Altered Relationship between Functional Connectivity and Fiber-Bundle Structure in High-Functioning Male Adults with Autism Spectrum Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Imaging Data
2.3. Image Preprocessing
2.4. Functional and Structural Network Construction
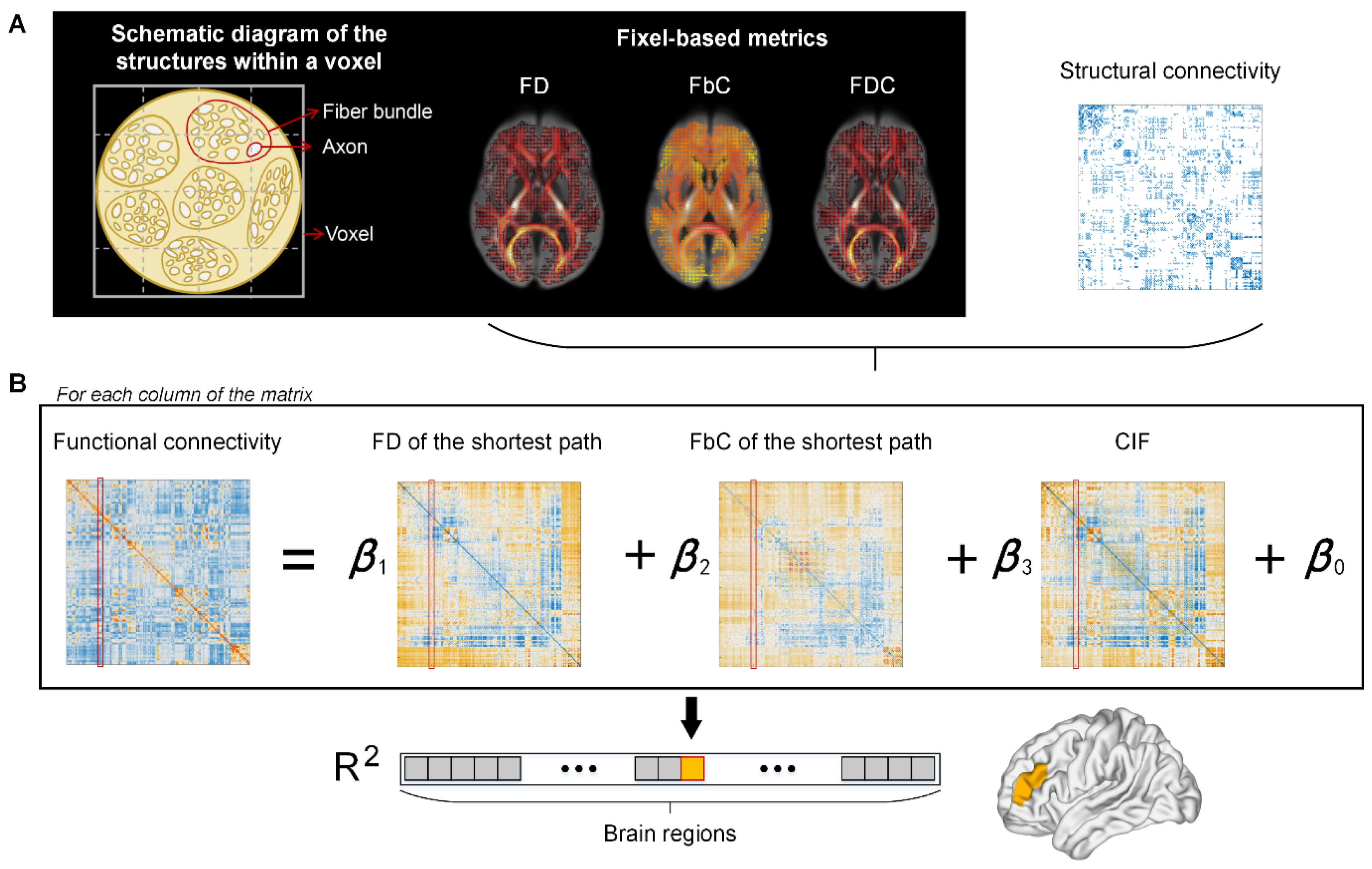
2.5. Fixel-Based Fiber-Bundle Analysis
2.6. Multilinear Model
2.7. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
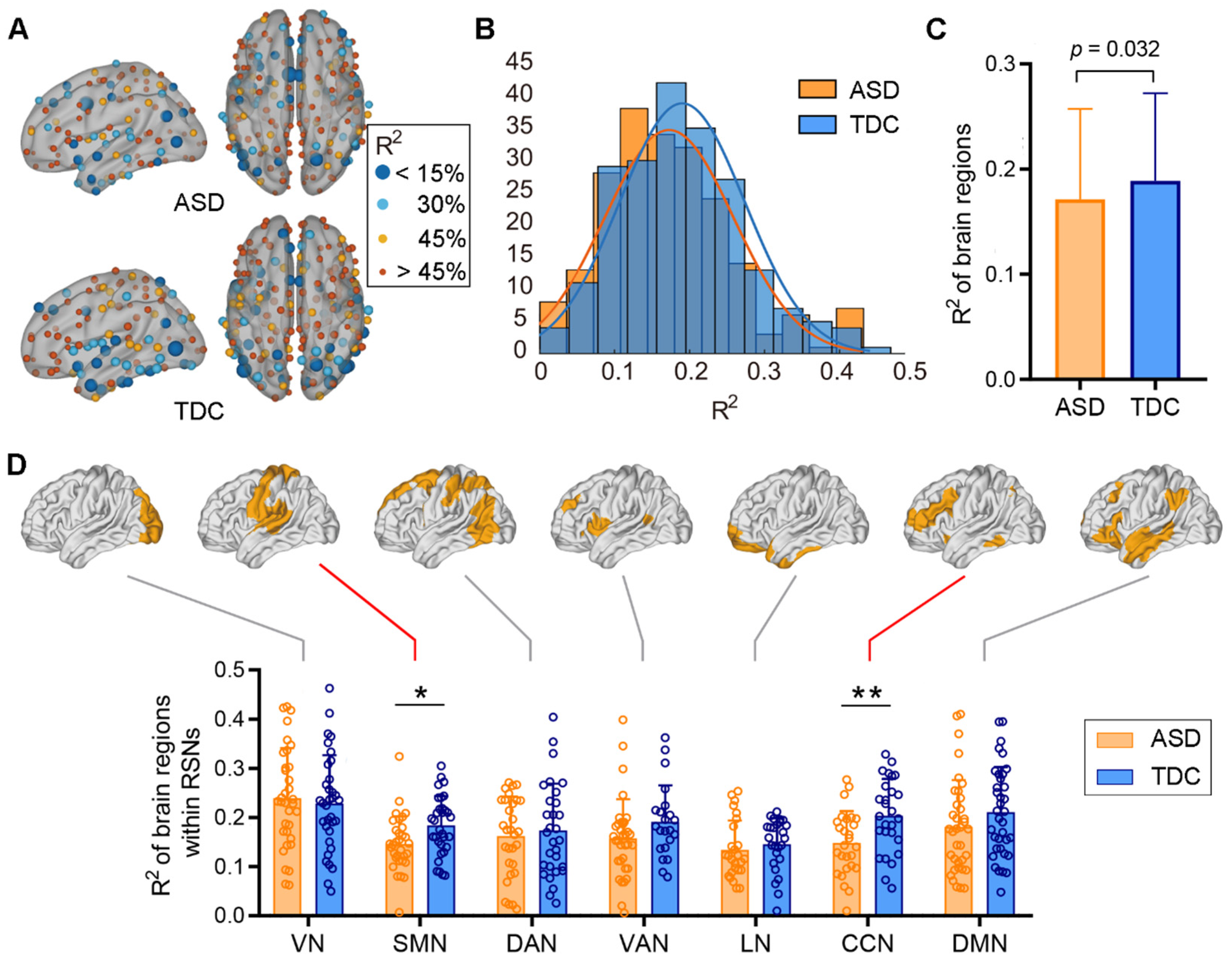
3.2. Comparisons between ASD and TDC at the Template Level
3.3. Comparisons between ASD and TDC at the Individual Difference Level
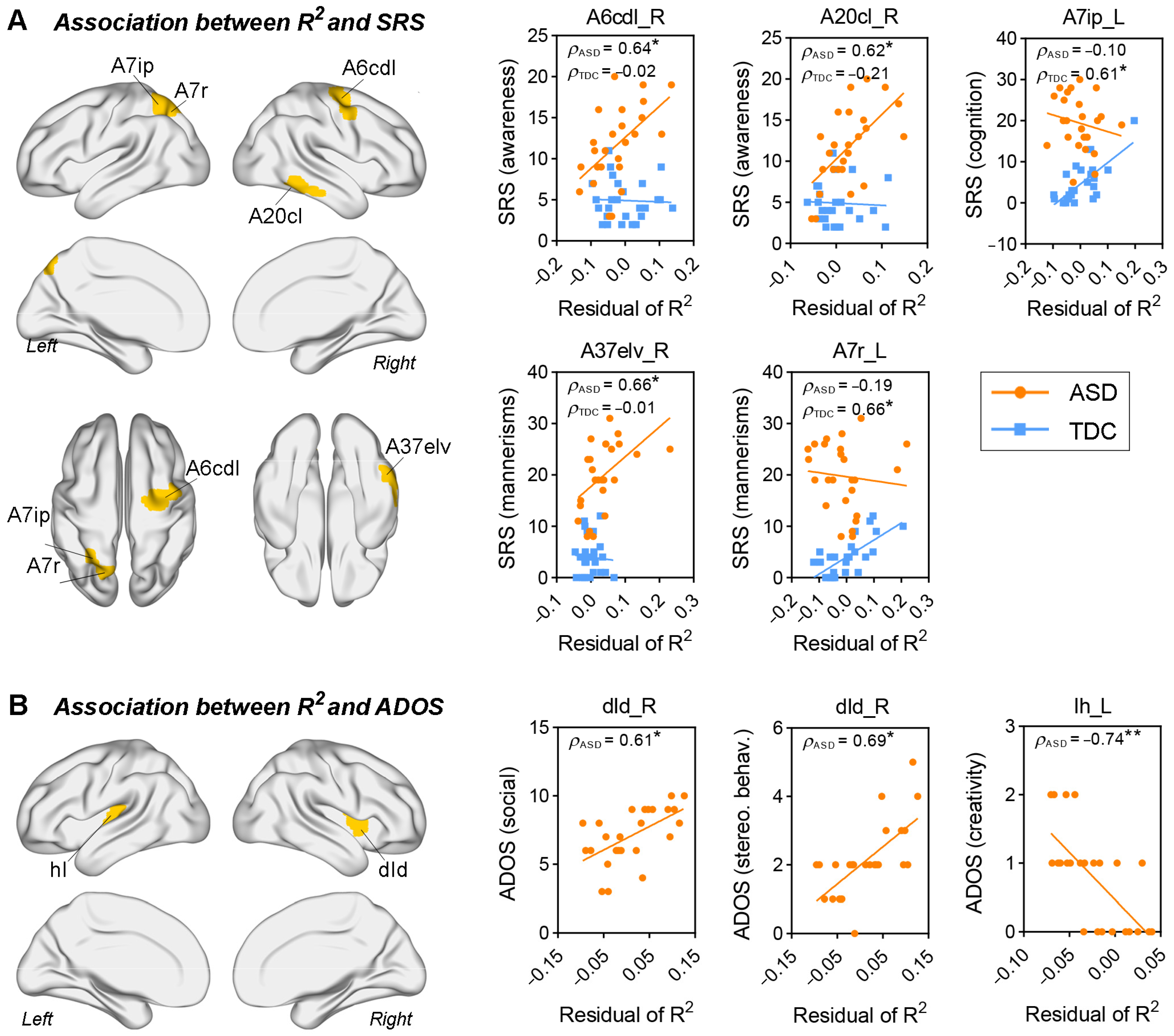
3.4. Correlations between Structure–Function Correspondence and Clinical Assessments
4. Discussion
4.1. Divergent Structure–Function Relationships in Patients with ASD
4.2. Autistic Clinical Symptoms Were Related to Subtle Changes in Structure–Function Relationships
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, M.C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Lawson, R.P.; Mathys, C.; Rees, G. Adults with autism overestimate the volatility of the sensory environment. Nat. Neurosci. 2017, 20, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, G.; Masi, G.; Toni, C.; Dell’Osso, L.; Marazziti, D.; Perugi, G. Clinical features, developmental course, and psychiatric comorbidity of adult autism spectrum disorders. CNS Spectr. 2014, 19, 157–164. [Google Scholar] [CrossRef]
- Brugha, T.S.; McManus, S.; Bankart, J.; Scott, F.; Purdon, S.; Smith, J.; Bebbington, P.; Jenkins, R.; Meltzer, H. Epidemiology of autism spectrum disorders in adults in the community in England. Arch. Gen. Psychiatry 2011, 68, 459–465. [Google Scholar] [CrossRef]
- Fusar-Poli, L.; Brondino, N.; Politi, P.; Aguglia, E. Missed diagnoses and misdiagnoses of adults with autism spectrum disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Lombardo, M.V.; Pasco, G.; Ruigrok, A.N.; Wheelwright, S.J.; Sadek, S.A.; Chakrabarti, B.; Baron-Cohen, S. A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PLoS ONE 2011, 6, e20835. [Google Scholar] [CrossRef]
- Lai, M.C.; Baron-Cohen, S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry 2015, 2, 1013–1027. [Google Scholar] [CrossRef]
- Courchesne, E.; Karns, C.M.; Davis, H.R.; Ziccardi, R.; Carper, R.A.; Tigue, Z.D.; Chisum, H.J.; Moses, P.; Pierce, K.; Lord, C.; et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology 2001, 57, 245–254. [Google Scholar] [CrossRef]
- Zheng, W.; Eilamstock, T.; Wu, T.; Spagna, A.; Chen, C.; Hu, B.; Fan, J. Multi-feature based network revealing the structural abnormalities in autism spectrum disorder. IEEE Trans. Affect. Comput. 2018, 12, 732–742. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, Z.; Zhang, Z.; Liu, T.; Zhang, Y.; Fan, J.; Wu, D. Developmental pattern of the cortical topology in high-functioning individuals with autism spectrum disorder. Hum. Brain Mapp. 2020, 42, 660–675. [Google Scholar] [CrossRef] [PubMed]
- Palmen, S.J.; Hulshoff Pol, H.E.; Kemner, C.; Schnack, H.G.; Durston, S.; Lahuis, B.E.; Kahn, R.S.; Van Engeland, H. Increased gray-matter volume in medication-naive high-functioning children with autism spectrum disorder. Psychol. Med. 2005, 35, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Hazlett, H.C.; Poe, M.D.; Gerig, G.; Smith, R.G.; Piven, J. Cortical gray and white brain tissue volume in adolescents and adults with autism. Biol. Psychiatry 2006, 59, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Carper, R.A.; Moses, P.; Tigue, Z.D.; Courchesne, E. Cerebral lobes in autism: Early hyperplasia and abnormal age effects. Neuroimage 2002, 16, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Levitt, J.G.; Blanton, R.E.; Smalley, S.; Thompson, P.M.; Guthrie, D.; McCracken, J.T.; Sadoun, T.; Heinichen, L.; Toga, A.W. Cortical sulcal maps in autism. Cereb. Cortex 2003, 13, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Nordahl, C.W.; Dierker, D.; Mostafavi, I.; Schumann, C.M.; Rivera, S.M.; Amaral, D.G.; Van Essen, D.C. Cortical folding abnormalities in autism revealed by surface-based morphometry. J. Neurosci. 2007, 27, 11725–11735. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.T.; Pearl, P.L. Imaging Data in Autism: From Structure to Malfunction, Seminars in Pediatric Neurology; Elsevier: Amsterdam, The Netherlands, 2004; pp. 205–213. [Google Scholar]
- Ecker, C.; Bookheimer, S.Y.; Murphy, D.G. Neuroimaging in autism spectrum disorder: Brain structure and function across the lifespan. Lancet Neurol. 2015, 14, 1121–1134. [Google Scholar] [CrossRef]
- Yao, Z.; Hu, B.; Xie, Y.; Zheng, F.; Liu, G.; Chen, X.; Zheng, W. Resting-State Time-Varying Analysis Reveals Aberrant Variations of Functional Connectivity in Autism. Front. Hum. Neurosci. 2016, 10, 463. [Google Scholar] [CrossRef]
- Di Martino, A.; Yan, C.G.; Li, Q.; Denio, E.; Castellanos, F.X.; Alaerts, K.; Anderson, J.S.; Assaf, M.; Bookheimer, S.Y.; Dapretto, M.; et al. The autism brain imaging data exchange: Towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol. Psychiatry 2014, 19, 659–667. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, X.; Jiao, J.; Yuan, D.; Li, S.; Luo, T.; Wang, M.; Situ, M.; Sun, X.; Huang, Y. Brain white matter microstructure abnormalities in children with optimal outcome from autism: A four-year follow-up study. Sci. Rep. 2022, 12, 20151. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; Keynton, R.S.; Mostapha, M.M.; ElTanboly, A.H.; Casanova, M.F.; Gimel’farb, G.L.; El-Baz, A. Studying Autism Spectrum Disorder with Structural and Diffusion Magnetic Resonance Imaging: A Survey. Front. Hum. Neurosci. 2016, 10, 211. [Google Scholar] [CrossRef]
- Jiang, X.; Shou, X.-J.; Zhao, Z.; Chen, Y.; Meng, F.-C.; Le, J.; Song, T.-J.; Xu, X.-J.; Guo, W.; Ke, X.; et al. A brain structural connectivity biomarker for autism spectrum disorder diagnosis in early childhood. Psychoradiology 2023, 3, kkad005. [Google Scholar] [CrossRef]
- Yu, Y.; Zheng, W.; Tan, X.; Li, X.; Zhang, X.; Gao, J.; Pan, G.; Wu, D.; Luo, B. Microstructural profiles of thalamus and thalamocortical connectivity in patients with disorder of consciousness. J. Neurosci. Res. 2021, 99, 3261–3273. [Google Scholar] [CrossRef] [PubMed]
- Raffelt, D.A.; Tournier, J.D.; Smith, R.E.; Vaughan, D.N.; Jackson, G.; Ridgway, G.R.; Connelly, A. Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage 2017, 144 Pt A, 58–73. [Google Scholar] [CrossRef]
- Pierpaoli, C.; Barnett, A.; Pajevic, S.; Chen, R.; Penix, L.R.; Virta, A.; Basser, P. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage 2001, 13 Pt 1, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Tan, X.; Liu, T.; Li, X.; Gao, J.; Hong, L.; Zhang, X.; Zhao, Z.; Yu, Y.; Zhang, Y.; et al. Individualized Thalamic Parcellation Reveals Alterations in Shape and Microstructure of Thalamic Nuclei in Patients with Disorder of Consciousness. Cereb. Cortex Commun. 2021, 2, tgab024. [Google Scholar] [CrossRef] [PubMed]
- Raffelt, D.A.; Smith, R.E.; Ridgway, G.R.; Tournier, J.D.; Vaughan, D.N.; Rose, S.; Henderson, R.; Connelly, A. Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. Neuroimage 2015, 117, 40–55. [Google Scholar] [CrossRef]
- Dimond, D.; Schuetze, M.; Smith, R.E.; Dhollander, T.; Cho, I.; Vinette, S.; Ten Eycke, K.; Lebel, C.; McCrimmon, A.; Dewey, D.; et al. Reduced White Matter Fiber Density in Autism Spectrum Disorder. Cereb. Cortex 2019, 29, 1778–1788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, W. The Discriminative Power of White Matter Microstructures for Autism Diagnosis. IFAC-PapersOnLine 2020, 53, 446–451. [Google Scholar] [CrossRef]
- Cocchi, L.; Harding, I.H.; Lord, A.; Pantelis, C.; Yucel, M.; Zalesky, A. Disruption of structure-function coupling in the schizophrenia connectome. NeuroImage Clin. 2014, 4, 779–787. [Google Scholar] [CrossRef]
- Jirsa, V.K.; Proix, T.; Perdikis, D.; Woodman, M.M.; Wang, H.; Gonzalez-Martinez, J.; Bernard, C.; Bénar, C.; Guye, M.; Chauvel, P.; et al. The Virtual Epileptic Patient: Individualized whole-brain models of epilepsy spread. Neuroimage 2017, 145 Pt B, 377–388. [Google Scholar] [CrossRef]
- Medaglia, J.D.; Huang, W.; Karuza, E.A.; Kelkar, A.; Thompson-Schill, S.L.; Ribeiro, A.; Bassett, D.S. Functional Alignment with Anatomical Networks is Associated with Cognitive Flexibility. Nat. Hum. Behav. 2018, 2, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Mottron, L.; Park, B.Y.; Benkarim, O.; Valk, S.L.; Paquola, C.; Larivière, S.; Vos de Wael, R.; Degré-Pelletier, J.; Soulieres, I.; et al. A convergent structure-function substrate of cognitive imbalances in autism. Cereb. Cortex 2023, 33, 1566–1580. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.C.; Chou, T.L.; Fan, L.Y.; Gau, S.S.; Chiu, Y.N.; Tseng, W.Y. Altered structure-function relations of semantic processing in youths with high-functioning autism: A combined diffusion and functional MRI study. Autism Res. Off. J. Int. Soc. Autism Res. 2013, 6, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zheng, W.; Liu, H.; Guo, M.; Ma, L.; Hu, W.; Ke, M.; Sun, Y.; Zhang, J.; Zhang, Z. Aberrant dynamic structure-function relationship of rich-club organization in treatment-naïve newly diagnosed juvenile myoclonic epilepsy. Hum. Brain Mapp. 2022, 43, 3633–3645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wan, X.; Ai, K.; Zheng, W.; Liu, G.; Wang, J.; Huang, W.; Fan, F.; Yao, Z.; Zhang, J. Rich-club reorganization and related network disruptions are associated with the symptoms and severity in classic trigeminal neuralgia patients. NeuroImage Clin. 2022, 36, 103160. [Google Scholar] [CrossRef]
- Suárez, L.E.; Markello, R.D.; Betzel, R.F.; Misic, B. Linking Structure and Function in Macroscale Brain Networks. Trends Cogn. Sci. 2020, 24, 302–315. [Google Scholar] [CrossRef]
- Vázquez-Rodríguez, B.; Suárez, L.E.; Markello, R.D.; Shafiei, G.; Paquola, C.; Hagmann, P.; Van den Heuvel, M.P.; Bernhardt, B.C.; Spreng, R.N.; Misic, B. Gradients of structure-function tethering across neocortex. Proc. Natl. Acad. Sci. USA 2019, 116, 21219–21227. [Google Scholar] [CrossRef]
- Lord, C.; Rutter, M.; DiLavore, P.; Risi, S.; Gotham, K.; Bishop, S. Autism Diagnostic Observation Schedule; ADOS Western Psychological Services: Los Angeles, CA, USA, 2002. [Google Scholar]
- Gruber, J. Social Responsiveness Scale, 2nd ed.; (SRS-2); Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Kaufman, A.S. Kaufman Brief Intelligence Test, 2nd ed.; (KBIT-2); American Guidance Service: Circle Pines, MN, USA, 2004. [Google Scholar]
- Yan, C.; Zang, Y. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Front. Syst. Neurosci. 2010, 4, 13. [Google Scholar] [CrossRef]
- Tournier, J.-D.; Calamante, F.; Connelly, A. MRtrix: Diffusion tractography in crossing fiber regions. Int. J. Imaging Syst. Technol. 2012, 22, 53–66. [Google Scholar] [CrossRef]
- Veraart, J.; Novikov, D.S.; Christiaens, D.; Ades-Aron, B.; Sijbers, J.; Fieremans, E. Denoising of diffusion MRI using random matrix theory. Neuroimage 2016, 142, 394–406. [Google Scholar] [CrossRef]
- Tustison, N.J.; Avants, B.B.; Cook, P.A.; Zheng, Y.; Egan, A.; Yushkevich, P.A.; Gee, J.C. N4ITK: Improved N3 bias correction. IEEE Trans. Med. Imaging 2010, 29, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Raffelt, D.; Tournier, J.D.; Crozier, S.; Connelly, A.; Salvado, O. Reorientation of fiber orientation distributions using apodized point spread functions. Magn. Reson. Med. 2012, 67, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Dhollander, T.; Connelly, A. A novel iterative approach to reap the benefits of multi-tissue CSD from just single-shell (+b = 0) diffusion MRI data. In Proceedings of the 2016 Annual Meeting & Exhibition, Singapore, 7–13 May 2016; p. 3010. [Google Scholar]
- Raffelt, D.; Tournier, J.D.; Fripp, J.; Crozier, S.; Connelly, A.; Salvado, O. Symmetric diffeomorphic registration of fibre orientation distributions. Neuroimage 2011, 56, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Li, H.; Zhuo, J.; Zhang, Y.; Wang, J.; Chen, L.; Yang, Z.; Chu, C.; Xie, S.; Laird, A.R.; et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb. Cortex 2016, 26, 3508–3526. [Google Scholar] [CrossRef] [PubMed]
- Tournier, J.-D.; Calamante, F.; Connelly, A. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. In Proceedings of the International Society for Magnetic Resonance in Medicine; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Yeo, B.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar]
- Colom, R.; Karama, S.; Jung, R.E.; Haier, R.J. Human intelligence and brain networks. Dialogues Clin. Neurosci. 2010, 12, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Malagurski, B.; Liem, F.; Oschwald, J.; Mérillat, S.; Jäncke, L. Longitudinal functional brain network reconfiguration in healthy aging. Hum. Brain Mapp. 2020, 41, 4829–4845. [Google Scholar] [CrossRef]
- Porcu, M.; Wintermark, M.; Suri, J.S.; Saba, L. The influence of the volumetric composition of the intracranial space on neural activity in healthy subjects: A resting-state functional magnetic resonance study. Eur. J. Neurosci. 2020, 51, 1944–1961. [Google Scholar] [CrossRef]
- Baum, G.L.; Cui, Z.; Roalf, D.R.; Ciric, R.; Betzel, R.F.; Larsen, B.; Cieslak, M.; Cook, P.A.; Xia, C.H.; Moore, T.M.; et al. Development of structure-function coupling in human brain networks during youth. Proc. Natl. Acad. Sci. USA 2020, 117, 771–778. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, C.; Cui, S.; Wang, R.; Cai, H.; Zhao, W.; Zhu, J.; Yu, Y. Transcriptional substrates underlying functional connectivity profiles of subregions within the human sensorimotor cortex. Hum. Brain Mapp. 2022, 43, 5562–5578. [Google Scholar] [CrossRef]
- Eisenberg, M.; Shmuelof, L.; Vaadia, E.; Zohary, E. The representation of visual and motor aspects of reaching movements in the human motor cortex. J. Neurosci. 2011, 31, 12377–12384. [Google Scholar] [CrossRef] [PubMed]
- Soman, S.M.; Raghavan, S.; Rajesh, P.G.; Mohanan, N.; Thomas, B.; Kesavadas, C.; Menon, R.N. Does resting state functional connectivity differ between mild cognitive impairment and early Alzheimer’s dementia? J. Neurol. Sci. 2020, 418, 117093. [Google Scholar] [CrossRef]
- Mostofsky, S.H.; Ewen, J.B. Altered connectivity and action model formation in autism is autism. Neuroscientist 2011, 17, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Gabrielsen, T.P.; Anderson, J.S.; Stephenson, K.G.; Beck, J.; King, J.B.; Kellems, R.; Top, D.N., Jr.; Russell, N.C.C.; Anderberg, E.; Lundwall, R.A.; et al. Functional MRI connectivity of children with autism and low verbal and cognitive performance. Mol. Autism 2018, 9, 67. [Google Scholar] [CrossRef]
- Mostofsky, S.H.; Powell, S.K.; Simmonds, D.J.; Goldberg, M.C.; Caffo, B.; Pekar, J.J. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain 2009, 132 Pt 9, 2413–2425. [Google Scholar] [CrossRef] [PubMed]
- Oldehinkel, M.; Mennes, M.; Marquand, A.; Charman, T.; Tillmann, J.; Ecker, C.; Dell’Acqua, F.; Brandeis, D.; Banaschewski, T.; Baumeister, S.; et al. Altered Connectivity Between Cerebellum, Visual, and Sensory-Motor Networks in Autism Spectrum Disorder: Results from the EU-AIMS Longitudinal European Autism Project. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 260–270. [Google Scholar] [CrossRef]
- Paquola, C.; Vos De Wael, R.; Wagstyl, K.; Bethlehem, R.A.I.; Hong, S.J.; Seidlitz, J.; Bullmore, E.T.; Evans, A.C.; Misic, B.; Margulies, D.S.; et al. Microstructural and functional gradients are increasingly dissociated in transmodal cortices. PLoS Biol. 2019, 17, e3000284. [Google Scholar] [CrossRef]
- Long, Z.; Duan, X.; Mantini, D.; Chen, H. Alteration of functional connectivity in autism spectrum disorder: Effect of age and anatomical distance. Sci. Rep. 2016, 6, 26527. [Google Scholar] [CrossRef]
- Koshino, H.; Carpenter, P.A.; Minshew, N.J.; Cherkassky, V.L.; Keller, T.A.; Just, M.A. Functional connectivity in an fMRI working memory task in high-functioning autism. NeuroImage 2005, 24, 810–821. [Google Scholar] [CrossRef]
- Lepping, R.J.; McKinney, W.S.; Magnon, G.C.; Keedy, S.K.; Wang, Z.; Coombes, S.A.; Vaillancourt, D.E.; Sweeney, J.A.; Mosconi, M.W. Visuomotor brain network activation and functional connectivity among individuals with autism spectrum disorder. Hum. Brain Mapp. 2022, 43, 844–859. [Google Scholar] [CrossRef]
- Masi, A.; DeMayo, M.M.; Glozier, N.; Guastella, A.J. An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options. Neurosci. Bull. 2017, 33, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.J.M.; Wallace, G.L.; Gallegos, S.M.; Braden, B.B. Brain-based sex differences in autism spectrum disorder across the lifespan: A systematic review of structural MRI, fMRI, and DTI findings. Neuroimage Clin. 2021, 31, 102719. [Google Scholar] [CrossRef] [PubMed]

| ASD | TDC | p Value | |
|---|---|---|---|
| Age | 38.15 ± 16.18 | 40 ± 15.35 | 0.67 a |
| Gender | 26 male | 26 male | - |
| Full-scale IQ | 109.31 ± 13.66 | 111.27 ± 12.18 | 0.58 a |
| ADOS | |||
| Communication | 3.80 ± 1.32 | - | - |
| Social | 7.12 ± 2.01 | - | - |
| Stereotyped behavior | 2.12 ± 1.09 | - | - |
| Creativity | 0.80 ± 0.71 | - | - |
| Total | 10.92 ± 2.99 | - | - |
| SRS (raw scores) | |||
| Awareness | 11.68 ± 4.71 | 4.92 ± 2.46 | <0.0001 a |
| Cognition | 19.52 ± 6.60 | 4.54 ± 4.62 | <0.0001 a |
| Communication | 35.64 ± 11.51 | 6.65 ± 5.82 | <0.0001 a |
| Motivation | 19.16 ± 6.39 | 5.77 ± 4.49 | <0.0001 a |
| Mannerisms | 19.76 ± 6.63 | 4.00 ± 3.64 | <0.0001 a |
| Total | 105.76 ± 31.92 | 25.88 ± 16.71 | <0.0001 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Q.; Li, J.; Ju, Y.; Xiao, C.; Li, K.; Shi, B.; Zheng, W.; Zhang, Y. Altered Relationship between Functional Connectivity and Fiber-Bundle Structure in High-Functioning Male Adults with Autism Spectrum Disorder. Brain Sci. 2023, 13, 1098. https://doi.org/10.3390/brainsci13071098
Dong Q, Li J, Ju Y, Xiao C, Li K, Shi B, Zheng W, Zhang Y. Altered Relationship between Functional Connectivity and Fiber-Bundle Structure in High-Functioning Male Adults with Autism Spectrum Disorder. Brain Sciences. 2023; 13(7):1098. https://doi.org/10.3390/brainsci13071098
Chicago/Turabian StyleDong, Qiangli, Jialong Li, Yumeng Ju, Chuman Xiao, Kangning Li, Bin Shi, Weihao Zheng, and Yan Zhang. 2023. "Altered Relationship between Functional Connectivity and Fiber-Bundle Structure in High-Functioning Male Adults with Autism Spectrum Disorder" Brain Sciences 13, no. 7: 1098. https://doi.org/10.3390/brainsci13071098
APA StyleDong, Q., Li, J., Ju, Y., Xiao, C., Li, K., Shi, B., Zheng, W., & Zhang, Y. (2023). Altered Relationship between Functional Connectivity and Fiber-Bundle Structure in High-Functioning Male Adults with Autism Spectrum Disorder. Brain Sciences, 13(7), 1098. https://doi.org/10.3390/brainsci13071098







