress Hyperglycemia Is Associated with Malignant Cerebral Edema, Hemorrhage, and Poor Functional Outcome After Mechanical Thrombectomy. Neurosurgery 2022, 90, 66–71. [Google Scholar] [CrossRef]
- Takeda, H.; Spatz, M.; Ruetzler, C.; McCarron, R.; Becker, K.; Hallenbeck, J. Induction of mucosal tolerance to E-selectin prevents is-chemic and hemorrhagic stroke in spontaneously hypertensive genetically stroke-prone rats. Stroke 2002, 33, 2156–2163. [Google Scholar] [CrossRef]
- Telen, M.J. Cellular adhesion and the endothelium: E-selectin, L-selectin, and pan-selectin inhibitors. Hematol. Oncol. Clin. 2014, 28, 341–354. [Google Scholar] [CrossRef]
- Bitsch, A.; Klene, W.; Murtada, L.; Prange, H.; Rieckmann, P. A Longitudinal Prospective Study of Soluble Adhesion Molecules in Acute Stroke. Stroke 1998, 29, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Choudhri, T.; Winfree, C.; McTaggart, R.; Kiss, S.; Mocco, J.; Kim, L.J.; Protopsaltis, T.S.; Zhang, Y.; Pinsky, D.J.; et al. Postischemic cerebrovascular E-selectin expression mediates tissue injury in murine stroke. Stroke 2000, 31, 3047–3053. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Pinto, A.; Corrao, S.; Di Raimondo, D.; Fernandez, P.; Di Sciacca, R.; Arnao, V.; Licata, G. Immuno-inflammatory and thrombot-ic/fibrinolytic variables associated with acute ischemic stroke diagnosis. Atherosclerosis 2009, 203, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Licata, G.; Tuttolomondo, A.; Corrao, S.; Di Raimondo, D.; Fernandez, P.; Caruso, C.; Avellone, G.; Pinto, A. Immunoinflammatory Activation during the Acute Phase of Lacunar and Non-Lacunar Ischemic Stroke: Association with Time of Onset and Diabetic State. Int. J. Immunopathol. Pharmacol. 2006, 19, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yin, Q.; Sun, W.; Zhu, W.; Li, Y.; Liu, W.; Xiao, L.; Duan, Z.; Cai, Q.; Liu, D.; et al. Microbleeds in ischemic stroke are associated with lower serum adiponectin and higher soluble E-selectin levels. J. Neurol. Sci. 2013, 334, 83–87. [Google Scholar] [CrossRef]
- Sucharew, H.; Khoury, J.; Moomaw, C.J.; Alwell, K.; Kissela, B.M.; Belagaje, S.; Adeoye, O.; Khatri, P.; Woo, D.; Flaherty, M.L.; et al. Profiles of the National Institutes of Health Stroke Scale Items as a Predictor of Patient Outcome. Stroke 2013, 44, 2182–2187. [Google Scholar] [CrossRef]
- Barber, P.; Demchuk, A.M.; Zhang, J.; Buchan, A.M. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 2000, 355, 1670–1674. [Google Scholar] [CrossRef]
- Adams, H.J.; Bendixen, B.; Kappelle, L.; Biller, J.; Love, B.; Gordon, D. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Zaidat, O.O.; Yoo, A.J.; Khatri, P.; Tomsick, T.A.; von Kummer, R.; Saver, J.L.; Marks, M.P.; Prabhakaran, S.; Kallmes, D.F.; Fitzsimmons, B.-F.M.; et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: A consensus statement. Stroke 2013, 44, 2650–2663. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, M.; Feng, C.; Wang, H.; Gong, P.; Jiang, T.; Xie, Y.; Yang, D.; Yuan, K.; Chen, J.; et al. Nomogram predicting early neurological improvement in ischaemic stroke patients treated with endovascular thrombectomy. Eur. J. Neurol. 2021, 28, 152–160. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, K.; Wang, H.; Gong, P.; Jiang, T.; Xie, Y.; Sheng, L.; Liu, D.; Liu, X.; Xu, G. Nomogram to Predict Mortality of Endovascular Thrombectomy for Ischemic Stroke Despite Successful Recanalization. J. Am. Heart Assoc. 2020, 9, e014899. [Google Scholar] [CrossRef]
- Kimberly, W.T.; Dutra, B.G.; Boers, A.M.M.; Alves, H.C.B.R.; Berkhemer, O.A.; Berg, L.V.D.; Sheth, K.N.; Roos, Y.B.W.E.M.; Van Der Lugt, A.; Beenen, L.F.M.; et al. Association of Reperfusion with Brain Edema in Patients With Acute Ischemic Stroke: A Secondary Analysis of the MR CLEAN Trial. JAMA Neurol. 2018, 75, 453–461. [Google Scholar] [CrossRef]
- Ong, C.; Gluckstein, J.; Laurido-Soto, O.; Yan, Y.; Dhar, R.; Lee, J. Enhanced Detection of Edema in Malignant Anterior Circulation Stroke (EDEMA) Score: A Risk Prediction Tool. Stroke 2017, 48, 1969–1972. [Google Scholar] [CrossRef]
- Durrleman, S.; Simon, R. Flexible regression models with cubic splines. Stat. Med. 1998, 8, 551–561. [Google Scholar] [CrossRef]
- Davoli, A.; Motta, C.; Koch, G.; Diomedi, M.; Napolitano, S.; Giordano, A.; Panella, M.; Morosetti, D.; Fabiano, S.; Floris, R.; et al. Pretreatment predictors of malignant evolution in patients with ischemic stroke undergoing mechanical thrombectomy. J. NeuroInterv. Surg. 2018, 10, 340–344. [Google Scholar] [CrossRef]
- Wiącek, M.; Szymański, M.; Walewska, K.; Bartosik-Psujek, H. Blood Pressure Changes During Mechanical Thrombectomy for Acute Ischemic Stroke Are Associated with Serious Early Treatment Complications: Symptomatic Intracerebral Hemorrhage and Malignant Brain Edema. Front. Neurol. 2022, 13, 884519. [Google Scholar] [CrossRef]
- Chiu, J.-J.; Chen, L.-J.; Lee, C.-I.; Lee, P.-L.; Lee, D.-Y.; Tsai, M.-C.; Lin, C.-W.; Usami, S.; Chien, S. Mechanisms of induction of endothelial cell E-selectin expression by smooth muscle cells and its inhibition by shear stress. Blood 2007, 110, 519–528. [Google Scholar] [CrossRef]
- Richard, S.; Lagerstedt, L.; Burkhard, P.; Debouverie, M.; Turck, N.; Sanchez, J. E-selectin and vascular cell adhesion molecule-1 as bi-omarkers of 3-month outcome in cerebrovascular diseases. J. Inflamm. 2015, 12, 61. [Google Scholar] [CrossRef]
- Schram, M.T.; Stehouwer, C.D.A. Endothelial Dysfunction, Cellular Adhesion Molecules and the Metabolic Syndrome. Horm. Metab. Res. 2005, 37, 49–55. [Google Scholar] [CrossRef]
- Ling, S.; Nheu, L.; Komesaroff, P.A. Cell adhesion molecules as pharmaceutical target in atherosclerosis. Mini-Reviews Med. Chem. 2012, 12, 175–183. [Google Scholar] [CrossRef]
- Chen, Y.; Ruetzler, C.; Pandipati, S.; Spatz, M.; McCarron, R.M.; Becker, K.; Hallenbeck, J.M. Mucosal tolerance to E-selectin provides cell-mediated protection against ischemic brain injury. Proc. Natl. Acad. Sci. USA 2003, 100, 15107–15112. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Upadhyay, U.M.; Tamargo, R.J. Inflammation in stroke and focal cerebral ischemia. Surg. Neurol. 2006, 66, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-J.; Cheng, J.-W.; Zhang, J.; Liu, A.-J.; Liu, W.; Guo, W.; Shen, F.-M.; Lu, G.-C. E-selectin Deficiency Attenuates Brain Ischemia in Mice. CNS Neurosci. Ther. 2012, 18, 903–908. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).