Operational Modal Analysis of Near-Infrared Spectroscopy Measure of 2-Month Exercise Intervention Effects in Sedentary Older Adults with Diabetes and Cognitive Impairment
Abstract
1. Introduction
2. Methods
2.1. Study Design
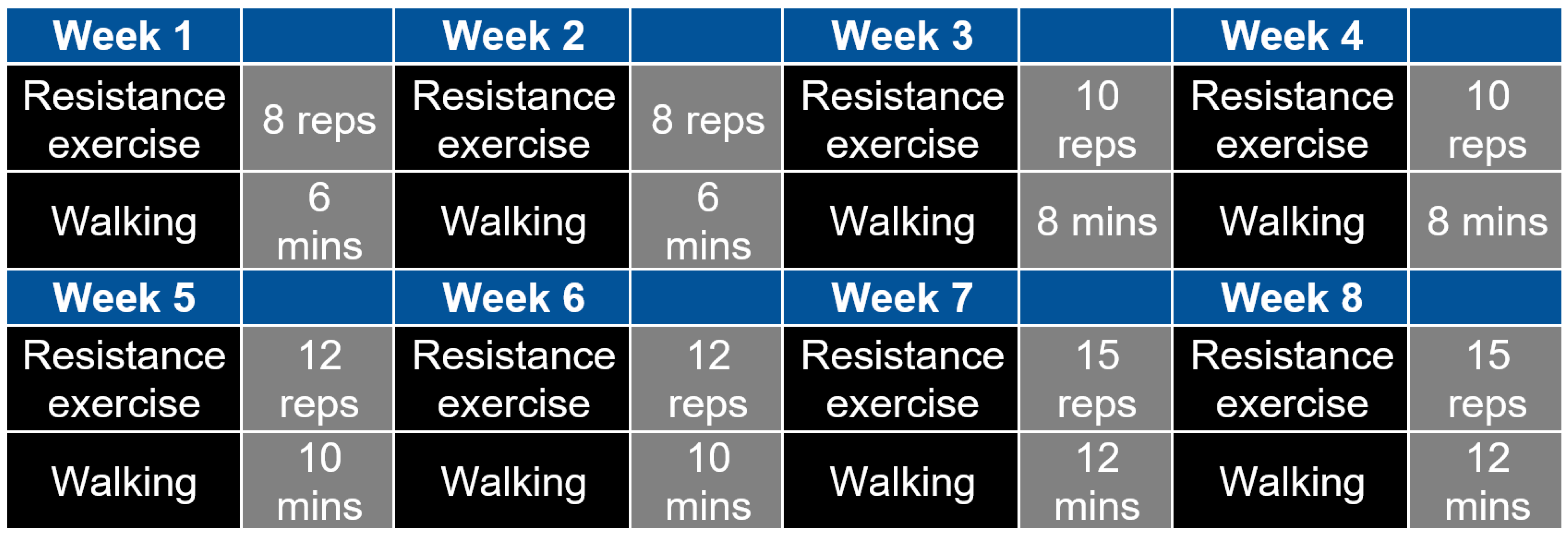
2.2. Development of the 2-Month Exercise Intervention
2.3. Sample Size
2.4. Inclusion and Exclusion Criteria
2.5. Experimental Test Protocol
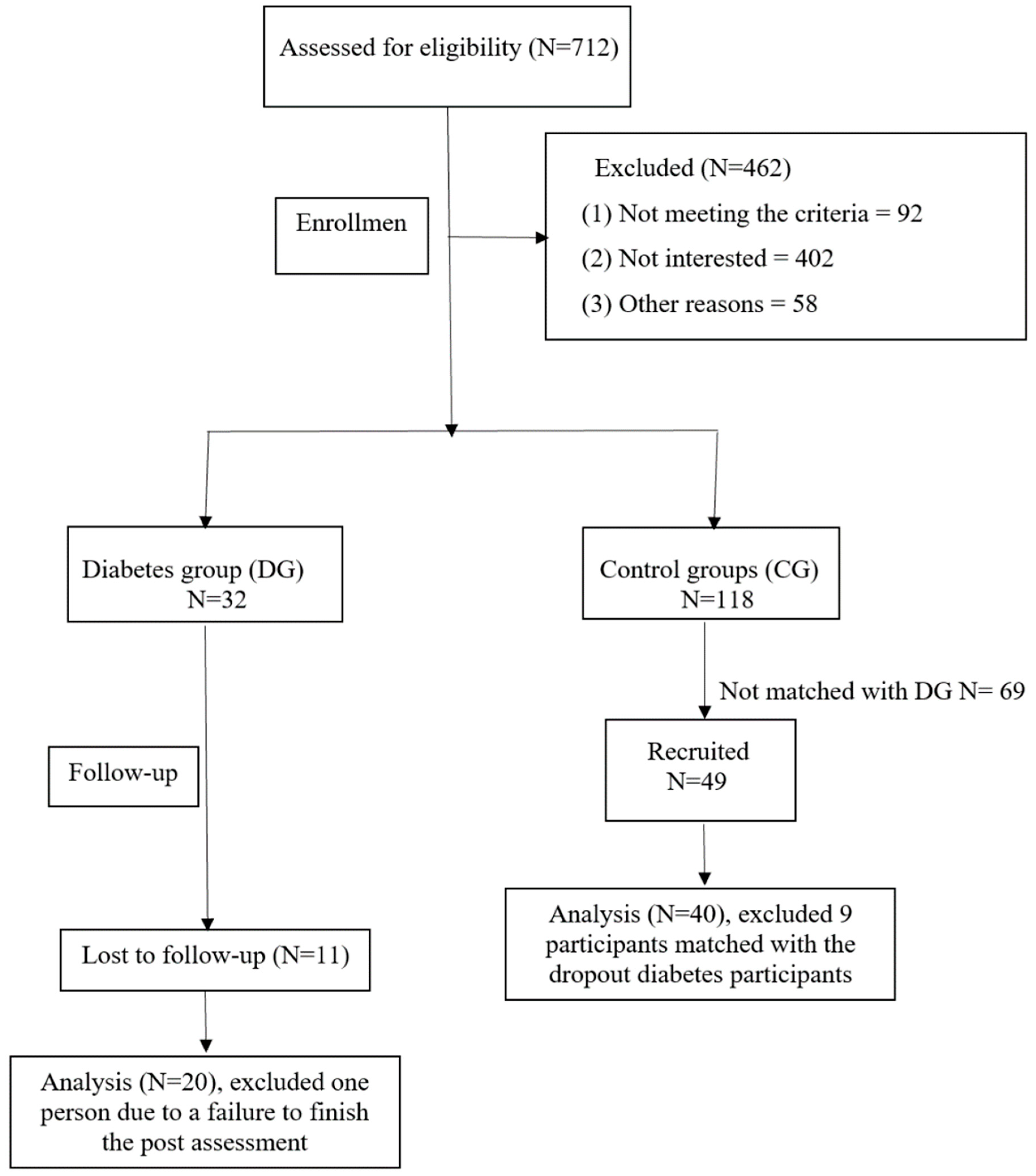
2.5.1. Recruitment
2.5.2. Pre- and Post-Intervention Measurements
Pre-Intervention Cognitive Performance
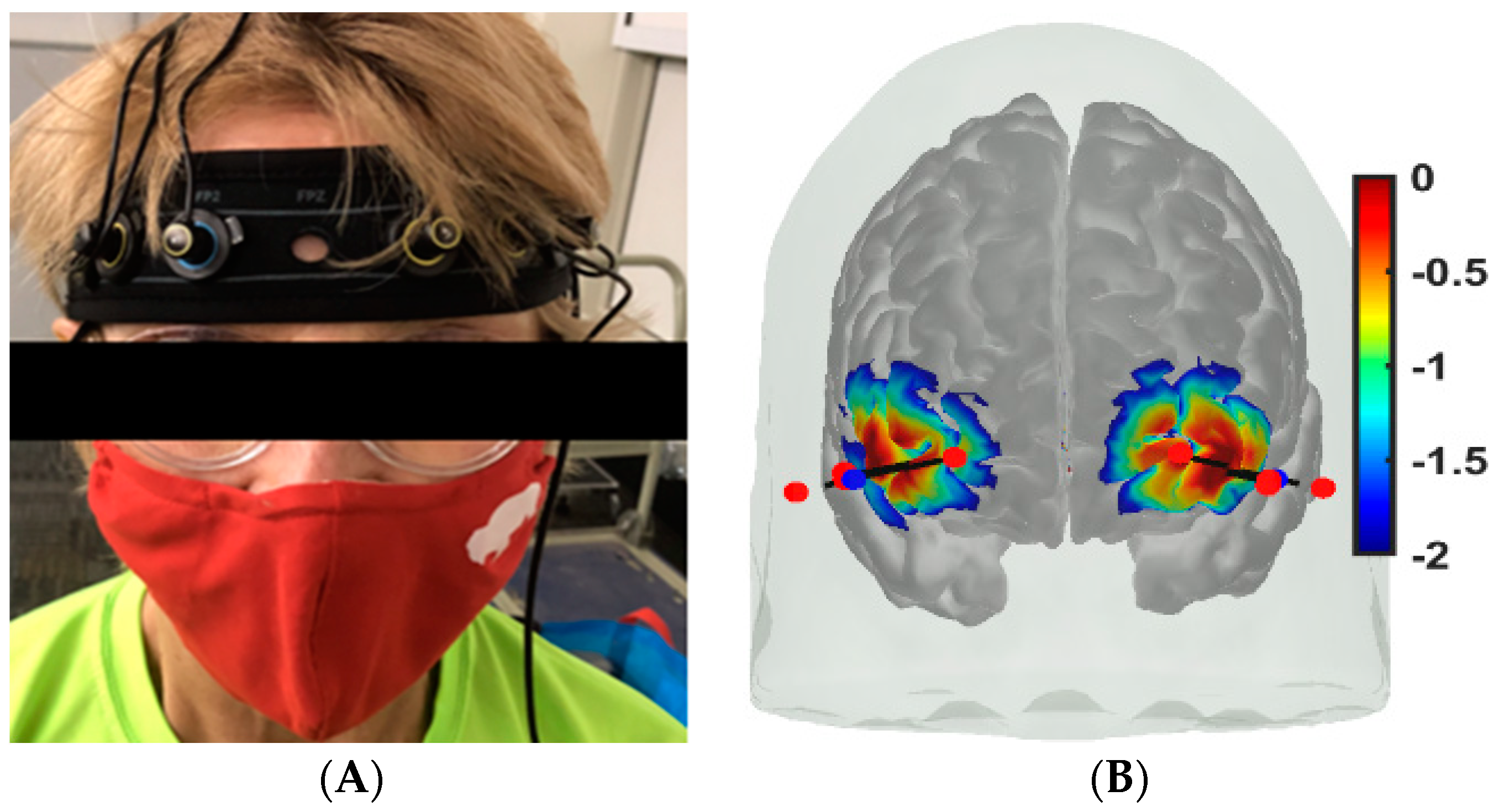
Cerebral Oxygenation Measures
Muscle Oxygenation Measures
Cognitive and Physical Function Tasks
2.6. Statistical Analysis
3. Results
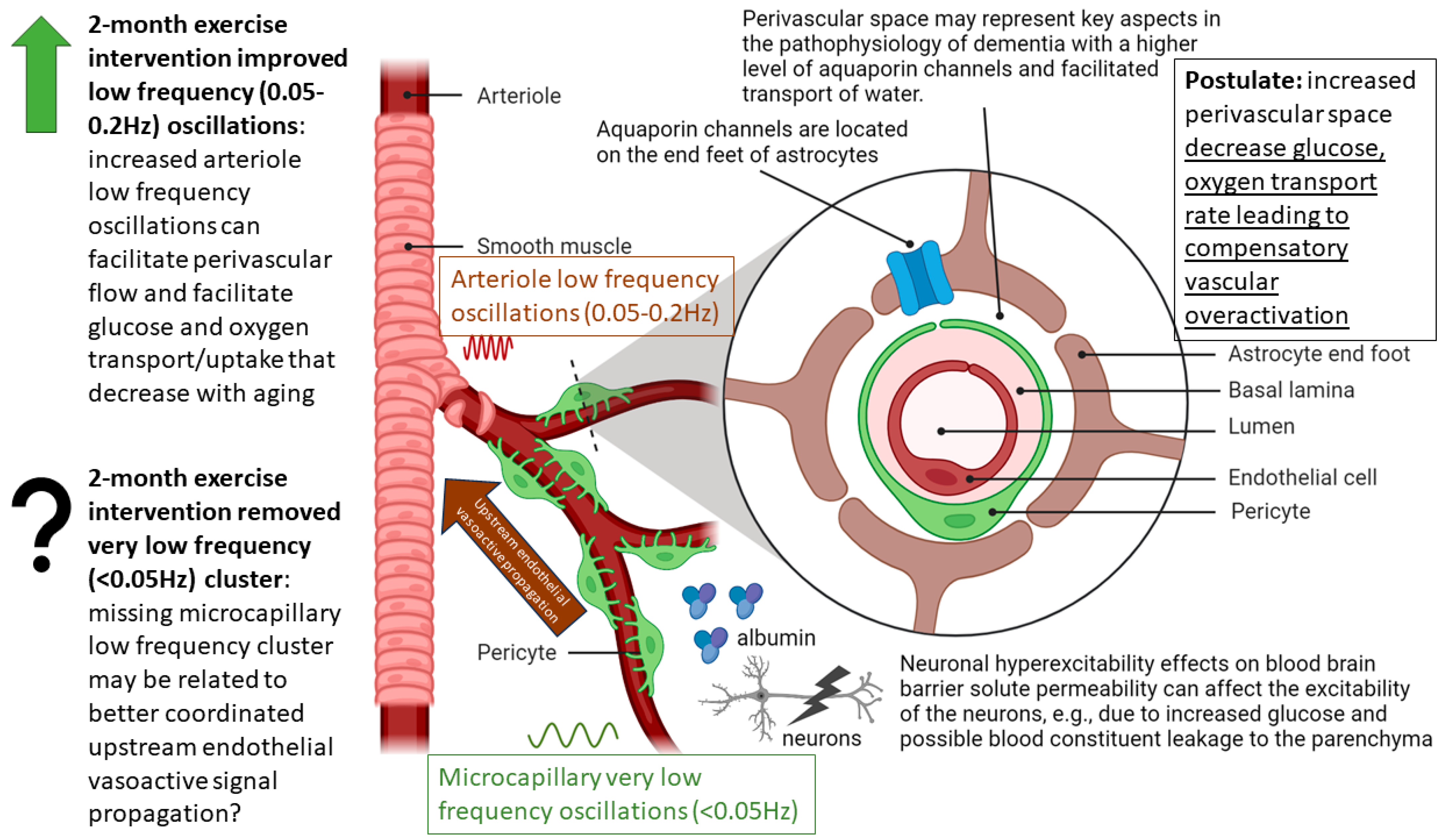
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Meisl, G.; Hidari, E.; Allinson, K.; Rittman, T.; DeVos, S.L.; Sanchez, J.S.; Xu, C.K.; Duff, K.E.; Johnson, K.A.; Rowe, J.B.; et al. In Vivo Rate-Determining Steps of Tau Seed Accumulation in Alzheimer’s Disease. Sci. Adv. 2021, 7, eabh1448. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; Thirumala, V.; Reddy, P.H. Is Alzheimer’s Disease a Type 3 Diabetes? A Critical Appraisal. Biochim. Et Biophys. Acta 2017, 1863, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and Exercise in the Prevention and Treatment of Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes—2016 Abridged for Primary Care Providers. Clin. Diabetes 2016, 34, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Ford, E.S.; Li, C.; Balluz, L.S. Physical Activity in U.S. Older Adults with Diabetes Mellitus: Prevalence and Correlates of Meeting Physical Activity Recommendations. J. Am. Geriatr. Soc. 2011, 59, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Cuff, D.J.; Meneilly, G.S.; Martin, A.; Ignaszewski, A.; Tildesley, H.D.; Frohlich, J.J. Effective Exercise Modality to Reduce Insulin Resistance in Women with Type 2 Diabetes. Diabetes Care 2003, 26, 2977–2982. [Google Scholar] [CrossRef]
- Hamasaki, H. Daily Physical Activity and Type 2 Diabetes: A Review. World J. Diabetes 2016, 7, 243–251. [Google Scholar] [CrossRef]
- Özdirenç, M.; Biberoğlu, S.; Özcan, A. Evaluation of Physical Fitness in Patients with Type 2 Diabetes Mellitus. Diabetes Res. Clin. Pract. 2003, 60, 171–176. [Google Scholar] [CrossRef]
- Advika, T.S.; Idiculla, J.; Kumari, S.J. Exercise in Patients with Type 2 Diabetes: Facilitators and Barriers—A Qualitative Study. J. Fam. Med. Prim. Care 2017, 6, 288–292. [Google Scholar] [CrossRef]
- Fagour, C.; Gonzalez, C.; Pezzino, S.; Florenty, S.; Rosette-Narece, M.; Gin, H.; Rigalleau, V. Low Physical Activity in Patients with Type 2 Diabetes: The Role of Obesity. Diabetes Metab. 2013, 39, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.T.; Hamilton, D.G.; Zderic, T.W. Sedentary Behavior as a Mediator of Type 2 Diabetes. Med. Sport. Sci. 2014, 60, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Cheung, M.; Dutta, A.; Fisher, N.; Tomita, M. Exercises to Determine Older Adults’ Muscle Oxygenation Change Rate by Various Physical Performance Levels. Arch. Phys. Med. Rehabil. 2019, 100, e178–e179. [Google Scholar] [CrossRef]
- Dutta, A.; Zhao, F.; Cheung, M.; Das, A.; Tomita, M.; Chatterjee, K. Cerebral and Muscle Near-Infrared Spectroscopy during Lower-Limb Muscle Activity—Volitional and Neuromuscular Electrical Stimulation. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Online, 1–5 November 2021; pp. 6577–6580. [Google Scholar]
- Zhao, F.; Dutta, A.; Tomita, M. Reduced Muscle Oxidative Capacity During and After Exercise in Older Adults with Obesity. Innov. Aging 2021, 5, 681. [Google Scholar] [CrossRef]
- Zhao, F.; Tomita, M.R.; Dutta, A. Functional Near-Infrared Spectroscopy of Prefrontal Cortex during Memory Encoding and Recall in Elderly with Type 2 Diabetes Mellitus. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2022, 2022, 3323–3326. [Google Scholar] [CrossRef] [PubMed]
- Bicciato, G.; Keller, E.; Wolf, M.; Brandi, G.; Schulthess, S.; Friedl, S.G.; Willms, J.F.; Narula, G. Increase in Low-Frequency Oscillations in FNIRS as Cerebral Response to Auditory Stimulation with Familiar Music. Brain Sci. 2021, 12, 42. [Google Scholar] [CrossRef]
- Arora, Y.; Dutta, A. Human-in-the-Loop Optimization of Transcranial Electrical Stimulation at the Point of Care: A Computational Perspective. Brain Sci. 2022, 12, 1294. [Google Scholar] [CrossRef] [PubMed]
- Arora, Y.; Walia, P.; Hayashibe, M.; Muthalib, M.; Chowdhury, S.R.; Perrey, S.; Dutta, A. Grey-Box Modeling and Hypothesis Testing of Functional near-Infrared Spectroscopy-Based Cerebrovascular Reactivity to Anodal High-Definition TDCS in Healthy Humans. PLoS Comput. Biol. 2021, 17, e1009386. [Google Scholar] [CrossRef]
- Hagan, B.; Mujumdar, R.; Sahoo, J.P.; Das, A.; Dutta, A. Technical Feasibility of Multimodal Imaging in Neonatal Hypoxic-Ischemic Encephalopathy from an Ovine Model to a Human Case Series. Front. Pediatr. 2023, 11, 1072663. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; Al-Jiffri, O.H.; Al-Shreef, F.M. Aerobic Exercises Alleviate Symptoms of Fatigue Related to Inflammatory Cytokines in Obese Patients with Type 2 Diabetes. Afr. Health Sci. 2015, 15, 1142–1148. [Google Scholar] [CrossRef]
- Radaelli, R.; Fleck, S.J.; Leite, T.; Leite, R.D.; Pinto, R.S.; Fernandes, L.; Simão, R. Dose-Response of 1, 3, and 5 Sets of Resistance Exercise on Strength, Local Muscular Endurance, and Hypertrophy. J. Strength. Cond. Res. 2015, 29, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Peterson, M.D.; Ogborn, D.; Contreras, B.; Sonmez, G.T. Effects of Low- vs. High-Load Resistance Training on Muscle Strength and Hypertrophy in Well-Trained Men. J. Strength. Cond. Res. 2015, 29, 2954–2963. [Google Scholar] [CrossRef]
- Poirier, P.; Garneau, C.; Bogaty, P.; Nadeau, A.; Marois, L.; Brochu, C.; Gingras, C.; Fortin, C.; Jobin, J.; Dumesnil, J.G. Impact of Left Ventricular Diastolic Dysfunction on Maximal Treadmill Performance in Normotensive Subjects with Well-Controlled Type 2 Diabetes Mellitus. Am. J. Cardiol. 2000, 85, 473–477. [Google Scholar] [CrossRef] [PubMed]
- DeVan, A.E.; Anton, M.M.; Cook, J.N.; Neidre, D.B.; Cortez-Cooper, M.Y.; Tanaka, H. Acute Effects of Resistance Exercise on Arterial Compliance. J. Appl. Physiol. 2005, 98, 2287–2291. [Google Scholar] [CrossRef] [PubMed]
- Karanasios, E.; Ryan-Stewart, H.; Faulkner, J. The Acute Effects of Resistance Training on Arterial Stiffness: A Systematic Review. J. Trainology 2023, 12, 5–13. [Google Scholar] [CrossRef]
- Tanaka, H.; Dinenno, F.A.; Monahan, K.D.; Clevenger, C.M.; DeSouza, C.A.; Seals, D.R. Aging, Habitual Exercise, and Dynamic Arterial Compliance. Circulation 2000, 102, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Zhu, L. The Crosstalk between Autonomic Nervous System and Blood Vessels. Int. J. Physiol. Pathophysiol. Pharmacol. 2018, 10, 17–28. [Google Scholar]
- Miyachi, M.; Kawano, H.; Sugawara, J.; Takahashi, K.; Hayashi, K.; Yamazaki, K.; Tabata, I.; Tanaka, H. Unfavorable Effects of Resistance Training on Central Arterial Compliance. Circulation 2004, 110, 2858–2863. [Google Scholar] [CrossRef]
- Boutouyrie, P.; Lacolley, P.; Girerd, X.; Beck, L.; Safar, M.; Laurent, S. Sympathetic Activation Decreases Medium-Sized Arterial Compliance in Humans. Am. J. Physiol. 1994, 267, H1368–H1376. [Google Scholar] [CrossRef]
- Canna, A.; Esposito, F.; Tedeschi, G.; Trojsi, F.; Passaniti, C.; di Meo, I.; Polito, R.; Maiorino, M.I.; Paolisso, G.; Cirillo, M.; et al. Neurovascular Coupling in Patients with Type 2 Diabetes Mellitus. Front. Aging Neurosci. 2022, 14, 976340. [Google Scholar] [CrossRef] [PubMed]
- Rorbach-Dolata, A.; Piwowar, A. Neurometabolic Evidence Supporting the Hypothesis of Increased Incidence of Type 3 Diabetes Mellitus in the 21st Century. Biomed. Res. Int. 2019, 2019, 1435276. [Google Scholar] [CrossRef] [PubMed]
- Filosa, J.A.; Bonev, A.D.; Nelson, M.T. Calcium Dynamics in Cortical Astrocytes and Arterioles During Neurovascular Coupling. Circ. Res. 2004, 95, e73–e81. [Google Scholar] [CrossRef] [PubMed]
- Leybaert, L. Neurobarrier Coupling in the Brain: A Partner of Neurovascular and Neurometabolic Coupling? J. Cereb. Blood Flow. Metab. 2005, 25, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Freeman, R.D. Neurometabolic Coupling between Neural Activity, Glucose and Lactate in Activated Visual Cortex. J. Neurochem. 2015, 135, 742–754. [Google Scholar] [CrossRef]
- Barloese, M.C.J.; Bauer, C.; Petersen, E.T.; Hansen, C.S.; Madsbad, S.; Siebner, H.R. Neurovascular Coupling in Type 2 Diabetes with Cognitive Decline. A Narrative Review of Neuroimaging Findings and Their Pathophysiological Implications. Front. Endocrinol. 2022, 13, 874007. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.J.; Botella, J.; Genders, A.J.; Lee, M.J.-C.; Saner, N.J.; Kuang, J.; Yan, X.; Granata, C. High-Intensity Exercise and Mitochondrial Biogenesis: Current Controversies and Future Research Directions. Physiology 2019, 34, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Oonthonpan, L.; Sheldon, R.D.; Rauckhorst, A.J.; Zhu, Z.; Tompkins, S.C.; Cho, K.; Grzesik, W.J.; Gray, L.R.; Scerbo, D.A.; et al. Impaired Skeletal Muscle Mitochondrial Pyruvate Uptake Rewires Glucose Metabolism to Drive Whole-Body Leanness. eLife 2019, 8, e45873. [Google Scholar] [CrossRef]
- Hody, S.; Warren, B.E.; Votion, D.-M.; Rogister, B.; Lemieux, H. Eccentric Exercise Causes Specific Adjustment in Pyruvate Oxidation by Mitochondria. Med. Sci. Sports Exerc. 2022, 54, 1300–1308. [Google Scholar] [CrossRef]
- Limpawattana, P.; Manjavong, M. The Mini-Cog, Clock Drawing Test, and Three-Item Recall Test: Rapid Cognitive Screening Tools with Comparable Performance in Detecting Mild NCD in Older Patients. Geriatrics 2021, 6, 91. [Google Scholar] [CrossRef]
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The Present and Future Use of Functional Near-infrared Spectroscopy (FNIRS) for Cognitive Neuroscience. Ann. N. Y. Acad. Sci. 2020, 1464, 5–29. [Google Scholar] [CrossRef]
- Belardinelli, R.; Georgiou, D.; Scocco, V.; Barstow, T.J.; Purcaro, A. Low Intensity Exercise Training in Patients with Chronic Heart Failure. J. Am. Coll. Cardiol. 1995, 26, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Grassi, B.; Quaresima, V.; Marconi, C.; Ferrari, M.; Cerretelli, P. Blood Lactate Accumulation and Muscle Deoxygenation during Incremental Exercise. J. Appl. Physiol. 1999, 87, 348–355. [Google Scholar] [CrossRef] [PubMed]
- van der Zwaard, S.; de Ruiter, C.J.; Noordhof, D.A.; Sterrenburg, R.; Bloemers, F.W.; de Koning, J.J.; Jaspers, R.T.; van der Laarse, W.J. Maximal Oxygen Uptake Is Proportional to Muscle Fiber Oxidative Capacity, from Chronic Heart Failure Patients to Professional Cyclists. J. Appl. Physiol. 2016, 121, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.; Zierath, J.R. Exercise Metabolism and the Molecular Regulation of Skeletal Muscle Adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, A.S.; Lin, C.-T.; Sanchez, D.M.; Laskin, J.; Spangenburg, E.E.; Neufer, P.D.; Fisher–Wellman, K.; Ellis, J.M. Skeletal Muscle Undergoes Fiber Type Metabolic Switch without Myosin Heavy Chain Switch in Response to Defective Fatty Acid Oxidation. Mol. Metab. 2022, 59, 101456. [Google Scholar] [CrossRef]
- Jacobs, R.A.; Díaz, V.; Soldini, L.; Haider, T.; Thomassen, M.; Nordsborg, N.B.; Gassmann, M.; Lundby, C. Fast-Twitch Glycolytic Skeletal Muscle Is Predisposed to Age-Induced Impairments in Mitochondrial Function. J. Gerontol. Ser. A 2013, 68, 1010–1022. [Google Scholar] [CrossRef]
- Bourdeau Julien, I.; Sephton, C.F.; Dutchak, P.A. Metabolic Networks Influencing Skeletal Muscle Fiber Composition. Front. Cell Dev. Biol. 2018, 6, 125. [Google Scholar] [CrossRef]
- Barstow, T.J. Understanding near Infrared Spectroscopy and Its Application to Skeletal Muscle Research. J. Appl. Physiol. 2019, 126, 1360–1376. [Google Scholar] [CrossRef]
- Farzam, P.; Starkweather, Z.; Franceschini, M.A. Validation of a Novel Wearable, Wireless Technology to Estimate Oxygen Levels and Lactate Threshold Power in the Exercising Muscle. Physiol. Rep. 2018, 6, e13664. [Google Scholar] [CrossRef]
- Jones, S.; Chiesa, S.T.; Chaturvedi, N.; Hughes, A.D. Recent Developments in Near-Infrared Spectroscopy (NIRS) for the Assessment of Local Skeletal Muscle Microvascular Function and Capacity to Utilise Oxygen. Artery Res. 2016, 16, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Lagerwaard, B.; Nieuwenhuizen, A.G.; de Boer, V.C.J.; Keijer, J. In Vivo Assessment of Mitochondrial Capacity Using NIRS in Locomotor Muscles of Young and Elderly Males with Similar Physical Activity Levels. GeroScience 2019, 42, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Boone, J.; Celie, B.; Dumortier, J.; Barstow, T.J.; De Bleecker, J.; Smet, J.; Van Lander, A.; Van Coster, R.; Bourgois, J. Forearm Muscle Oxygenation Responses during and Following Arterial Occlusion in Patients with Mitochondrial Myopathy. Respir. Physiol. Neurobiol. 2014, 190, 70–75. [Google Scholar] [CrossRef]
- Malagoni, A.M.; Felisatti, M.; Mandini, S.; Mascoli, F.; Manfredini, R.; Basaglia, N.; Zamboni, P.; Manfredini, F. Resting Muscle Oxygen Consumption by Near-Infrared Spectroscopy in Peripheral Arterial Disease: A Parameter to Be Considered in a Clinical Setting? Angiology 2010, 61, 530–536. [Google Scholar] [CrossRef]
- Vardi, M.; Nini, A. Near-Infrared Spectroscopy for Evaluation of Peripheral Vascular Disease. A Systematic Review of Literature. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 68–74. [Google Scholar] [CrossRef]
- Malagoni, A.M.; Felisatti, M.; Lamberti, N.; Basaglia, N.; Manfredini, R.; Salvi, F.; Zamboni, P.; Manfredini, F. Muscle Oxygen Consumption by NIRS and Mobility in Multiple Sclerosis Patients. BMC Neurol. 2013, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.-C.; Wang, C.-H.; Lin, P.-S.; Hsu, C.-C.; Cherng, W.-J.; Huang, S.-C.; Liu, M.-H.; Chiang, C.-L.; Wang, J.-S. Aerobic Interval Training Improves Oxygen Uptake Efficiency by Enhancing Cerebral and Muscular Hemodynamics in Patients with Heart Failure. Int. J. Cardiol. 2013, 167, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Southern, W.M.; Ryan, T.E.; Kepple, K.; Murrow, J.R.; Nilsson, K.R.; McCully, K.K. Reduced Skeletal Muscle Oxidative Capacity and Impaired Training Adaptations in Heart Failure. Physiol. Rep. 2015, 3, e12353. [Google Scholar] [CrossRef]
- Belardinelli, R.; Barstow, T.J.; Porszasz, J.; Wasserman, K. Changes in Skeletal Muscle Oxygenation during Incremental Exercise Measured with near Infrared Spectroscopy. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 70, 487–492. [Google Scholar] [CrossRef]
- van der Zwaard, S.; Jaspers, R.T.; Blokland, I.J.; Achterberg, C.; Visser, J.M.; den Uil, A.R.; Hofmijster, M.J.; Levels, K.; Noordhof, D.A.; de Haan, A.; et al. Oxygenation Threshold Derived from Near-Infrared Spectroscopy: Reliability and Its Relationship with the First Ventilatory Threshold. PLoS ONE 2016, 11, e0162914. [Google Scholar] [CrossRef]
- Wahl, M.P.; Scalzo, R.L.; Regensteiner, J.G.; Reusch, J.E.B. Mechanisms of Aerobic Exercise Impairment in Diabetes: A Narrative Review. Front. Endocrinol. 2018, 9, 181. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Seifert, T.; Brassard, P.; Rasmussen, P.; Vaag, A.; Nielsen, H.B.; Secher, N.H.; van Lieshout, J.J. Impaired Cerebral Blood Flow and Oxygenation during Exercise in Type 2 Diabetic Patients. Physiol. Rep. 2015, 3, e12430. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.J. Microvascular and Macrovascular Complications of Diabetes. Clin. Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef]
- Rask-Madsen, C.; King, G.L. Vascular Complications of Diabetes: Mechanisms of Injury and Protective Factors. Cell Metab. 2013, 17, 20–33. [Google Scholar] [CrossRef] [PubMed]
- McClatchey, P.M.; Schafer, M.; Hunter, K.S.; Reusch, J.E.B. The Endothelial Glycocalyx Promotes Homogenous Blood Flow Distribution within the Microvasculature. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H168–H176. [Google Scholar] [CrossRef]
- Dogné, S.; Flamion, B.; Caron, N. Endothelial Glycocalyx as a Shield Against Diabetic Vascular Complications. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1427–1439. [Google Scholar] [CrossRef]
- Kröpfl, J.M.; Beltrami, F.G.; Rehm, M.; Gruber, H.-J.; Stelzer, I.; Spengler, C.M. Acute Exercise-Induced Glycocalyx Shedding Does Not Differ between Exercise Modalities, but Is Associated with Total Antioxidative Capacity. J. Sci. Med. Sport. 2021, 24, 689–695. [Google Scholar] [CrossRef]
- Schmitz, B.; Niehues, H.; Lenders, M.; Thorwesten, L.; Klose, A.; Krüger, M.; Brand, E.; Brand, S.-M. Effects of High-Intensity Interval Training on Microvascular Glycocalyx and Associated MicroRNAs. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H1538–H1551. [Google Scholar] [CrossRef]
- Machin, D.R.; Phuong, T.T.T.; Donato, A.J. The Role of the Endothelial Glycocalyx in Advanced Age and Cardiovascular Disease. Curr. Opin. Pharmacol. 2019, 45, 66–71. [Google Scholar] [CrossRef]
- Hahn, R.G.; Patel, V.; Dull, R.O. Human Glycocalyx Shedding: Systematic Review and Critical Appraisal. Acta Anaesthesiol. Scand. 2021, 65, 590–606. [Google Scholar] [CrossRef]
- Targosz-Korecka, M.; Jaglarz, M.; Malek-Zietek, K.E.; Gregorius, A.; Zakrzewska, A.; Sitek, B.; Rajfur, Z.; Chlopicki, S.; Szymonski, M. AFM-Based Detection of Glycocalyx Degradation and Endothelial Stiffening in the Db/Db Mouse Model of Diabetes. Sci. Rep. 2017, 7, 15951. [Google Scholar] [CrossRef]
- Sandoo, A.; van Zanten, J.J.C.S.V.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The Endothelium and Its Role in Regulating Vascular Tone. Open Cardiovasc. Med. J. 2010, 4, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.G. Endothelial Control of Vasomotion and Nitric Oxide Production: A Potential Target for Risk Factor Management. Cardiol. Clin. 1996, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Haselden, W.D.; Kedarasetti, R.T.; Drew, P.J. Spatial and Temporal Patterns of Nitric Oxide Diffusion and Degradation Drive Emergent Cerebrovascular Dynamics. PLoS Comput. Biol. 2020, 16, e1008069. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.G.; Widder, J.; Grumbach, I.; Chen, W.; Weber, M.; Searles, C. Endothelial Mechanotransduction, Nitric Oxide and Vascular Inflammation. J. Intern. Med. 2006, 259, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Shannon, O.M.; Clifford, T.; Seals, D.R.; Craighead, D.H.; Rossman, M.J. Nitric Oxide, Aging and Aerobic Exercise: Sedentary Individuals to Master’s Athletes. Nitric Oxide 2022, 125–126, 31–39. [Google Scholar] [CrossRef]
- Pogoda, K.; Mannell, H.; Blodow, S.; Schneider, H.; Schubert, K.M.; Qiu, J.; Schmidt, A.; Imhof, A.; Beck, H.; Tanase, L.I.; et al. NO Augments Endothelial Reactivity by Reducing Myoendothelial Calcium Signal Spreading. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2280–2290. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, J.; He, X.; Cao, W.; Peng, X.; Li, G. Pulsatility Protects the Endothelial Glycocalyx during Extracorporeal Membrane Oxygenation. Microcirculation 2021, 28, e12722. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.L.; Palta, P.; Tanaka, H.; Deal, J.A.; Wright, J.; Knopman, D.S.; Griswold, M.E.; Mosley, T.H.; Heiss, G. Association of Central Arterial Stiffness and Pressure Pulsatility with Mild Cognitive Impairment and Dementia: The Atherosclerosis Risk in Communities Study-Neurocognitive Study (ARIC-NCS). J. Alzheimers Dis. 2017, 57, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Murphy, K.; Drew, P.J. Rude Mechanicals in Brain Haemodynamics: Non-Neural Actors That Influence Blood Flow. Philos. Trans. R. Soc. B Biol. Sci. 2020, 376, 20190635. [Google Scholar] [CrossRef] [PubMed]
- Aalkjær, C.; Nilsson, H. Vasomotion: Cellular Background for the Oscillator and for the Synchronization of Smooth Muscle Cells. Br. J. Pharmacol. 2005, 144, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, O.A.; Bryant, M.B.; Panza, J.A. Role of Endothelial Nitric Oxide in Shear Stress–Induced Vasodilation of Human Microvasculature. Circulation 2001, 103, 1752–1758. [Google Scholar] [CrossRef]
- Jiang, X.Z.; Goligorsky, M.S. Biomechanical Properties of Endothelial Glycocalyx: An Imperfect Pendulum. Matrix Biol. Plus 2021, 12, 100087. [Google Scholar] [CrossRef]
- Farina, A.; Rosso, F.; Fasano, A. A Continuum Mechanics Model for the Fåhræus-Lindqvist Effect. J. Biol. Phys. 2021, 47, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Forouzan, O.; Yang, X.; Sosa, J.M.; Burns, J.M.; Shevkoplyas, S.S. Spontaneous Oscillations of Capillary Blood Flow in Artificial Microvascular Networks. Microvasc. Res. 2012, 84, 123–132. [Google Scholar] [CrossRef]
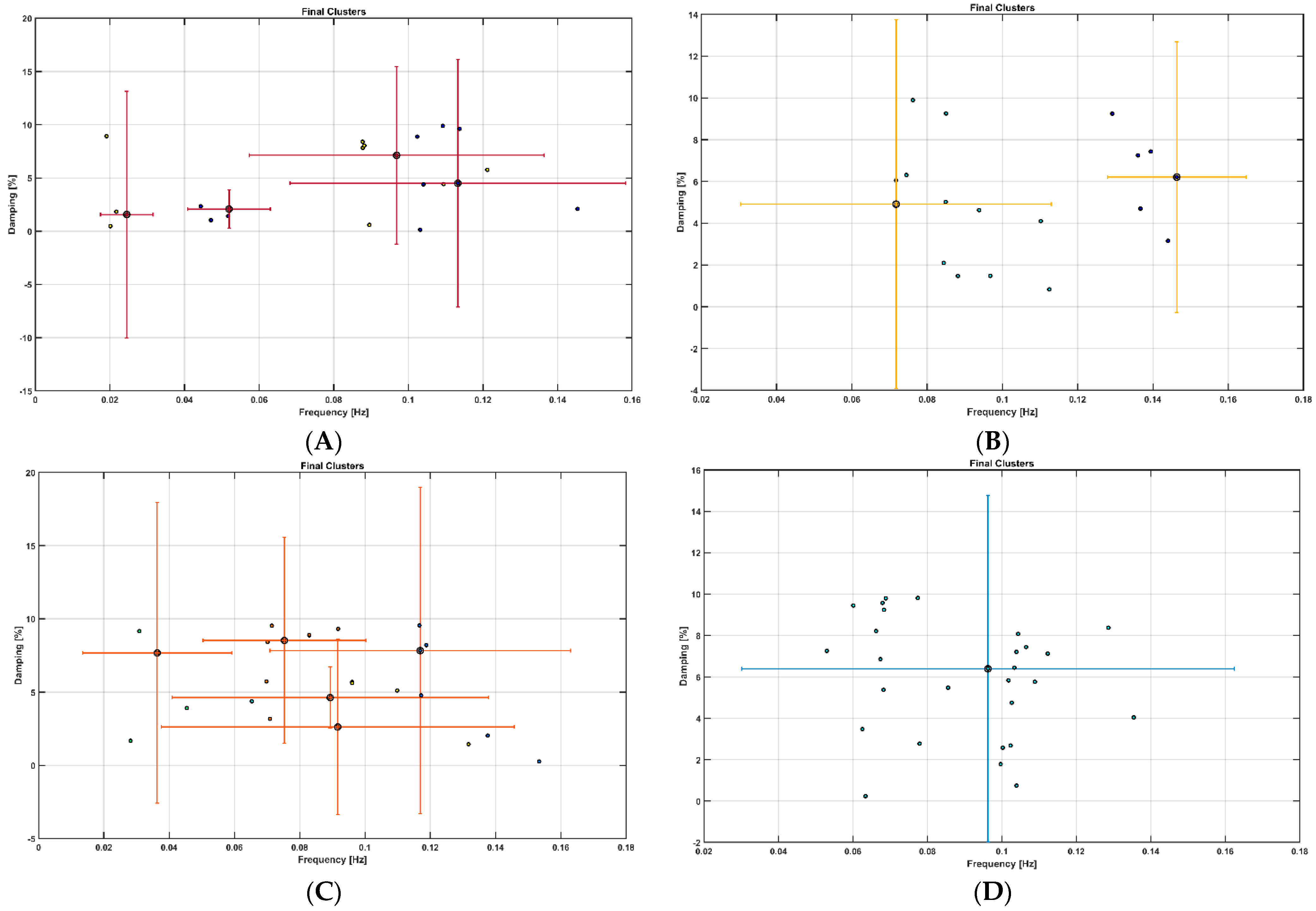
- Au, S.-K.; Brownjohn, J.M.W.; Li, B.; Raby, A. Understanding and Managing Identification Uncertainty of Close Modes in Operational Modal Analysis. Mech. Syst. Signal Process. 2021, 147, 107018. [Google Scholar] [CrossRef]
- Akazawa, N.; Tanahashi, K.; Kosaki, K.; Ra, S.; Matsubara, T.; Choi, Y.; Zempo-Miyaki, A.; Maeda, S. Aerobic Exercise Training Enhances Cerebrovascular Pulsatility Response to Acute Aerobic Exercise in Older Adults. Physiol. Rep. 2018, 6, e13681. [Google Scholar] [CrossRef]
- Nieuwdorp, M.; van Haeften, T.W.; Gouverneur, M.C.L.G.; Mooij, H.L.; van Lieshout, M.H.P.; Levi, M.; Meijers, J.C.M.; Holleman, F.; Hoekstra, J.B.L.; Vink, H.; et al. Loss of Endothelial Glycocalyx During Acute Hyperglycemia Coincides with Endothelial Dysfunction and Coagulation Activation In Vivo. Diabetes 2006, 55, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Jahani, S.; Fantana, A.L.; Harper, D.; Ellison, J.M.; Boas, D.A.; Forester, B.P.; Yücel, M.A. FNIRS Can Robustly Measure Brain Activity during Memory Encoding and Retrieval in Healthy Subjects. Sci. Rep. 2017, 7, 9533. [Google Scholar] [CrossRef]
- Sun, S.; Yang, B.; Zhang, Q.; Wüchner, R.; Pan, L.; Zhu, H. Fast Online Implementation of Covariance-Driven Stochastic Subspace Identification. Mech. Syst. Signal Process. 2023, 197, 110326. [Google Scholar] [CrossRef]
- How Accurate Is the Mini-Cog Test When Used to Assess Dementia in General Practice? Available online: https://www.cochrane.org/CD011415/DEMENTIA_how-accurate-mini-cog-test-when-used-assess-dementia-general-practice (accessed on 30 May 2023).
- Zhao, F. Cerebral and Muscular Oxygenation Changes after Moderate-Intensity Exercise in Sedentary Older Adults with Type 2 Diabetes. Ph.D. Thesis, State University of New York at Buffalo, New York, NY, USA, 2022. [Google Scholar]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B.; American College of Sports Medicine; et al. Exercise and Type 2 Diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint Position Statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef]
- Irvine, C.; Taylor, N.F. Progressive Resistance Exercise Improves Glycaemic Control in People with Type 2 Diabetes Mellitus: A Systematic Review. Aust. J. Physiother. 2009, 55, 237–246. [Google Scholar] [CrossRef]
- Bennett, J.A.; Winters-Stone, K.; Nail, L.M.; Scherer, J. Definitions of Sedentary in Physical-Activity-Intervention Trials: A Summary of the Literature. J. Aging Phys. Act. 2006, 14, 456–477. [Google Scholar] [CrossRef]
- Bohannon, R.W. The Heel-Raise Test for Ankle Plantarflexor Strength: A Scoping Review and Meta-Analysis of Studies Providing Norms. J. Phys. Ther. Sci. 2022, 34, 528–531. [Google Scholar] [CrossRef]
- Matos Casano, H.A.; Anjum, F. Six-Minute Walk Test. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ashendorf, L.; Jefferson, A.L.; O’Connor, M.K.; Chaisson, C.; Green, R.C.; Stern, R.A. Trail Making Test Errors in Normal Aging, Mild Cognitive Impairment, and Dementia. Arch. Clin. Neuropsychol. 2008, 23, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Murkin, J.M.; Arango, M. Near-Infrared Spectroscopy as an Index of Brain and Tissue Oxygenation. Br. J. Anaesth. 2009, 103, i3–i13. [Google Scholar] [CrossRef] [PubMed]
- Huppert, T.J.; Hoge, R.D.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. A Temporal Comparison of BOLD, ASL, and NIRS Hemodynamic Responses to Motor Stimuli in Adult Humans. Neuroimage 2006, 29, 368–382. [Google Scholar] [CrossRef] [PubMed]
- Mehagnoul-Schipper, D.J.; van der Kallen, B.F.W.; Colier, W.N.J.M.; van der Sluijs, M.C.; van Erning, L.J.T.O.; Thijssen, H.O.M.; Oeseburg, B.; Hoefnagels, W.H.L.; Jansen, R.W.M.M. Simultaneous Measurements of Cerebral Oxygenation Changes during Brain Activation by Near-Infrared Spectroscopy and Functional Magnetic Resonance Imaging in Healthy Young and Elderly Subjects. Hum. Brain Mapp. 2002, 16, 14–23. [Google Scholar] [CrossRef]
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. HomER: A Review of Time-Series Analysis Methods for near-Infrared Spectroscopy of the Brain. Appl. Opt. 2009, 48, D280–D298. [Google Scholar] [CrossRef]
- Arora, Y.; Dutta, A. Perspective: Disentangling the Effects of TES on Neurovascular Unit. Front. Neurol. 2023, 13, 1038700. [Google Scholar] [CrossRef] [PubMed]
- Santosa, H.; Zhai, X.; Fishburn, F.; Huppert, T. The NIRS Brain AnalyzIR Toolbox. Algorithms 2018, 11, 73. [Google Scholar] [CrossRef]
- Barker, J.W.; Aarabi, A.; Huppert, T.J. Autoregressive Model Based Algorithm for Correcting Motion and Serially Correlated Errors in FNIRS. Biomed. Opt. Express 2013, 4, 1366–1379. [Google Scholar] [CrossRef]
- Brincker, R.; Andersen, P.; Jacobsen, N.-J. Automated Frequency Domain Decomposition for Operational Modal Analysis. In Proceedings of the IMAC-XXIV: A Conference & Exposition on Structural Dynamics, St Louis, Missouri, USA, 30 January–2 February 2006. [Google Scholar]
- Brincker, R.; Zhang, L.; Andersen, P. Modal Identification of Output-Only Systems Using Frequency Domain Decomposition. Smart Mater. Struct. 2001, 10, 441. [Google Scholar] [CrossRef]
- Neu, E.; Janser, F.; Khatibi, A.A.; Orifici, A.C. Fully Automated Operational Modal Analysis Using Multi-Stage Clustering. Mech. Syst. Signal Process. 2017, 84, 308–323. [Google Scholar] [CrossRef]
- Crum, E.M.; O’Connor, W.J.; Van Loo, L.; Valckx, M.; Stannard, S.R. Validity and Reliability of the Moxy Oxygen Monitor during Incremental Cycling Exercise. Eur. J. Sport. Sci. 2017, 17, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Borson, S.; Scanlan, J.; Brush, M.; Vitaliano, P.; Dokmak, A. The Mini-Cog: A Cognitive “vital Signs” Measure for Dementia Screening in Multi-Lingual Elderly. Int. J. Geriatr. Psychiatry 2000, 15, 1021–1027. [Google Scholar] [CrossRef]
- Tsoi, K.K.F.; Chan, J.Y.C.; Hirai, H.W.; Wong, S.Y.S.; Kwok, T.C.Y. Cognitive Tests to Detect Dementia: A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2015, 175, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Balke, B. A Simple Field Test for the Assessment of Physical Fitness; Rep 63-6; Civil Aeromedical Research Institute: Oklahoma, OK, USA, 1963; pp. 1–8. [Google Scholar]
- Chan, W.L.; Chan, H.L.; Chen, K.M.; Fan, H.L.; Lai, W.C.; Yu, S.W. Reliability and Validity of Walk Tests for Older Adults with Dementia: A Systematic Review. Alzheimer’s Dement. 2021, 17, e050371. [Google Scholar] [CrossRef]
- Lunsford, B.R.; Perry, J. The Standing Heel-Rise Test for Ankle Plantar Flexion: Criterion for Normal. Phys. Ther. 1995, 75, 694–698. [Google Scholar] [CrossRef]
- Fairclough, S.H.; Burns, C.; Kreplin, U. FNIRS Activity in the Prefrontal Cortex and Motivational Intensity: Impact of Working Memory Load, Financial Reward, and Correlation-Based Signal Improvement. Neurophotonics 2018, 5, 035001. [Google Scholar] [CrossRef]
- du Boisgueheneuc, F.; Levy, R.; Volle, E.; Seassau, M.; Duffau, H.; Kinkingnehun, S.; Samson, Y.; Zhang, S.; Dubois, B. Functions of the Left Superior Frontal Gyrus in Humans: A Lesion Study. Brain 2006, 129, 3315–3328. [Google Scholar] [CrossRef]
- Rolls, E.T.; Huang, C.-C.; Lin, C.-P.; Feng, J.; Joliot, M. Automated Anatomical Labelling Atlas 3. NeuroImage 2020, 206, 116189. [Google Scholar] [CrossRef]
- Obrig, H.; Neufang, M.; Wenzel, R.; Kohl, M.; Steinbrink, J.; Einhäupl, K.; Villringer, A. Spontaneous Low Frequency Oscillations of Cerebral Hemodynamics and Metabolism in Human Adults. Neuroimage 2000, 12, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Hocke, L.M.; Frederick, B.B. Low Frequency Systemic Hemodynamic “Noise” in Resting State BOLD FMRI: Characteristics, Causes, Implications, Mitigation Strategies, and Applications. Front. Neurosci. 2019, 13, 787. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D.; Corfield, D.R.; Chandler, M.P.; Mathias, C.J.; Dolan, R.J. Cerebral Correlates of Autonomic Cardiovascular Arousal: A Functional Neuroimaging Investigation in Humans. J. Physiol. 2000, 523, 259–270. [Google Scholar] [CrossRef]
- Andersen, A.V.; Simonsen, S.A.; Schytz, H.W.; Iversen, H.K. Assessing Low-Frequency Oscillations in Cerebrovascular Diseases and Related Conditions with near-Infrared Spectroscopy: A Plausible Method for Evaluating Cerebral Autoregulation? Neurophotonics 2018, 5, 030901. [Google Scholar] [CrossRef]
- Brigadoi, S.; Cooper, R.J. How Short Is Short? Optimum Source-Detector Distance for Short-Separation Channels in Functional near-Infrared Spectroscopy. Neurophotonics 2015, 2, 025005. [Google Scholar] [CrossRef] [PubMed]
- ter Laan, M.; van Dijk, J.M.C.; Elting, J.W.J.; Staal, M.J.; Absalom, A.R. Sympathetic Regulation of Cerebral Blood Flow in Humans: A Review. Br. J. Anaesth. 2013, 111, 361–367. [Google Scholar] [CrossRef]
- Vinik, A.I.; Ziegler, D. Diabetic Cardiovascular Autonomic Neuropathy. Circulation 2007, 115, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Stefanovska, A.; Bracic, M.; Kvernmo, H.D. Wavelet Analysis of Oscillations in the Peripheral Blood Circulation Measured by Laser Doppler Technique. IEEE Trans. Biomed. Eng. 1999, 46, 1230–1239. [Google Scholar] [CrossRef]
- Gibbons, C.H.; Freeman, R. Treatment-Induced Neuropathy of Diabetes: An Acute, Iatrogenic Complication of Diabetes. Brain 2015, 138, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Geddes, J.B.; Carr, R.T.; Wu, F.; Lao, Y.; Maher, M. Blood Flow in Microvascular Networks: A Study in Nonlinear Biology. Chaos 2010, 20, 045123. [Google Scholar] [CrossRef]
- Irace, C.; Carallo, C.; Scavelli, F.; De Franceschi, M.S.; Esposito, T.; Gnasso, A. Blood Viscosity in Subjects with Normoglycemia and Prediabetes. Diabetes Care 2014, 37, 488–492. [Google Scholar] [CrossRef]
- Hu, S.; Ide, J.S.; Zhang, S.; Li, C.R. The Right Superior Frontal Gyrus and Individual Variation in Proactive Control of Impulsive Response. J. Neurosci. 2016, 36, 12688–12696. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Goodyear, L.J. Muscle-Adipose Tissue Cross Talk. Cold Spring Harb. Perspect. Med. 2018, 8, a029801. [Google Scholar] [CrossRef] [PubMed]
- Rosano, C.; Newman, A.; Santanasto, A.; Zhu, X.; Goodpaster, B.; Miljkovic, I. Increase in Skeletal Muscular Adiposity and Cognitive Decline in a Biracial Cohort of Older Men and Women. J. Am. Geriatr. Soc. 2023. [Google Scholar] [CrossRef] [PubMed]
- Leitner, D.R.; Frühbeck, G.; Yumuk, V.; Schindler, K.; Micic, D.; Woodward, E.; Toplak, H. Obesity and Type 2 Diabetes: Two Diseases with a Need for Combined Treatment Strategies—EASO Can Lead the Way. Obes. Facts 2017, 10, 483–492. [Google Scholar] [CrossRef]
- Smith, J.C.; Nielson, K.A.; Antuono, P.; Lyons, J.-A.; Hanson, R.J.; Butts, A.M.; Hantke, N.C.; Verber, M.D. Semantic Memory Functional MRI and Cognitive Function after Exercise Intervention in Mild Cognitive Impairment. J. Alzheimer’s Dis. 2013, 37, 197–215. [Google Scholar] [CrossRef]
- Liao, Y.-Y.; Chen, I.-H.; Hsu, W.-C.; Tseng, H.-Y.; Wang, R.-Y. Effect of Exergaming versus Combined Exercise on Cognitive Function and Brain Activation in Frail Older Adults: A Randomised Controlled Trial. Ann. Phys. Rehabil. Med. 2021, 64, 101492. [Google Scholar] [CrossRef]
- Silveira-Rodrigues, J.G.; Pires, W.; Gomes, P.F.; Ogando, P.H.M.; Melo, B.P.; Aleixo, I.M.S.; Soares, D.D. Combined Exercise Training Improves Specific Domains of Cognitive Functions and Metabolic Markers in Middle-Aged and Older Adults with Type 2 Diabetes Mellitus. Diabetes Res. Clin. Pract. 2021, 173, 108700. [Google Scholar] [CrossRef]
- Rane, D.; Dash, D.P.; Dutt, A.; Dutta, A.; Das, A.; Lahiri, U. Distinctive visual tasks for characterizing mild cognitive impairment and dementia using oculomotor behavior. Front. Aging Neurosci. 2023, 15, 1125651. [Google Scholar] [CrossRef]
- Leischik, R.; Schwarz, K.; Bank, P.; Brzek, A.; Dworrak, B.; Strauss, M.; Litwitz, H.; Gerlach, C.E. Exercise Improves Cognitive Function-A Randomized Trial on the Effects of Physical Activity on Cognition in Type 2 Diabetes Patients. J. Pers. Med. 2021, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Berchicci, M.; Lucci, G.; Di Russo, F. Benefits of Physical Exercise on the Aging Brain: The Role of the Prefrontal Cortex. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1337–1341. [Google Scholar] [CrossRef]
- Liao, Y.-Y.; Chen, I.-H.; Wang, R.-Y. Effects of Kinect-Based Exergaming on Frailty Status and Physical Performance in Prefrail and Frail Elderly: A Randomized Controlled Trial. Sci. Rep. 2019, 9, 9353. [Google Scholar] [CrossRef] [PubMed]
- Bertram, S.; Brixius, K.; Brinkmann, C. Exercise for the Diabetic Brain: How Physical Training May Help Prevent Dementia and Alzheimer’s Disease in T2DM Patients. Endocrine 2016, 53, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Lustig, C.; Shah, P.; Seidler, R.; Reuter-Lorenz, P.A. Aging, Training, and the Brain: A Review and Future Directions. Neuropsychol. Rev. 2009, 19, 504–522. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 5th ed.; Freeman, W.H., Ed.; NCBI: Bethesda, MD, USA, 2002. [Google Scholar]
- Kemp, G.J.; Hands, L.J.; Ramaswami, G.; Taylor, D.J.; Nicolaides, A.; Amato, A.; Radda, G.K. Calf Muscle Mitochondrial and Glycogenolytic Atp Synthesis in Patients with Claudication Due to Peripheral Vascular Disease Analysed Using 31P Magnetic Resonance Spectroscopy. Clin. Sci. 1995, 89, 581–590. [Google Scholar] [CrossRef]
- Bauer, T.A.; Reusch, J.E.B.; Levi, M.; Regensteiner, J.G. Skeletal Muscle Deoxygenation After the Onset of Moderate Exercise Suggests Slowed Microvascular Blood Flow Kinetics in Type 2 Diabetes. Diabetes Care 2007, 30, 2880–2885. [Google Scholar] [CrossRef]
- Kalra, S.; Sahay, R. Diabetes Fatigue Syndrome. Diabetes Ther. 2018, 9, 1421–1429. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Hillman, C. The Influence of Exercise on Cognitive Abilities. Compr. Physiol. 2013, 3, 403–428. [Google Scholar] [CrossRef]
- Mendez Colmenares, A.; Voss, M.W.; Fanning, J.; Salerno, E.A.; Gothe, N.P.; Thomas, M.L.; McAuley, E.; Kramer, A.F.; Burzynska, A.Z. White Matter Plasticity in Healthy Older Adults: The Effects of Aerobic Exercise. NeuroImage 2021, 239, 118305. [Google Scholar] [CrossRef]
- Allen, K.V.; Frier, B.M.; Strachan, M.W.J. The Relationship between Type 2 Diabetes and Cognitive Dysfunction: Longitudinal Studies and Their Methodological Limitations. Eur. J. Pharmacol. 2004, 490, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, Z.; Wilson, R.S.; Bienias, J.L.; Evans, D.A.; Bennett, D.A. Diabetes Mellitus and Risk of Alzheimer Disease and Decline in Cognitive Function. Arch. Neurol. 2004, 61, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, T.; Gerstein, H.C.; Williamson, J.D. Cognitive Decline and Dementia in Diabetes--Systematic Overview of Prospective Observational Studies. Diabetologia 2005, 48, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- CHOI, S.E.; ROY, B.; FREEBY, M.; MULLUR, R.; WOO, M.A.; KUMAR, R. Prefrontal Cortex Brain Damage and Glycemic Control in Patients with Type 2 Diabetes. J. Diabetes 2020, 12, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Reuter-Lorenz, P.A.; Cappell, K.A. Neurocognitive Aging and the Compensation Hypothesis. Curr. Dir. Psychol. Sci. 2008, 17, 177–182. [Google Scholar] [CrossRef]
- Wood, A.G.; Chen, J.; Moran, C.; Phan, T.; Beare, R.; Cooper, K.; Litras, S.; Srikanth, V. Brain Activation during Memory Encoding in Type 2 Diabetes Mellitus: A Discordant Twin Pair Study. J. Diabetes Res. 2016, 2016, 3978428. [Google Scholar] [CrossRef]
- He, X.-S.; Wang, Z.-X.; Zhu, Y.-Z.; Wang, N.; Hu, X.; Zhang, D.-R.; Zhu, D.-F.; Zhou, J.-N. Hyperactivation of Working Memory-Related Brain Circuits in Newly Diagnosed Middle-Aged Type 2 Diabetics. Acta Diabetol. 2015, 52, 133–142. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Liu, C.; Zhang, H.; Zhou, X.; Ni, C.; Qin, W.; Zhang, Q. Altered Brain Activation and Functional Connectivity in Working Memory Related Networks in Patients with Type 2 Diabetes: An ICA-Based Analysis. Sci. Rep. 2016, 6, 23767. [Google Scholar] [CrossRef]
- Sorond, F.A.; Schnyer, D.M.; Serrador, J.M.; Milberg, W.P.; Lipsitz, L.A. Cerebral Blood Flow Regulation during Cognitive Tasks: Effects of Healthy Aging. Cortex 2008, 44, 179–184. [Google Scholar] [CrossRef]
- Beishon, L.C.; Hosford, P.; Gurung, D.; Brassard, P.; Minhas, J.S.; Robinson, T.G.; Haunton, V.; Panerai, R.B. The Role of the Autonomic Nervous System in Cerebral Blood Flow Regulation in Dementia: A Review. Auton. Neurosci. 2022, 240, 102985. [Google Scholar] [CrossRef]
- Kisler, K.; Nelson, A.R.; Montagne, A.; Zlokovic, B.V. Cerebral Blood Flow Regulation and Neurovascular Dysfunction in Alzheimer Disease. Nat. Rev. Neurosci. 2017, 18, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Binder, J.R.; Rao, S.M.; Hammeke, T.A.; Frost, J.A.; Bandettini, P.A.; Hyde, J.S. Effects of Stimulus Rate on Signal Response during Functional Magnetic Resonance Imaging of Auditory Cortex. Cogn. Brain Res. 1994, 2, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Lu, H. Cerebral Oxygen Extraction Fraction MRI: Techniques and Applications. Magn. Reson. Med. 2022, 88, 575–600. [Google Scholar] [CrossRef]
- Buxton, R.B.; Griffeth, V.E.M.; Simon, A.B.; Moradi, F. Variability of the Coupling of Blood Flow and Oxygen Metabolism Responses in the Brain: A Problem for Interpreting BOLD Studies but Potentially a New Window on the Underlying Neural Activity. Front. Neurosci. 2014, 8, 139. [Google Scholar] [CrossRef]
- Buxton, R.B. Interpreting Oxygenation-Based Neuroimaging Signals: The Importance and the Challenge of Understanding Brain Oxygen Metabolism. Front. Neuroenergetics 2010, 2, 8. [Google Scholar] [CrossRef]
- Buxton, R.B.; Frank, L.R. A Model for the Coupling between Cerebral Blood Flow and Oxygen Metabolism during Neural Stimulation. J. Cereb. Blood Flow. Metab. 1997, 17, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Van Ryckeghem, L.; Keytsman, C.; Verboven, K.; Verbaanderd, E.; Frederix, I.; Bakelants, E.; Petit, T.; Jogani, S.; Stroobants, S.; Dendale, P.; et al. Exercise Capacity Is Related to Attenuated Responses in Oxygen Extraction and Left Ventricular Longitudinal Strain in Asymptomatic Type 2 Diabetes Patients. Eur. J. Prev. Cardiol. 2021, 28, 1756–1766. [Google Scholar] [CrossRef]
- Van Ryckeghem, L.; Keytsman, C.; De Brandt, J.; Verboven, K.; Verbaanderd, E.; Marinus, N.; Franssen, W.M.A.; Frederix, I.; Bakelants, E.; Petit, T.; et al. Impact of Continuous vs. Interval Training on Oxygen Extraction and Cardiac Function during Exercise in Type 2 Diabetes Mellitus. Eur. J. Appl. Physiol. 2022, 122, 875–887. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, M.; Ahn, Y.-B.; Lim, H.-K.; Kang, S.-G.; Cho, J.; Park, S.-J.; Song, S.-W. Effect of Dance Exercise on Cognitive Function in Elderly Patients with Metabolic Syndrome: A Pilot Study. J. Sports Sci. Med. 2011, 10, 671–678. [Google Scholar]
- Holwerda, S.W.; Restaino, R.M.; Manrique, C.; Lastra, G.; Fisher, J.P.; Fadel, P.J. Augmented Pressor and Sympathetic Responses to Skeletal Muscle Metaboreflex Activation in Type 2 Diabetes Patients. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H300–H309. [Google Scholar] [CrossRef]
- Pinna, V.; Doneddu, A.; Roberto, S.; Magnani, S.; Ghiani, G.; Mulliri, G.; Sanna, I.; Serra, S.; Hosseini Kakhak, S.A.; Milia, R.; et al. Combined Mental Task and Metaboreflex Impair Cerebral Oxygenation in Patients with Type 2 Diabetes Mellitus. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2021, 320, R488–R499. [Google Scholar] [CrossRef]
- Toth, P.; Tarantini, S.; Csiszar, A.; Ungvari, Z. Functional Vascular Contributions to Cognitive Impairment and Dementia: Mechanisms and Consequences of Cerebral Autoregulatory Dysfunction, Endothelial Impairment, and Neurovascular Uncoupling in Aging. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1–H20. [Google Scholar] [CrossRef] [PubMed]
- Bherer, L.; Erickson, K.I.; Liu-Ambrose, T. A Review of the Effects of Physical Activity and Exercise on Cognitive and Brain Functions in Older Adults. J. Aging Res. 2013, 2013, 657508. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, B.C.; Salat, D.H.; Greve, D.N.; Chua, E.F.; Rand-Giovannetti, E.; Rentz, D.M.; Bertram, L.; Mullin, K.; Tanzi, R.E.; Blacker, D.; et al. Increased Hippocampal Activation in Mild Cognitive Impairment Compared to Normal Aging and AD. Neurology 2005, 65, 404–411. [Google Scholar] [CrossRef]
- Reuter-Lorenz, P.A.; Lustig, C. Brain Aging: Reorganizing Discoveries about the Aging Mind. Curr. Opin. Neurobiol. 2005, 15, 245–251. [Google Scholar] [CrossRef]
- Scholey, A.B.; Harper, S.; Kennedy, D.O. Cognitive Demand and Blood Glucose. Physiol. Behav. 2001, 73, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Bruckmaier, M.; Tachtsidis, I.; Phan, P.; Lavie, N. Attention and Capacity Limits in Perception: A Cellular Metabolism Account. J. Neurosci. 2020, 40, 6801–6811. [Google Scholar] [CrossRef]
- Barbiellini Amidei, C.; Fayosse, A.; Dumurgier, J.; Machado-Fragua, M.D.; Tabak, A.G.; van Sloten, T.; Kivimäki, M.; Dugravot, A.; Sabia, S.; Singh-Manoux, A. Association Between Age at Diabetes Onset and Subsequent Risk of Dementia. JAMA 2021, 325, 1640–1649. [Google Scholar] [CrossRef]
- Kim, J.; Wei, Y.; Sowers, J.R. Role of Mitochondrial Dysfunction in Insulin Resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef]
- Yassine, H.N.; Solomon, V.; Thakral, A.; Sheikh-Bahaei, N.; Chui, H.C.; Braskie, M.N.; Schneider, L.S.; Talbot, K. Brain Energy Failure in Dementia Syndromes: Opportunities and Challenges for Glucagon-like Peptide-1 Receptor Agonists. Alzheimer’s Dement. 2022, 18, 478–497. [Google Scholar] [CrossRef]
- Salehpour, F.; Khademi, M.; Hamblin, M.R. Photobiomodulation Therapy for Dementia: A Systematic Review of Pre-Clinical and Clinical Studies. J. Alzheimers Dis. 2021, 83, 1431–1452. [Google Scholar] [CrossRef]
- Ludwig, B.; Bender, E.; Arnold, S.; Hüttemann, M.; Lee, I.; Kadenbach, B. Cytochrome c Oxidase and the Regulation of Oxidative Phosphorylation. ChemBioChem 2001, 2, 392–403. [Google Scholar] [CrossRef]
- Li, Y.; Park, J.-S.; Deng, J.-H.; Bai, Y. Cytochrome c Oxidase Subunit IV Is Essential for Assembly and Respiratory Function of the Enzyme Complex. J. Bioenerg. Biomembr. 2006, 38, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Karanth, S.S.; Mujumdar, R.; Sahoo, J.P.; Das, A.; Stachowiak, M.K.; Dutta, A. Human Brain Organoid Platform for Neuroengineering Optical Theranostics in Neonatal Sepsis. In Proceedings of the Converging Clinical and Engineering Research on Neurorehabilitation IV, Pisa, Italy, 16–20 October 2018; Torricelli, D., Akay, M., Pons, J.L., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 753–757. [Google Scholar]
- Zhao, X.; Xu, Z.; Xiao, L.; Shi, T.; Xiao, H.; Wang, Y.; Li, Y.; Xue, F.; Zeng, W. Review on the Vascularization of Organoids and Organoids-on-a-Chip. Front. Bioeng. Biotechnol. 2021, 9, 637048. [Google Scholar] [CrossRef] [PubMed]
- Dagar, S.; Chowdhury, S.R.; Bapi, R.S.; Dutta, A.; Roy, D. Near-Infrared Spectroscopy—Electroencephalography-Based Brain-State-Dependent Electrotherapy: A Computational Approach Based on Excitation–Inhibition Balance Hypothesis. Front. Neurol. 2016, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- A ‘Phase Zero’ Human Brain Organoid Platform for Neuroengineering Optical Theranostics. NYC Neuromodulation Online. 2020. Available online: https://neuromodec.com/nyc-neuromodulation-online-2020 (accessed on 19 April 2021).
- Grubb, S.; Cai, C.; Hald, B.O.; Khennouf, L.; Murmu, R.P.; Jensen, A.G.K.; Fordsmann, J.; Zambach, S.; Lauritzen, M. Precapillary Sphincters Maintain Perfusion in the Cerebral Cortex. Nat. Commun. 2020, 11, 395. [Google Scholar] [CrossRef]
- Cai, C.; Zambach, S.A.; Grubb, S.; Thomsen, K.J.; Lind, B.L.; Hald, B.O.; Lønstrup, M.; Nielsen, R.M.; Lauritzen, M.J. Impaired Dynamics of Brain Precapillary Sphincters and Pericytes at First Order Capillaries Explains Reduced Neurovascular Functions in Aging. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lombardi, F.; Herrmann, H.J.; de Arcangelis, L. Balance of Excitation and Inhibition Determines 1/f Power Spectrum in Neuronal Networks. Chaos 2017, 27, 047402. [Google Scholar] [CrossRef]
- Dutta, A.; Karanth, S.S.; Bhattacharya, M.; Liput, M.; Augustyniak, J.; Cheung, M.; Stachowiak, E.K.; Stachowiak, M.K. A Proof of Concept ‘Phase Zero’ Study of Neurodevelopment Using Brain Organoid Models with Vis/near-Infrared Spectroscopy and Electrophysiology. Sci. Rep. 2020, 10, 20987. [Google Scholar] [CrossRef]
- Zhang, L.; Su, F.; Buizer, S.; Lu, H.; Gao, W.; Tian, Y.; Meldrum, D. A Dual Sensor for Real-Time Monitoring of Glucose and Oxygen. Biomaterials 2013, 34, 10–1016. [Google Scholar] [CrossRef]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.-F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-Responsive Genes Involved in Oxidative Phosphorylation Are Coordinately Downregulated in Human Diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef]
- Patti, M.E.; Butte, A.J.; Crunkhorn, S.; Cusi, K.; Berria, R.; Kashyap, S.; Miyazaki, Y.; Kohane, I.; Costello, M.; Saccone, R.; et al. Coordinated Reduction of Genes of Oxidative Metabolism in Humans with Insulin Resistance and Diabetes: Potential Role of PGC1 and NRF1. Proc. Natl. Acad. Sci. USA 2003, 100, 8466–8471. [Google Scholar] [CrossRef]
- Petersen, K.F.; Befroy, D.; Dufour, S.; Dziura, J.; Ariyan, C.; Rothman, D.L.; DiPietro, L.; Cline, G.W.; Shulman, G.I. Mitochondrial Dysfunction in the Elderly: Possible Role in Insulin Resistance. Science 2003, 300, 1140–1142. [Google Scholar] [CrossRef]
- Schrauwen-Hinderling, V.B.; Kooi, M.E.; Hesselink, M.K.C.; Jeneson, J.a.L.; Backes, W.H.; van Echteld, C.J.A.; van Engelshoven, J.M.A.; Mensink, M.; Schrauwen, P. Impaired in Vivo Mitochondrial Function but Similar Intramyocellular Lipid Content in Patients with Type 2 Diabetes Mellitus and BMI-Matched Control Subjects. Diabetologia 2007, 50, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Szendroedi, J.; Schmid, A.I.; Chmelik, M.; Toth, C.; Brehm, A.; Krssak, M.; Nowotny, P.; Wolzt, M.; Waldhausl, W.; Roden, M. Muscle Mitochondrial ATP Synthesis and Glucose Transport/Phosphorylation in Type 2 Diabetes. PLoS Med. 2007, 4, e154. [Google Scholar] [CrossRef]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The Blood-Brain Barrier: An Engineering Perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef]
- Celaya-Alcala, J.T.; Lee, G.V.; Smith, A.F.; Li, B.; Sakadžić, S.; Boas, D.A.; Secomb, T.W. Simulation of Oxygen Transport and Estimation of Tissue Perfusion in Extensive Microvascular Networks: Application to Cerebral Cortex. J. Cereb. Blood Flow. Metab. 2021, 41, 656–669. [Google Scholar] [CrossRef]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.-Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain Insulin Resistance in Type 2 Diabetes and Alzheimer Disease: Concepts and Conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef]
- Demarest, T.G.; Varma, V.R.; Estrada, D.; Babbar, M.; Basu, S.; Mahajan, U.V.; Moaddel, R.; Croteau, D.L.; Thambisetty, M.; Mattson, M.P.; et al. Biological Sex and DNA Repair Deficiency Drive Alzheimer’s Disease via Systemic Metabolic Remodeling and Brain Mitochondrial Dysfunction. Acta Neuropathol. 2020, 140, 25–47. [Google Scholar] [CrossRef]
- Zilberter, Y.; Zilberter, M. The Vicious Circle of Hypometabolism in Neurodegenerative Diseases: Ways and Mechanisms of Metabolic Correction. J. Neurosci. Res. 2017, 95, 2217–2235. [Google Scholar] [CrossRef]
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain Energy Metabolism: Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef]
- Li, W.; Roy Choudhury, G.; Winters, A.; Prah, J.; Lin, W.; Liu, R.; Yang, S.-H. Hyperglycemia Alters Astrocyte Metabolism and Inhibits Astrocyte Proliferation. Aging Dis. 2018, 9, 674–684. [Google Scholar] [CrossRef]
- Shen, Z.; Li, Z.-Y.; Yu, M.-T.; Tan, K.-L.; Chen, S. Metabolic Perspective of Astrocyte Dysfunction in Alzheimer’s Disease and Type 2 Diabetes Brains. Biomed. Pharmacother. 2023, 158, 114206. [Google Scholar] [CrossRef] [PubMed]
- Mullins, R.; Reiter, D.; Kapogiannis, D. Magnetic Resonance Spectroscopy Reveals Abnormalities of Glucose Metabolism in the Alzheimer’s Brain. Ann. Clin. Transl. Neurol. 2018, 5, 262–272. [Google Scholar] [CrossRef]
- Vermeij, A.; Meel-van den Abeelen, A.S.S.; Kessels, R.P.C.; van Beek, A.H.E.A.; Claassen, J.A.H.R. Very-Low-Frequency Oscillations of Cerebral Hemodynamics and Blood Pressure Are Affected by Aging and Cognitive Load. Neuroimage 2014, 85 Pt 1, 608–615. [Google Scholar] [CrossRef]
- Chan, C.C.; Fage, B.A.; Burton, J.K.; Smailagic, N.; Gill, S.S.; Herrmann, N.; Nikolaou, V.; Quinn, T.J.; Noel-Storr, A.H.; Seitz, D.P. Mini-Cog for the Diagnosis of Alzheimer’s Disease Dementia and Other Dementias within a Secondary Care Setting. Cochrane Database Syst. Rev. 2019, 9, CD011414. [Google Scholar] [CrossRef]
- Attwell, D.; Gibb, A. Neuroenergetics and the Kinetic Design of Excitatory Synapses. Nat. Rev. Neurosci. 2005, 6, 841–849. [Google Scholar] [CrossRef]
- Holper, L.; Mann, J.J. Test–Retest Reliability of Brain Mitochondrial Cytochrome-c-Oxidase Assessed by Functional near-Infrared Spectroscopy. JBO 2018, 23, 056006. [Google Scholar] [CrossRef]
- Dutta, A. Bidirectional Interactions between Neuronal and Hemodynamic Responses to Transcranial Direct Current Stimulation (TDCS): Challenges for Brain-State Dependent TDCS. Front. Syst. Neurosci. 2015, 9, 107. [Google Scholar] [CrossRef]
- Shaw, K.; Bell, L.; Boyd, K.; Grijseels, D.M.; Clarke, D.; Bonnar, O.; Crombag, H.S.; Hall, C.N. Neurovascular Coupling and Oxygenation Are Decreased in Hippocampus Compared to Neocortex Because of Microvascular Differences. Nat. Commun. 2021, 12, 3190. [Google Scholar] [CrossRef]
- Arora, Y.; Chowdhury, S.R.; Dutta, A. Physiological Neurovascular Modeling of Cerebrovascular Effects of Transcranial Electrical Current Stimulation. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2021, 14, 1597–1598. [Google Scholar] [CrossRef]
- Kistenmacher, A.; Manneck, S.; Wardzinski, E.K.; Martens, J.C.; Gohla, G.; Melchert, U.H.; Jauch-Chara, K.; Oltmanns, K.M. Persistent Blood Glucose Reduction upon Repeated Transcranial Electric Stimulation in Men. Brain Stimul. 2017, 10, 780–786. [Google Scholar] [CrossRef]
- Sood, M.; Besson, P.; Muthalib, M.; Jindal, U.; Perrey, S.; Dutta, A.; Hayashibe, M. NIRS-EEG Joint Imaging during Transcranial Direct Current Stimulation: Online Parameter Estimation with an Autoregressive Model. J. Neurosci. Methods 2016, 274, 71–80. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability Changes Induced in the Human Motor Cortex by Weak Transcranial Direct Current Stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef]
- Jamil, A.; Batsikadze, G.; Kuo, H.-I.; Meesen, R.L.J.; Dechent, P.; Paulus, W.; Nitsche, M.A. Current Intensity- and Polarity-Specific Online and Aftereffects of Transcranial Direct Current Stimulation: An FMRI Study. Human. Brain Mapp. 2020, 41, 1644–1666. [Google Scholar] [CrossRef]
- Bahr-Hosseini, M.; Bikson, M. Neurovascular-Modulation: A Review of Primary Vascular Responses to Transcranial Electrical Stimulation as a Mechanism of Action. Brain Stimul. 2021, 14, 837–847. [Google Scholar] [CrossRef]
- Dutta, A.; Jacob, A.; Chowdhury, S.R.; Das, A.; Nitsche, M.A. EEG-NIRS Based Assessment of Neurovascular Coupling during Anodal Transcranial Direct Current Stimulation--a Stroke Case Series. J. Med. Syst. 2015, 39, 205. [Google Scholar] [CrossRef]
- Dutta, A. Simultaneous Functional Near-Infrared Spectroscopy (FNIRS) and Electroencephalogram (EEG) to Elucidate Neurovascular Modulation by Transcranial Electrical Stimulation (TES). Brain Stimul. 2021, 14, 1093–1094. [Google Scholar] [CrossRef]
- Zambach, S.A.; Cai, C.; Helms, H.C.C.; Hald, B.O.; Dong, Y.; Fordsmann, J.C.; Nielsen, R.M.; Hu, J.; Lønstrup, M.; Brodin, B.; et al. Precapillary Sphincters and Pericytes at First-Order Capillaries as Key Regulators for Brain Capillary Perfusion. Proc. Natl. Acad. Sci. USA 2021, 118, e2023749118. [Google Scholar] [CrossRef]
- Lundgaard, I.; Li, B.; Xie, L.; Kang, H.; Sanggaard, S.; Haswell, J.D.R.; Sun, W.; Goldman, S.; Blekot, S.; Nielsen, M.; et al. Direct Neuronal Glucose Uptake Heralds Activity-Dependent Increases in Cerebral Metabolism. Nat. Commun. 2015, 6, 6807. [Google Scholar] [CrossRef]
- Fujii, H.; Ito, H.; Aihara, K.; Ichinose, N.; Tsukada, M. Dynamical Cell Assembly Hypothesis—Theoretical Possibility of Spatio-Temporal Coding in the Cortex. Neural Netw. 1996, 9, 1303–1350. [Google Scholar] [CrossRef]
- Drew, P.J.; Mateo, C.; Turner, K.L.; Yu, X.; Kleinfeld, D. Ultra-Slow Oscillations in FMRI and Resting-State Connectivity: Neuronal and Vascular Contributions and Technical Confounds. Neuron 2020, 107, 782–804. [Google Scholar] [CrossRef]
- van Veluw, S.J.; Hou, S.S.; Calvo-Rodriguez, M.; Arbel-Ornath, M.; Snyder, A.C.; Frosch, M.P.; Greenberg, S.M.; Bacskai, B.J. Vasomotion as a Driving Force for Paravascular Clearance in the Awake Mouse Brain. Neuron 2020, 105, 549–561.e5. [Google Scholar] [CrossRef]





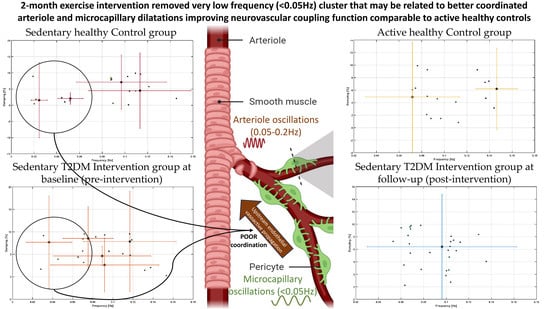
| Characteristics | T2DM Intervention Group at Baseline N (%) or Mean (Standard Deviation) | Sedentary Healthy (SH) Control Group N (%) or Mean (Standard Deviation) | Active Healthy (AH) Control Group N (%) or Mean (Standard Deviation) | F or χ2 |
|---|---|---|---|---|
| Age | 66.1 (4.5) | 66.6 (4.2) | 65.9 (4.2) | F = 0.055 (p = 0.947) |
| BMI | 34.8 (4.8) | 33.3 (5.3) | 26.1 (4.3) *** | F = 18.775 *** (p < 0.001) T2DM vs. AH |
| Sex | ||||
| Male | 10 (50.0%) | 10 (50.0%) | 10 (50.0%) | χ2 = 1.0 |
| Female | 10 (50.0%) | 10 (50.0%) | 10 (50.0%) | (p = 1.000) |
| Ethnicity | ||||
| Hispanic | 3 (15.0%) * | 0 | 0 | |
| Non-Hispanic | 17 (85.0%) | 100 (100.0%) | 100 (100.0%) | χ2 = 6.316 * (p = 0.043) |
| Race | ||||
| White | 14 (70.0%) | 17 (85.0%) | 19 (95.0%) | |
| Black/African American | 5 (25.0%) | 1 (5.0%) | 0 (0.0%) | χ2 = 8.260 (p = 0.083) |
| Other | 1 (3.0%) | 2 (10.0%) | 1 (5.0%) | |
| Living status | ||||
| Alone | 4 (20.0%) | 9 (45.0%) | 5 (25.0%) | χ2 = 3.333 |
| With someone | 16 (80.0%) | 11 (55.0%) | 15 (75.0%) | (p = 0.189) |
| Marital Status Single Married Widowed Separated/Divorced | 2 (10.0%) 15 (75.0%) 1 (5.0%) 2 (10.0%) | 5 (25.0%) 9 (45.0%) 2 (10.0%) 4 (20.0%) | 6 (30.0%) 11 (55.0%) 1 (5.0%) 2 (10.0%) | χ2 = 5.100 (p = 0.513) |
| Home Ownership | ||||
| Rent | 2 (10.0%) | 2 (10.0%) | 2 (10.0%) | χ2 = 2.038 |
| Own | 17 (85.0%) | 18 (90.0%) | 18 (90.0%) | (p = 0.729) |
| Other | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) | |
| Work Status | ||||
| Full Time | 5 (25.0%) | 7 (35.0%) | 6 (30.0%) | |
| Part time | 5 (25.0%) | 3 (15.0%) | 2 (10.0%) | χ2 = 3.333 |
| Retired | 9 (45.0%) | 9 (45.0%) | 12 (60.0%) | (p = 0.737) |
| Unemployed | 1 (5.0%) | 1 (5.0%) | 0 (0.0%) | |
| Income | ||||
| Very Comfortable | 4 (20.0%) | 6 (30.0%) | 10 (50.0%) | |
| Comfortable | 14 (70.0%) | 13 (65.0%) | 9 (45.0%) | χ2 = 5.967 |
| Uncomfortable | 1 (5.0%) | 1 (5.0%) | 1 (5.0%) | (p = 0.427) |
| Not comfortable at all | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) | |
| Education | ||||
| High School | 3 (15.0%) | 2 (10.0%) | 5 (25.0%) | |
| 2 Year College | 9 (45.0%) | 8 (40.0%) | 1 (5.0%) | |
| BS/BA | 3 (15.0%) | 5 (25.0%) | 3 (15.0%) | χ2 = 12.261 |
| MA/MS | 4 (20.0%) | 3 (15.0%) | 8 (40.0%) | (p = 0.140) |
| More Advanced Degree | 1 (5.0%) | 2 (10.0%) | 3 (15.0%) |
| Characteristics | Intervention Group at Baseline Mean (Standard Deviation) | Control Group Mean (Standard Deviation) | Z | Cohen’s d |
|---|---|---|---|---|
| Mini-Cog | 12.79 (2.1) | 14.16 (0.9) | Z = 3.273 ** (p = 0.0005) | d = 0.967 |
| Trail Making Part A (s) | 39.55 (12.1) | 30.94 (6.8) | Z = 2.548 ** (p = 0.006) | d = 0.972 |
| Trail Making Part B (s) | 93.45 (26.58) | 69.08 (21.3) | Z = 3.293 *** (p < 0.001) | d = 1.053 |
| Characteristics | Baseline M(SD) | Follow-Up M (SD) | t or Z | Cohen’s d |
|---|---|---|---|---|
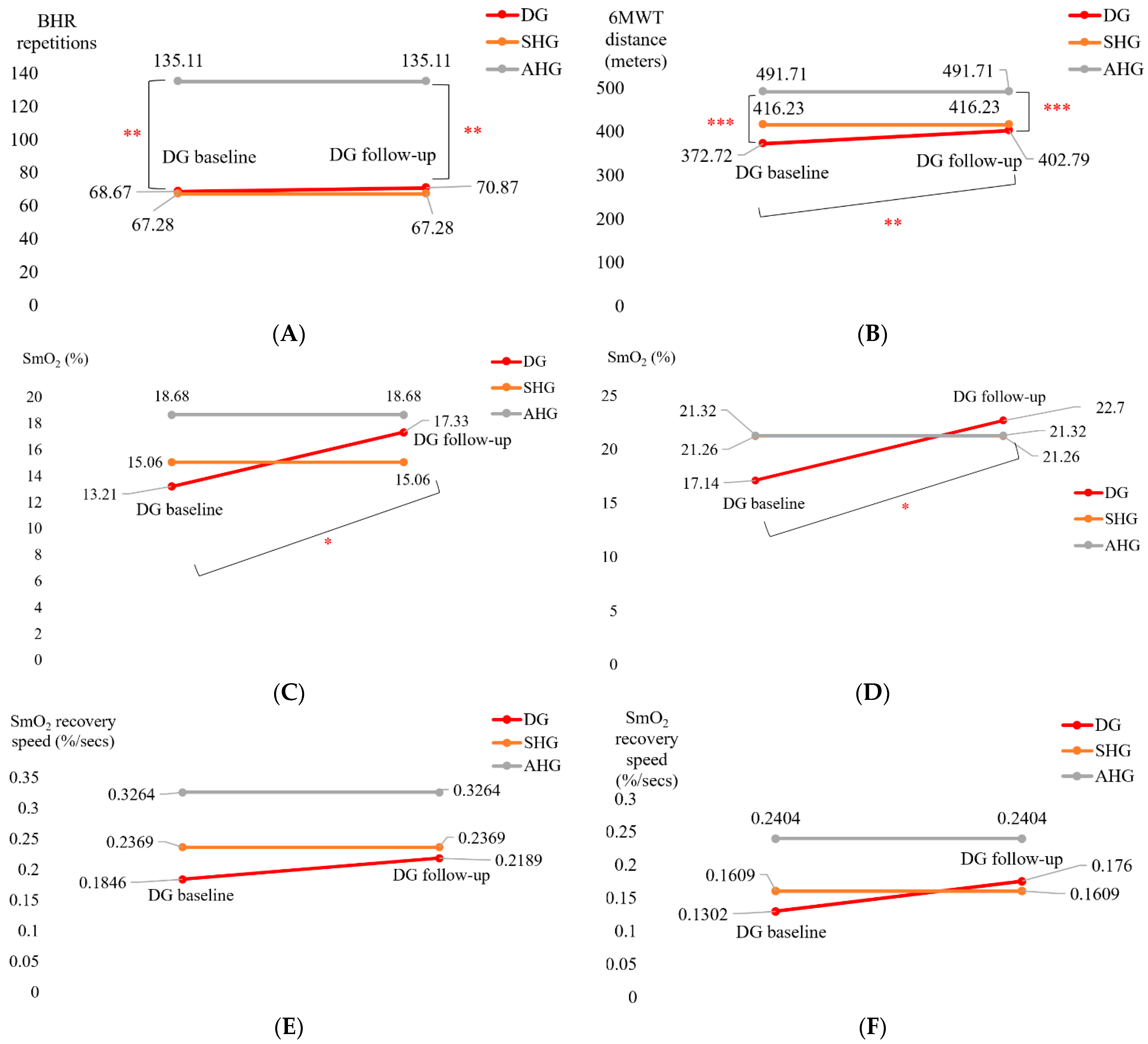
| SmO2 drop during BHR test (%) | 13.21 (7.5) | 17.33 (11.6) | t = 2.185 * (p = 0.022) | d = −0.515 |
| SmO2 drop during 6MWT (%) | 17.14 (9.1) | 22.70 (15.4) | t = 1.845 * (p = 0.041) | d = −0.435 |
| BHR SmO2 recovery speed (%/s) | 0.1846 (0.071) | 0.2189 (0.107) | t = 1.714 (p = 0.052) | d = −0.404 |
| 6MWT SmO2 recovery speed (%/s) | 0.1302 (0.087) | 0.1760 (0.174) | t = 1.094 (p = 0.145) | d = −0.258 |
| AAL Region | Source | Detector | Type | Factor | p | q |
|---|---|---|---|---|---|---|
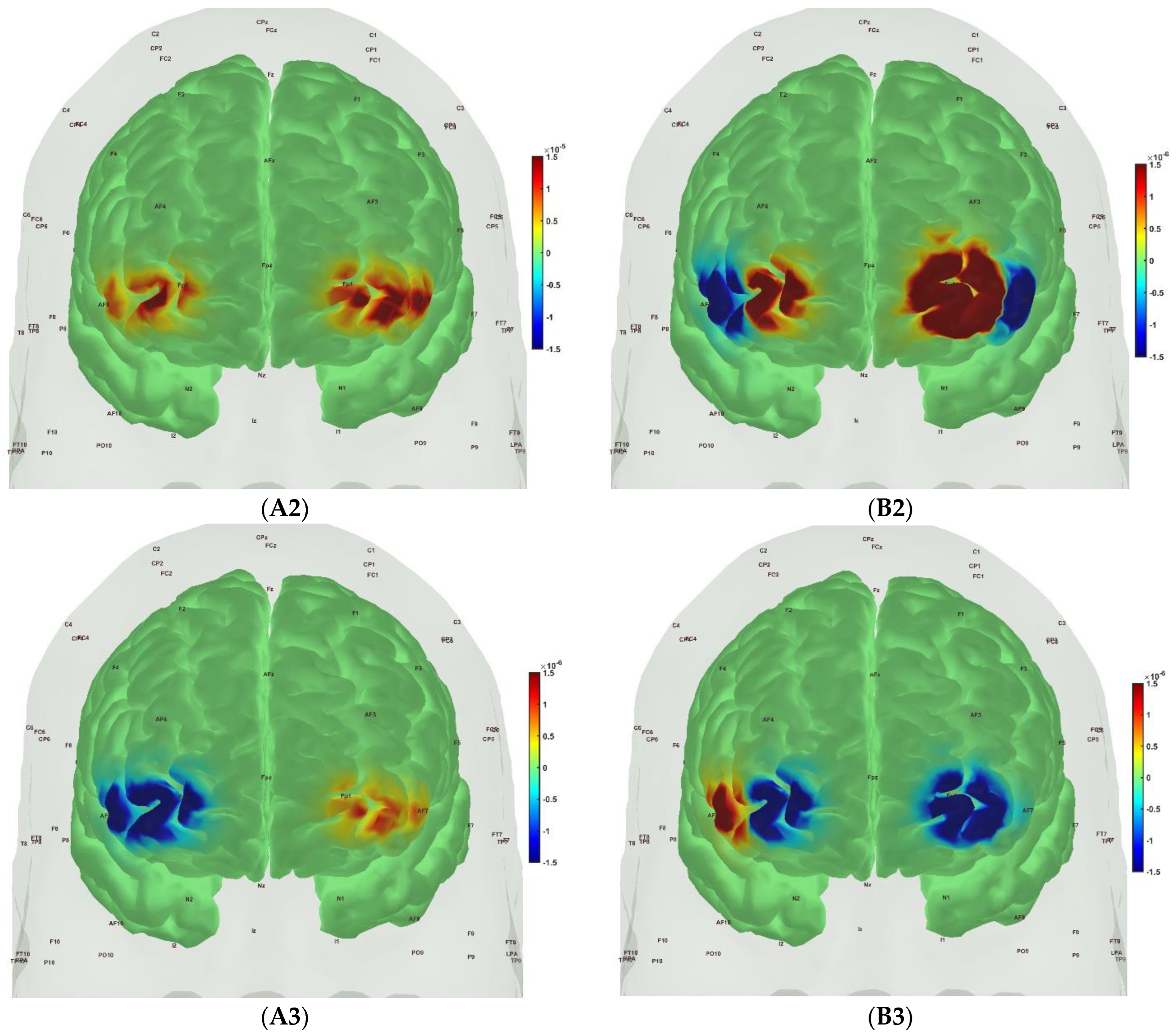
| Frontal_Inf_Tri_R | 1 | 1 | ‘hbo’ | ‘cond’ | 0.999999981 | 1 |
| Frontal_Inf_Tri_R | 1 | 1 | ‘hbo’ | ‘group’ | 0.083678248 | 0.205977227 |
| Frontal_Inf_Tri_R | 1 | 1 | ‘hbr’ | ‘cond’ | 0.947160368 | 1 |
| Frontal_Inf_Tri_R | 1 | 1 | ‘hbr’ | ‘group’ | 0.000314435 | 0.001437418 |
| Frontal_Sup_R | 3 | 1 | ‘hbo’ | ‘cond’ | 0.999987864 | 1 |
| Frontal_Sup_R | 3 | 1 | ‘hbo’ | ‘group’ | 0.003211619 | 0.012846478 |
| Frontal_Sup_R | 3 | 1 | ‘hbr’ | ‘cond’ | 1 | 1 |
| Frontal_Sup_R | 3 | 1 | ‘hbr’ | ‘group’ | 1.97 × 10−10 | 3.15 × 10−9 |
| Frontal_Inf_Tri_L | 5 | 2 | ‘hbo’ | ‘cond’ | 0.999999039 | 1 |
| Frontal_Inf_Tri_L | 5 | 2 | ‘hbo’ | ‘group’ | 0.064006828 | 0.170684874 |
| Frontal_Inf_Tri_L | 5 | 2 | ‘hbr’ | ‘cond’ | 0.994658711 | 1 |
| Frontal_Inf_Tri_L | 5 | 2 | ‘hbr’ | ‘group’ | 1.82 × 10−5 | 9.70 × 10−5 |
| Frontal_Sup_L | 7 | 2 | ‘hbo’ | ‘cond’ | 0.375322014 | 0.720721989 |
| Frontal_Sup_L | 7 | 2 | ‘hbo’ | ‘group’ | 6.15 × 10−7 | 4.92 × 10−6 |
| Frontal_Sup_L | 7 | 2 | ‘hbr’ | ‘cond’ | 0.999999845 | 1 |
| Frontal_Sup_L | 7 | 2 | ‘hbr’ | ‘group’ | 3.44 × 10−6 | 2.20 × 10−5 |
| AAL Region | Source | Detector | Type | Factor | p | q |
|---|---|---|---|---|---|---|
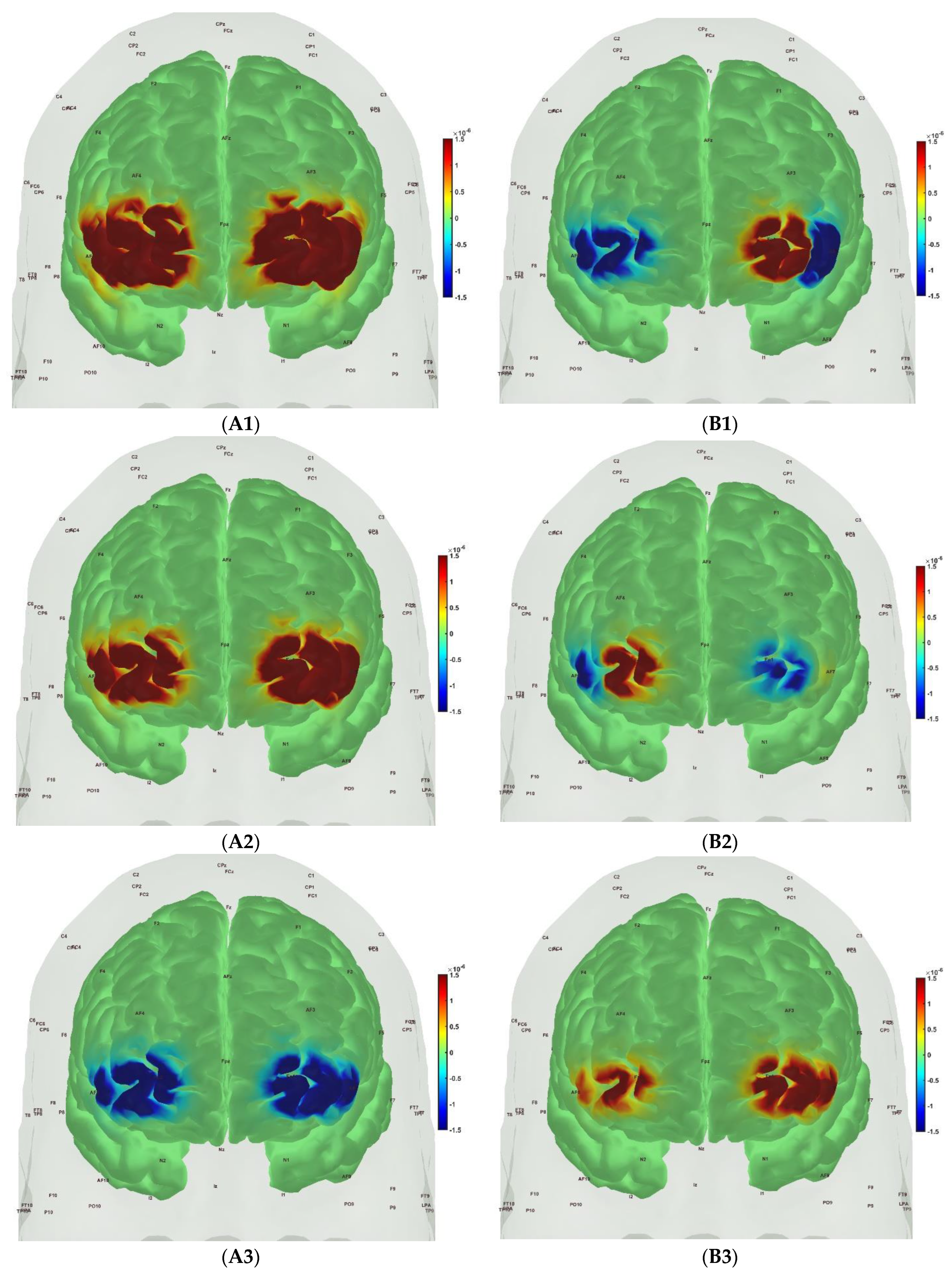
| Frontal_Inf_Tri_R | 1 | 1 | ‘hbo’ | ‘cond’ | 0.6481 | 1 |
| Frontal_Inf_Tri_R | 1 | 1 | ‘hbo’ | ‘group’ | 0.00587 | 0.01879 |
| Frontal_Inf_Tri_R | 1 | 1 | ‘hbr’ | ‘cond’ | 0.78319 | 1 |
| Frontal_Inf_Tri_R | 1 | 1 | ‘hbr’ | ‘group’ | 0.62103 | 1 |
| Frontal_Sup_R | 3 | 1 | ‘hbo’ | ‘cond’ | 0.97924 | 1 |
| Frontal_Sup_R | 3 | 1 | ‘hbo’ | ‘group’ | 2.39 × 10−6 | 8.50 × 10−6 |
| Frontal_Sup_R | 3 | 1 | ‘hbr’ | ‘cond’ | 1 | 1 |
| Frontal_Sup_R | 3 | 1 | ‘hbr’ | ‘group’ | 2.15 × 10−6 | 8.50 × 10−6 |
| Frontal_Inf_Tri_L | 5 | 2 | ‘hbo’ | ‘cond’ | 1 | 1 |
| Frontal_Inf_Tri_L | 5 | 2 | ‘hbo’ | ‘group’ | 0.7415 | 1 |
| Frontal_Inf_Tri_L | 5 | 2 | ‘hbr’ | ‘cond’ | 0.99998 | 1 |
| Frontal_Inf_Tri_L | 5 | 2 | ‘hbr’ | ‘group’ | 0.56073 | 1 |
| Frontal_Sup_L | 7 | 2 | ‘hbo’ | ‘cond’ | 1 | 1 |
| Frontal_Sup_L | 7 | 2 | ‘hbo’ | ‘group’ | 7.59 × 10−8 | 3.47 × 10−7 |
| Frontal_Sup_L | 7 | 2 | ‘hbr’ | ‘cond’ | 0.99781 | 1 |
| Frontal_Sup_L | 7 | 2 | ‘hbr’ | ‘group’ | 0.56618 | 1 |
| AAL Region | Source | Detector | Type | Factor | p | q |
|---|---|---|---|---|---|---|
| Frontal_Inf_Tri_R | 1 | 1 | ‘hbo’ | ‘cond’ | 0.99999259 | 1 |
| Frontal_Inf_Tri_R | 1 | 1 | ‘hbo’ | ‘group’ | 0.056004 | 0.17322566 |
| Frontal_Inf_Tri_R | 1 | 1 | ‘hbr’ | ‘cond’ | 1 | 1 |
| Frontal_Inf_Tri_R | 1 | 1 | ‘hbr’ | ‘group’ | 0.00543628 | 0.04349027 |
| Frontal_Sup_R | 3 | 1 | ‘hbo’ | ‘cond’ | 0.99999952 | 1 |
| Frontal_Sup_R | 3 | 1 | ‘hbo’ | ‘group’ | 0.01244236 | 0.05687937 |
| Frontal_Sup_R | 3 | 1 | ‘hbr’ | ‘cond’ | 1 | 1 |
| Frontal_Sup_R | 3 | 1 | ‘hbr’ | ‘group’ | 8.41 × 10−12 | 1.35 × 10−10 |
| Frontal_Inf_Tri_L | 5 | 2 | ‘hbo’ | ‘cond’ | 0.09426186 | 0.2320292 |
| Frontal_Inf_Tri_L | 5 | 2 | ‘hbo’ | ‘group’ | 0.06495962 | 0.17322566 |
| Frontal_Inf_Tri_L | 5 | 2 | ‘hbr’ | ‘cond’ | 0.97629652 | 1 |
| Frontal_Inf_Tri_L | 5 | 2 | ‘hbr’ | ‘group’ | 0.28616516 | 0.57233033 |
| Frontal_Sup_L | 7 | 2 | ‘hbo’ | ‘cond’ | 0.01672316 | 0.06689266 |
| Frontal_Sup_L | 7 | 2 | ‘hbo’ | ‘group’ | 0.96488565 | 1 |
| Frontal_Sup_L | 7 | 2 | ‘hbr’ | ‘cond’ | 1 | 1 |
| Frontal_Sup_L | 7 | 2 | ‘hbr’ | ‘group’ | 0.04105912 | 0.14598797 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Tomita, M.; Dutta, A. Operational Modal Analysis of Near-Infrared Spectroscopy Measure of 2-Month Exercise Intervention Effects in Sedentary Older Adults with Diabetes and Cognitive Impairment. Brain Sci. 2023, 13, 1099. https://doi.org/10.3390/brainsci13071099
Zhao F, Tomita M, Dutta A. Operational Modal Analysis of Near-Infrared Spectroscopy Measure of 2-Month Exercise Intervention Effects in Sedentary Older Adults with Diabetes and Cognitive Impairment. Brain Sciences. 2023; 13(7):1099. https://doi.org/10.3390/brainsci13071099
Chicago/Turabian StyleZhao, Fei, Machiko Tomita, and Anirban Dutta. 2023. "Operational Modal Analysis of Near-Infrared Spectroscopy Measure of 2-Month Exercise Intervention Effects in Sedentary Older Adults with Diabetes and Cognitive Impairment" Brain Sciences 13, no. 7: 1099. https://doi.org/10.3390/brainsci13071099
APA StyleZhao, F., Tomita, M., & Dutta, A. (2023). Operational Modal Analysis of Near-Infrared Spectroscopy Measure of 2-Month Exercise Intervention Effects in Sedentary Older Adults with Diabetes and Cognitive Impairment. Brain Sciences, 13(7), 1099. https://doi.org/10.3390/brainsci13071099