Prevotella histicola Transplantation Ameliorates Cognitive Impairment and Decreases Oxidative Stress in Vascular Dementia Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Administration
2.2. Establishment of Vascular Dementia (VaD) Model
2.3. Morris Water Maze
2.4. Nissl Staining
2.5. Oxidative Stress Parameters Measurement
2.6. ELISA
2.7. Western Blot
2.8. RT-qPCR
2.9. Statistical Analysis
3. Results
3.1. Prevotella Histicola Transplantation Improves Neurological Behavior of VaD Model Rats
3.2. Prevotella Histicola Transplantation Affects Hippocampal Pathological Morphology and Synapse-Associated Protein Expression in VaD Model Rats
3.3. Prevotella Histicola Transplantation Inhibits the Neuronal Apoptosis of Hippocampi in VaD Model Rats
3.4. Prevotella Histicola Transplantation Alleviates Oxidative Stress in Hippocampi of VaD Model Rats
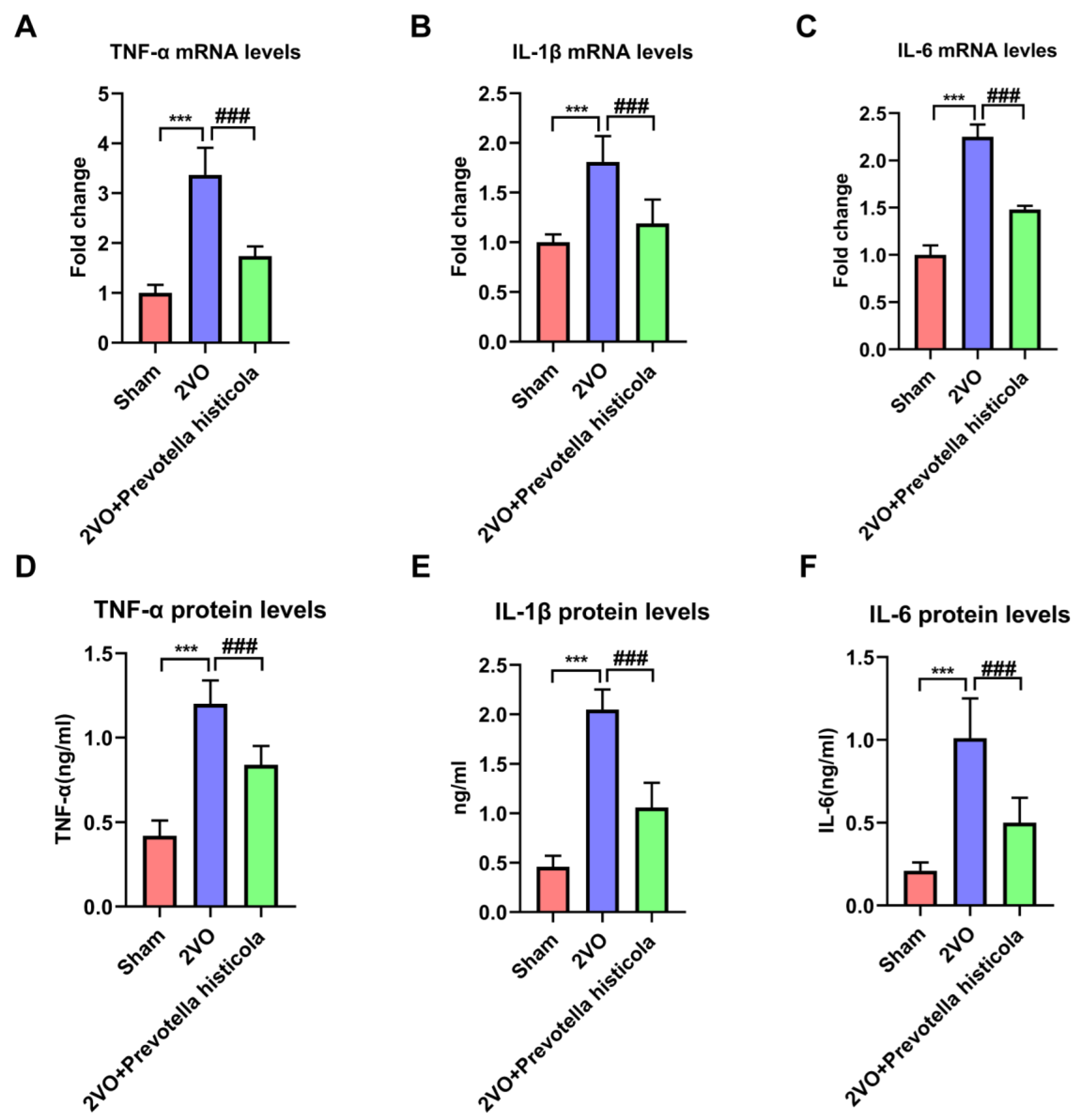
3.5. Prevotella Histicola Transplantation Regulated the Inflammatory Cytokines after VaD
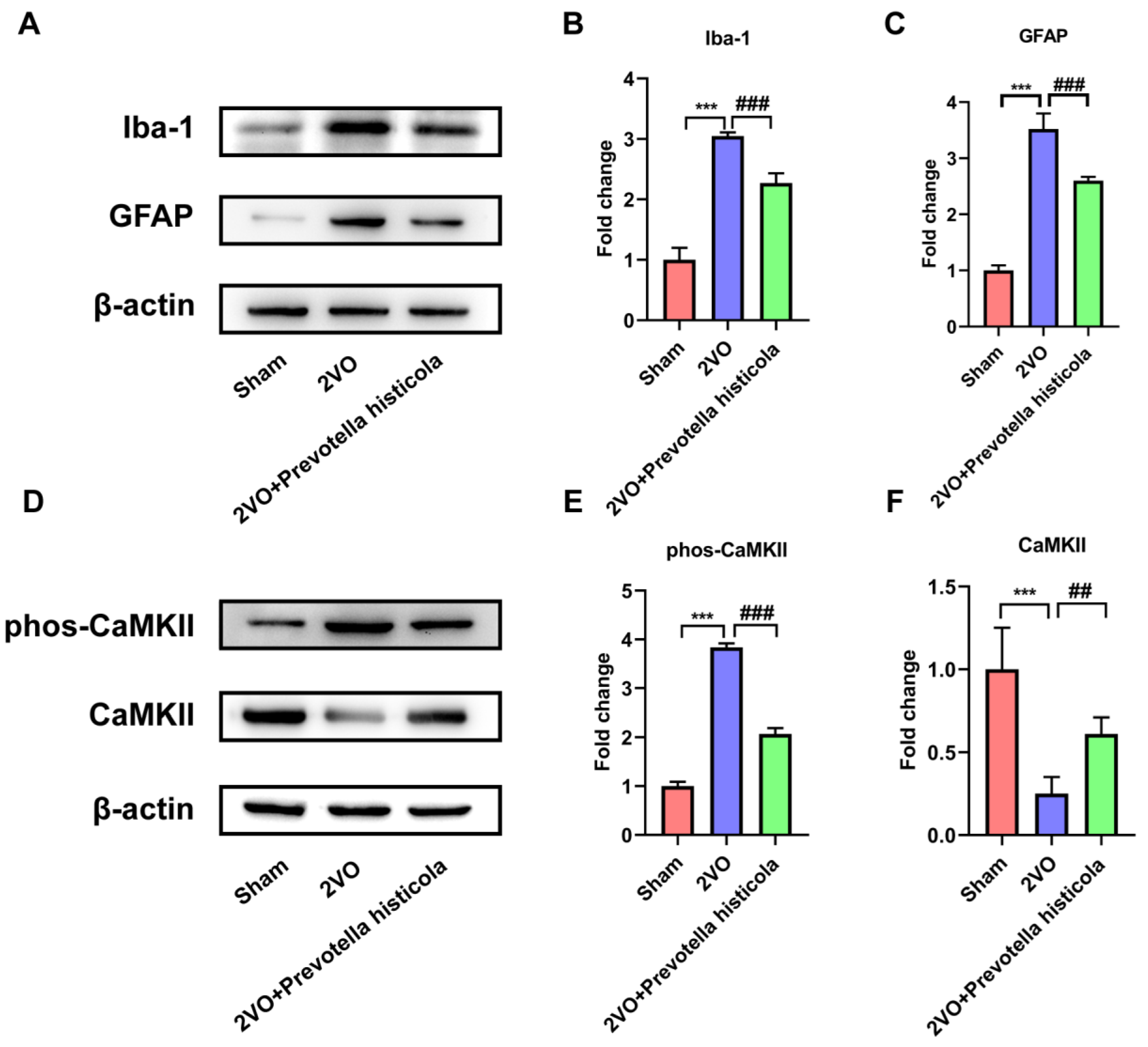
3.6. Prevotella Histicola Transplantation Regulates the Activation of Microglia and Astrocytes and Affects CaMKII Phosphorylation in the Hippocampi of VaD Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iadecola, C.; Duering, M.; Hachinski, V.; Joutel, A.; Pendlebury, S.T.; Schneider, J.A.; Dichgans, M. Vascular Cognitive Impairment and Dementia: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 3326–3344. [Google Scholar] [CrossRef] [PubMed]
- van der Flier, W.M.; Skoog, I.; Schneider, J.A.; Pantoni, L.; Mok, V.; Chen, C.L.H.; Scheltens, P. Vascular cognitive impairment. Nat. Rev. Dis. Primers 2018, 4, 18003. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.U.; Min, J.; Goshgarian, C.; Gorelick, P.B. Pharmacotherapy for Vascular Cognitive Impairment. CNS Drugs 2017, 31, 759–776. [Google Scholar] [CrossRef] [PubMed]
- Zanon Zotin, M.C.; Sveikata, L.; Viswanathan, A.; Yilmaz, P. Cerebral small vessel disease and vascular cognitive impairment: From diagnosis to management. Curr. Opin. Neurol. 2021, 34, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. CMLS 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Zhang, S.; Dong, L. Gut microbiota-mediated immunomodulation in tumor. J. Exp. Clin. Cancer Res. CR 2021, 40, 221. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, Y.; Choi, H.; Kim, W.; Park, S.; Lee, D.; Kim, D.K.; Kim, H.J.; Choi, H.; Hyun, D.W.; et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut 2020, 69, 283–294. [Google Scholar] [CrossRef]
- Zhao, Z.; Ning, J.; Bao, X.Q.; Shang, M.; Ma, J.; Li, G.; Zhang, D. Fecal microbiota transplantation protects rotenone-induced Parkinson’s disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome 2021, 9, 226. [Google Scholar] [CrossRef]
- Chen, C.; Fang, S.; Wei, H.; He, M.; Fu, H.; Xiong, X.; Zhou, Y.; Wu, J.; Gao, J.; Yang, H.; et al. Prevotella copri increases fat accumulation in pigs fed with formula diets. Microbiome 2021, 9, 175. [Google Scholar] [CrossRef]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Liu, X.; Xu, S.; Hu, S.; Wang, S.; Shi, D.; Wang, K.; Wang, Z.; Lin, Q.; Li, S.; et al. Prevotella histicola Mitigated Estrogen Deficiency-Induced Depression via Gut Microbiota-Dependent Modulation of Inflammation in Ovariectomized Mice. Front. Nutr. 2021, 8, 805465. [Google Scholar] [CrossRef] [PubMed]
- Poh, L.; Sim, W.L.; Jo, D.G.; Dinh, Q.N.; Drummond, G.R.; Sobey, C.G.; Chen, C.L.; Lai, M.K.P.; Fann, D.Y.; Arumugam, T.V. The role of inflammasomes in vascular cognitive impairment. Mol. Neurodegener. 2022, 17, 4. [Google Scholar] [CrossRef]
- Lee, J.; d’Aigle, J.; Atadja, L.; Quaicoe, V.; Honarpisheh, P.; Ganesh, B.P.; Hassan, A.; Graf, J.; Petrosino, J.; Putluri, N.; et al. Gut Microbiota-Derived Short-Chain Fatty Acids Promote Poststroke Recovery in Aged Mice. Circ. Res. 2020, 127, 453–465. [Google Scholar] [CrossRef]
- Jena, P.K.; Setayesh, T.; Sheng, L.; Di Lucente, J.; Jin, L.W.; Wan, Y.Y. Intestinal Microbiota Remodeling Protects Mice from Western Diet-Induced Brain Inflammation and Cognitive Decline. Cells 2022, 11, 504. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Luckey, D.; Bodhke, R.; Chen, J.; Marietta, E.; Jeraldo, P.; Murray, J.; Taneja, V. Prevotella histicola Protects From Arthritis by Expansion of Allobaculum and Augmenting Butyrate Production in Humanized Mice. Front. Immunol. 2021, 12, 609644. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The pathobiology of vascular dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef]
- Sharma, G.; Garg, N.; Hasan, S.; Shirodkar, S. Prevotella: An insight into its characteristics and associated virulence factors. Microb. Pathog. 2022, 169, 105673. [Google Scholar]
- Xie, A.; Ensink, E.; Li, P.; Gordevičius, J.; Marshall, L.L.; George, S.; Pospisilik, J.A.; Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; et al. Bacterial Butyrate in Parkinson’s Disease Is Linked to Epigenetic Changes and Depressive Symptoms. Mov. Disord. Off. J. Mov. Disord. Soc. 2022, 37, 1644–1653. [Google Scholar] [CrossRef]
- Shen, L.; Liu, L.; Ji, H.F. Alzheimer’s Disease Histological and Behavioral Manifestations in Transgenic Mice Correlate with Specific Gut Microbiome State. J. Alzheimer’s Dis. 2017, 56, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Raz, L.; Knoefel, J.; Bhaskar, K. The neuropathology and cerebrovascular mechanisms of dementia. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2016, 36, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Barone, E.; Di Domenico, F.; Perluigi, M.; Butterfield, D.A. The interplay among oxidative stress, brain insulin resistance and AMPK dysfunction contribute to neurodegeneration in type 2 diabetes and Alzheimer disease. Free. Radic. Biol. Med. 2021, 176, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, L.; Popescu-Olaru, I.; Cozma, L.; Tulbă, D.; Hinescu, M.E.; Ceafalan, L.C.; Gherghiceanu, M.; Popescu, B.O. Oxidative Stress and the Microbiota-Gut-Brain Axis. Oxidative Med. Cell. Longev. 2018, 2018, 2406594. [Google Scholar] [CrossRef]
- Merelli, A.; Repetto, M.; Lazarowski, A.; Auzmendi, J. Hypoxia, Oxidative Stress, and Inflammation: Three Faces of Neurodegenerative Diseases. J. Alzheimer’s Dis. 2021, 82, S109–S126. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.A.; Cole, M.P. Oxidative and nitrative stress in neurodegeneration. Neurobiol. Dis. 2015, 84, 4–21. [Google Scholar] [CrossRef]
- Hernández, J.A.; López-Sánchez, R.C.; Rendón-Ramírez, A. Lipids and Oxidative Stress Associated with Ethanol-Induced Neurological Damage. Oxidative Med. Cell. Longev. 2016, 2016, 1543809. [Google Scholar] [CrossRef]
- Yuan, T.F.; Gu, S.; Shan, C.; Marchado, S.; Arias-Carrión, O. Oxidative Stress and Adult Neurogenesis. Stem Cell Rev. Rep. 2015, 11, 706–709. [Google Scholar] [CrossRef]
- Du, S.Q.; Wang, X.R.; Zhu, W.; Ye, Y.; Yang, J.W.; Ma, S.M.; Ji, C.S.; Liu, C.Z. Acupuncture inhibits TXNIP-associated oxidative stress and inflammation to attenuate cognitive impairment in vascular dementia rats. CNS Neurosci. Ther. 2018, 24, 39–46. [Google Scholar] [CrossRef]
- Wang, X.R.; Shi, G.X.; Yang, J.W.; Yan, C.Q.; Lin, L.T.; Du, S.Q.; Zhu, W.; He, T.; Zeng, X.H.; Xu, Q.; et al. Acupuncture ameliorates cognitive impairment and hippocampus neuronal loss in experimental vascular dementia through Nrf2-mediated antioxidant response. Free. Radic. Biol. Med. 2015, 89, 1077–1084. [Google Scholar] [CrossRef]
- Custodero, C.; Ciavarella, A.; Panza, F.; Gnocchi, D.; Lenato, G.M.; Lee, J.; Mazzocca, A.; Sabbà, C.; Solfrizzi, V. Role of inflammatory markers in the diagnosis of vascular contributions to cognitive impairment and dementia: A systematic review and meta-analysis. GeroScience 2022, 44, 1373–1392. [Google Scholar] [PubMed]
- Karve, I.P.; Taylor, J.M.; Crack, P.J. The contribution of astrocytes and microglia to traumatic brain injury. Br. J. Pharmacol. 2016, 173, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xu, Z.; Wang, L.; Zhang, L.; Liu, Y.; Cao, J.; Fu, Q.; Liu, Y.; Li, H.; Lou, J.; et al. Cottonseed oil alleviates ischemic stroke injury by inhibiting the inflammatory activation of microglia and astrocyte. J. Neuroinflammation 2020, 17, 270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Pan, J.; Mamtilahun, M.; Zhu, Y.; Wang, L.; Venkatesh, A.; Shi, R.; Tu, X.; Jin, K.; Wang, Y.; et al. Microglia exacerbate white matter injury via complement C3/C3aR pathway after hypoperfusion. Theranostics 2020, 10, 74–90. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, R.; Hou, J.; Wang, X.; Huang, Z.; Cao, H.; Hu, J.; Peng, Q.; Duan, H.; Wang, Q.; Chen, X. Prevotella histicola Transplantation Ameliorates Cognitive Impairment and Decreases Oxidative Stress in Vascular Dementia Rats. Brain Sci. 2023, 13, 1136. https://doi.org/10.3390/brainsci13081136
Duan R, Hou J, Wang X, Huang Z, Cao H, Hu J, Peng Q, Duan H, Wang Q, Chen X. Prevotella histicola Transplantation Ameliorates Cognitive Impairment and Decreases Oxidative Stress in Vascular Dementia Rats. Brain Sciences. 2023; 13(8):1136. https://doi.org/10.3390/brainsci13081136
Chicago/Turabian StyleDuan, Rui, Jiankang Hou, Xixi Wang, Zhihang Huang, Haiming Cao, Junya Hu, Qiang Peng, Huijie Duan, Qingguang Wang, and Xiangliang Chen. 2023. "Prevotella histicola Transplantation Ameliorates Cognitive Impairment and Decreases Oxidative Stress in Vascular Dementia Rats" Brain Sciences 13, no. 8: 1136. https://doi.org/10.3390/brainsci13081136
APA StyleDuan, R., Hou, J., Wang, X., Huang, Z., Cao, H., Hu, J., Peng, Q., Duan, H., Wang, Q., & Chen, X. (2023). Prevotella histicola Transplantation Ameliorates Cognitive Impairment and Decreases Oxidative Stress in Vascular Dementia Rats. Brain Sciences, 13(8), 1136. https://doi.org/10.3390/brainsci13081136






