Improving Visual Working Memory with Cholinergic Deep Brain Stimulation
Abstract
1. Introduction
2. Cholinergic System
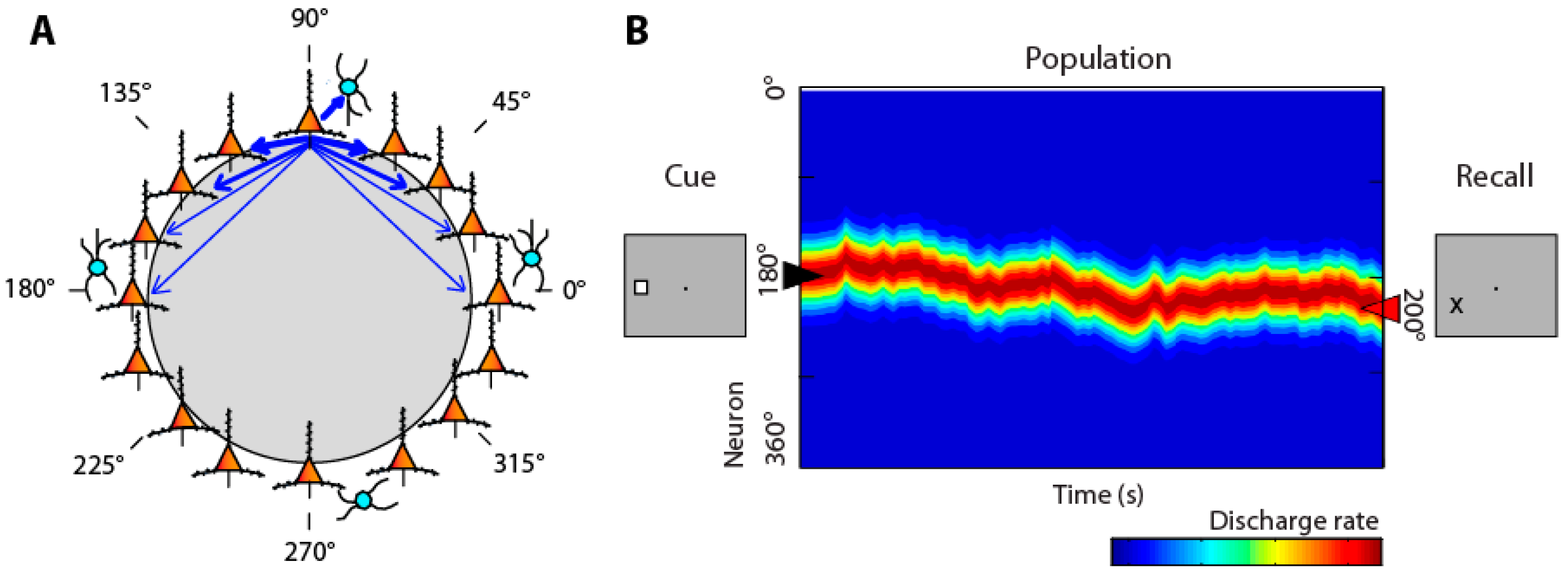
3. Neural Activity Underlying Visual Working Memory
4. Action of Acetylcholine on the Visual Cortex
5. Effects of Acetylcholine on the Prefrontal Cortex
6. Deep Brain Stimulation
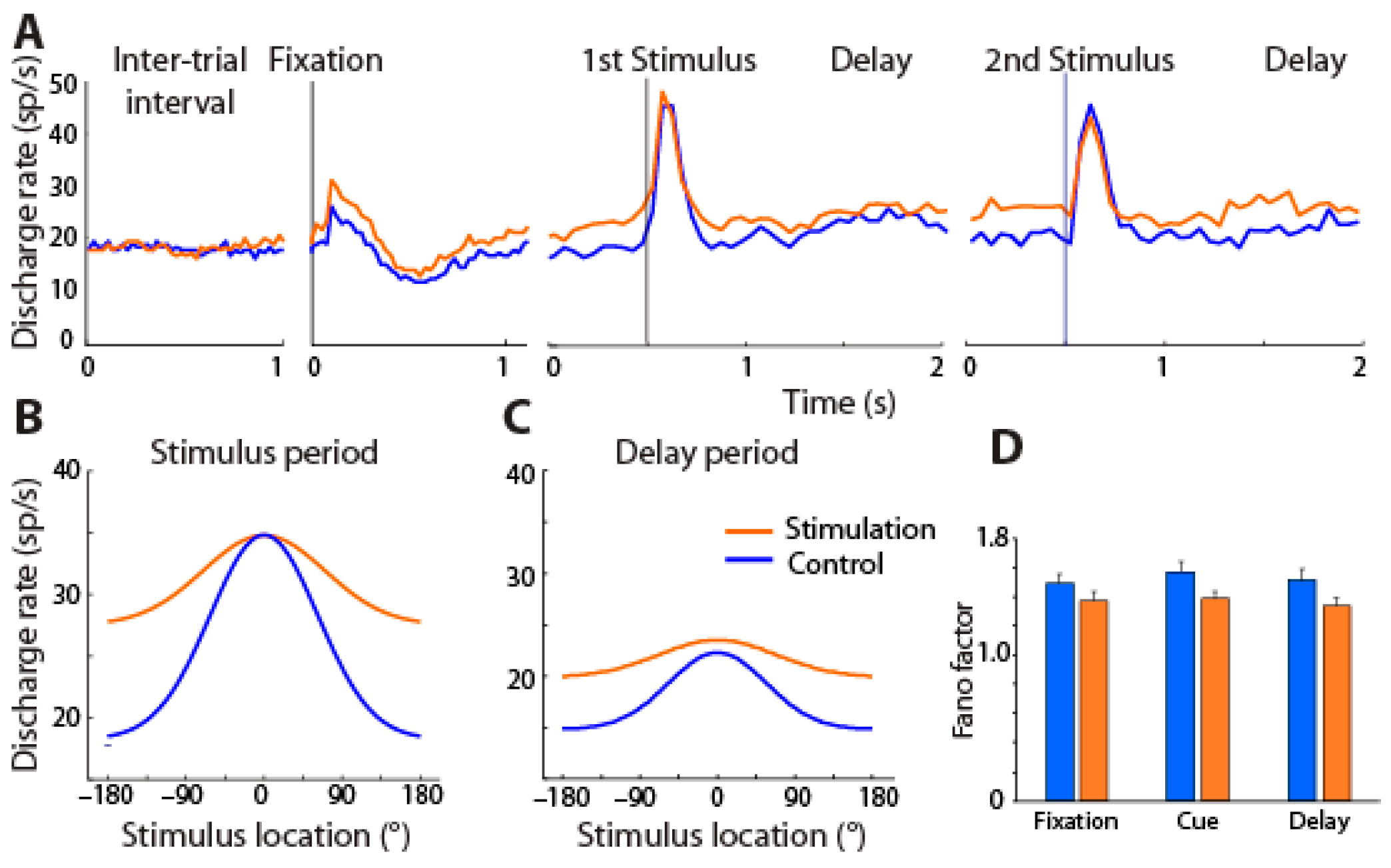
7. Cholinergic Deep Brain Stimulation in Visual Working Memory
8. Potential for Human Intervention
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baddeley, A. Working memory: Theories, models, and controversies. Annu. Rev. Psychol. 2012, 63, 1–29. [Google Scholar] [CrossRef]
- Constantinidis, C.; Klingberg, T. The neuroscience of working memory capacity and training. Nat. Rev. Neurosci. 2016, 17, 438–449. [Google Scholar] [CrossRef]
- Subramaniam, K.; Luks, T.L.; Fisher, M.; Simpson, G.V.; Nagarajan, S.; Vinogradov, S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron 2012, 73, 842–853. [Google Scholar] [CrossRef]
- Westerberg, H.; Jacobaeus, H.; Hirvikoski, T.; Clevberger, P.; Ostensson, M.L.; Bartfai, A.; Klingberg, T. Computerized working memory training after stroke—A pilot study. Brain Inj. 2007, 21, 21–29. [Google Scholar] [CrossRef]
- Hasselmo, M.E.; Sarter, M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology 2011, 36, 52–73. [Google Scholar] [CrossRef] [PubMed]
- Rountree, S.D.; Chan, W.; Pavlik, V.N.; Darby, E.J.; Siddiqui, S.; Doody, R.S. Persistent treatment with cholinesterase inhibitors and/or memantine slows clinical progression of Alzheimer disease. Alzheimers Res. Ther. 2009, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, E.; Pietrini, P.; Schapiro, M.B.; Rapoport, S.I.; Furey, M.L. Cholinergic modulation of visual working memory during aging: A parametric PET study. Brain Res. Bull. 2009, 79, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Blake, D.T.; Constantinidis, C. Cholinergic Deep Brain Stimulation for Memory and Cognitive Disorders. J. Alzheimers Dis. 2021, 83, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Cools, R.; Arnsten, A.F.T. Neuromodulation of prefrontal cortex cognitive function in primates: The powerful roles of monoamines and acetylcholine. Neuropsychopharmacology 2022, 47, 309–328. [Google Scholar] [CrossRef]
- Wang, M.; Datta, D.; Enwright, J.; Galvin, V.; Yang, S.T.; Paspalas, C.; Kozak, R.; Gray, D.L.; Lewis, D.A.; Arnsten, A.F.T. A novel dopamine D1 receptor agonist excites delay-dependent working memory-related neuronal firing in primate dorsolateral prefrontal cortex. Neuropharmacology 2019, 150, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Vijayraghavan, S.; Wang, M.; Birnbaum, S.G.; Williams, G.V.; Arnsten, A.F. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 2007, 10, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Galvin, V.C.; Arnsten, A.F.T.; Wang, M. Evolution in Neuromodulation—The Differential Roles of Acetylcholine in Higher Order Association vs. Primary Visual Cortices. Front. Neural Circuits 2018, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Hendry, S.H.; Jones, E.G.; Killackey, H.P.; Chalupa, L.M. Choline acetyltransferase-immunoreactive neurons in fetal monkey cerebral cortex. Brain Res. 1987, 465, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Mesulam, M.M.; Mufson, E.J.; Levey, A.I.; Wainer, B.H. Atlas of cholinergic neurons in the forebrain and upper brainstem of the macaque based on monoclonal choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry. Neuroscience 1984, 12, 669–686. [Google Scholar] [CrossRef]
- Saper, C.B.; Scammell, T.E.; Lu, J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005, 437, 1257–1263. [Google Scholar] [CrossRef]
- Ananth, M.R.; Rajebhosale, P.; Kim, R.; Talmage, D.A.; Role, L.W. Basal forebrain cholinergic signalling: Development, connectivity and roles in cognition. Nat. Rev. Neurosci. 2023, 24, 233–251. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T.; Price, D.L.; DeLong, M.R. Alzheimer’s disease: A disorder of cortical cholinergic innervation. Science 1983, 219, 1184–1190. [Google Scholar] [CrossRef]
- Terry, A.V., Jr.; Buccafusco, J.J. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 2003, 306, 821–827. [Google Scholar] [CrossRef]
- Sarter, M.; Bruno, J.P. Cognitive functions of cortical acetylcholine: Toward a unifying hypothesis. Brain Res. Rev. 1997, 23, 28–46. [Google Scholar] [CrossRef]
- Sarter, M.; Parikh, V. Choline transporters, cholinergic transmission and cognition. Nat. Rev. Neurosci. 2005, 6, 48–56. [Google Scholar] [CrossRef]
- Bentley, P.; Driver, J.; Dolan, R.J. Cholinergic modulation of cognition: Insights from human pharmacological functional neuroimaging. Prog. Neurobiol. 2011, 94, 360–388. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, Y.; Galvin, V.C.; Yang, S.; Arnsten, A.F.; Wang, M. Nicotinic α4β2 Cholinergic Receptor Influences on Dorsolateral Prefrontal Cortical Neuronal Firing during a Working Memory Task. J. Neurosci. 2017, 37, 5366–5377. [Google Scholar] [CrossRef] [PubMed]
- Gratton, C.; Yousef, S.; Aarts, E.; Wallace, D.L.; D’Esposito, M.; Silver, M.A. Cholinergic, But Not Dopaminergic or Noradrenergic, Enhancement Sharpens Visual Spatial Perception in Humans. J. Neurosci. 2017, 37, 4405–4415. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, E.C.; Ananth, M.; Talmage, D.A.; Role, L.W. Basal Forebrain Cholinergic Circuits and Signaling in Cognition and Cognitive Decline. Neuron 2016, 91, 1199–1218. [Google Scholar] [CrossRef] [PubMed]
- Blatt, J.; Vellage, A.; Baier, B.; Muller, N.G. The contribution of acetylcholine and dopamine to subprocesses of visual working memor—What patients with amnestic mild cognitive impairment and Parkinson’s disease can tell us. Neuropsychologia 2014, 61, 89–95. [Google Scholar] [CrossRef]
- Aigner, T.G.; Mitchell, S.J.; Aggleton, J.P.; DeLong, M.R.; Struble, R.G.; Price, D.L.; Wenk, G.L.; Pettigrew, K.D.; Mishkin, M. Transient impairment of recognition memory following ibotenic-acid lesions of the basal forebrain in macaques. Exp. Brain Res. 1991, 86, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Ridley, R.M.; Bowes, P.M.; Baker, H.F.; Crow, T.J. An involvement of acetylcholine in object discrimination learning and memory in the marmoset. Neuropsychologia 1984, 22, 253–263. [Google Scholar] [CrossRef]
- Voytko, M.L.; Olton, D.S.; Richardson, R.T.; Gorman, L.K.; Tobin, J.R.; Price, D.L. Basal forebrain lesions in monkeys disrupt attention but not learning and memory. J. Neurosci. 1994, 14, 167–186. [Google Scholar] [CrossRef]
- Croxson, P.L.; Kyriazis, D.A.; Baxter, M.G. Cholinergic modulation of a specific memory function of prefrontal cortex. Nat. Neurosci. 2011, 14, 1510–1512. [Google Scholar] [CrossRef]
- Deutsch, J.A. The cholinergic synapse and the site of memory. Science 1971, 174, 788–794. [Google Scholar] [CrossRef]
- Drachman, D.A. Memory and cognitive function in man: Does the cholinergic system have a specific role? Neurology 1977, 27, 783–790. [Google Scholar] [CrossRef]
- Everitt, B.J.; Robbins, T.W. Central cholinergic systems and cognition. Annu. Rev. Psychol. 1997, 48, 649–684. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Johnson, H.R. Short-term memory in the rhesus monkey: Disruption from the anti-cholinergic scopolamine. Pharmacol. Biochem. Behav. 1976, 5, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Buccafusco, J.J.; Terry, A.V., Jr.; Webster, S.J.; Martin, D.; Hohnadel, E.J.; Bouchard, K.A.; Warner, S.E. The scopolamine-reversal paradigm in rats and monkeys: The importance of computer-assisted operant-conditioning memory tasks for screening drug candidates. Psychopharmacology 2007, 199, 481–494. [Google Scholar] [CrossRef]
- Mesulam, M. The cholinergic lesion of Alzheimer’s disease: Pivotal factor or side show? Learn. Mem. 2004, 11, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T. On neurodegenerative diseases, models, and treatment strategies: Lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp. Neurol. 2000, 163, 495–529. [Google Scholar] [CrossRef]
- Rogers, S.L.; Farlow, M.R.; Doody, R.S.; Mohs, R.; Friedhoff, L.T. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology 1998, 50, 136–145. [Google Scholar] [CrossRef]
- Courtney, C.; Farrell, D.; Gray, R.; Hills, R.; Lynch, L.; Sellwood, E.; Edwards, S.; Hardyman, W.; Raftery, J.; Crome, P.; et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): Randomised double-blind trial. Lancet 2004, 363, 2105–2115. [Google Scholar]
- Birks, J.S.; Harvey, R. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 2018, 6, CD001190. [Google Scholar] [CrossRef] [PubMed]
- Doody, R.S.; Geldmacher, D.S.; Gordon, B.; Perdomo, C.A.; Pratt, R.D. Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer’s disease. Arch. Neurol. 2001, 58, 427–433. [Google Scholar] [CrossRef]
- Winblad, B.; Engedal, K.; Soininen, H.; Verhey, F.; Waldemar, G.; Wimo, A.; Wetterholm, A.L.; Zhang, R.; Haglund, A.; Subbiah, P. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 2001, 57, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Buccafusco, J.J.; Terry, A.V. Donepezil-induced improvement in delayed matching accuracy by young and old rhesus monkeys. J. Mol. Neurosci. 2004, 24, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Farlow, M.R.; Salloway, S.; Tariot, P.N.; Yardley, J.; Moline, M.L.; Wang, Q.; Brand-Schieber, E.; Zou, H.; Hsu, T.; Satlin, A. Effectiveness and tolerability of high-dose (23 mg/d) versus standard-dose (10 mg/d) donepezil in moderate to severe Alzheimer’s disease: A 24-week, randomized, double-blind study. Clin. Ther. 2010, 32, 1234–1251. [Google Scholar] [CrossRef]
- Yoshida, T.; Ha-Kawa, S.; Yoshimura, M.; Nobuhara, K.; Kinoshita, T.; Sawada, S. Effectiveness of treatment with donepezil hydrochloride and changes in regional cerebral blood flow in patients with Alzheimer’s disease. Ann. Nucl. Med. 2007, 21, 257–265. [Google Scholar] [CrossRef]
- Riley, M.R.; Constantinidis, C. Role of prefrontal persistent activity in working memory. Front. Syst. Neurosci. 2016, 9, 181. [Google Scholar] [CrossRef]
- Lundqvist, M.; Herman, P.; Miller, E.K. Working Memory: Delay Activity, Yes! Persistent Activity? Maybe Not. J. Neurosci. 2018, 38, 7013–7019. [Google Scholar] [CrossRef]
- Olesen, P.J.; Westerberg, H.; Klingberg, T. Increased prefrontal and parietal activity after training of working memory. Nat. Neurosci. 2004, 7, 75–79. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, C.; Wang, S.; Xue, G. The Neural Mechanism Underlying Visual Working Memory Training and Its Limited Transfer Effect. J. Cogn. Neurosci. 2022, 34, 2082–2099. [Google Scholar] [CrossRef]
- Bentley, P.; Driver, J.; Dolan, R.J. Cholinesterase inhibition modulates visual and attentional brain responses in Alzheimer’s disease and health. Brain 2008, 131, 409–424. [Google Scholar] [CrossRef]
- Funahashi, S.; Bruce, C.J.; Goldman-Rakic, P.S. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J. Neurophysiol. 1989, 61, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Qi, X.L.; Stanford, T.R.; Constantinidis, C. Stimulus selectivity in dorsal and ventral prefrontal cortex after training in working memory tasks. J. Neurosci. 2011, 31, 6266–6276. [Google Scholar] [CrossRef] [PubMed]
- Hart, E.; Huk, A.C. Recurrent circuit dynamics underlie persistent activity in the macaque frontoparietal network. eLife 2020, 9, e52460. [Google Scholar] [CrossRef] [PubMed]
- Compte, A.; Brunel, N.; Goldman-Rakic, P.S.; Wang, X.J. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb. Cortex 2000, 10, 910–923. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, Y.H.; Constantinidis, C.; Zhou, X. Neural Mechanisms of Working Memory Accuracy Revealed by Recurrent Neural Networks. Front. Syst. Neurosci. 2022, 16. [Google Scholar]
- Seung, H.S.; Lee, D.D.; Reis, B.Y.; Tank, D.W. Stability of the memory of eye position in a recurrent network of conductance-based model neurons. Neuron 2000, 26, 259–271. [Google Scholar] [CrossRef]
- Wimmer, K.; Nykamp, D.Q.; Constantinidis, C.; Compte, A. Bump attractor dynamics in prefrontal cortex explains behavioral precision in spatial working memory. Nat. Neurosci. 2014, 17, 431–439. [Google Scholar] [CrossRef]
- Jaffe, R.J.; Constantinidis, C. Working Memory: From Neural Activity to the Sentient Mind. Compr. Physiol. 2021, 11, 2547–2587. [Google Scholar] [CrossRef]
- Disney, A.A. Neuromodulatory Control of Early Visual Processing in Macaque. Annu. Rev. Vis. Sci. 2021, 7, 181–199. [Google Scholar] [CrossRef]
- Disney, A.A.; Aoki, C.; Hawken, M.J. Gain modulation by nicotine in macaque v1. Neuron 2007, 56, 701–713. [Google Scholar] [CrossRef]
- Herrero, J.L.; Roberts, M.J.; Delicato, L.S.; Gieselmann, M.A.; Dayan, P.; Thiele, A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature 2008, 454, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Veith, V.K.; Quigley, C.; Treue, S. Cholinergic manipulations affect sensory responses but not attentional enhancement in macaque MT. BMC Biol. 2021, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.J.; Dayan, P. Uncertainty, neuromodulation, and attention. Neuron 2005, 46, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.; Goard, M.J.; Estandian, D.; Xu, M.; Kwan, A.C.; Lee, S.H.; Harrison, T.C.; Feng, G.; Dan, Y. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat. Neurosci. 2013, 16, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- Disney, A.A.; Robert, J.S. Translational implications of the anatomical nonequivalence of functionally equivalent cholinergic circuit motifs. Proc. Natl. Acad. Sci. USA 2019, 116, 26181–26186. [Google Scholar] [CrossRef]
- Thiele, A.; Bellgrove, M.A. Neuromodulation of Attention. Neuron 2018, 97, 769–785. [Google Scholar] [CrossRef]
- Yang, Y.; Paspalas, C.D.; Jin, L.E.; Picciotto, M.R.; Arnsten, A.F.; Wang, M. Nicotinic α7 receptors enhance NMDA cognitive circuits in dorsolateral prefrontal cortex. Proc. Natl. Acad. Sci. USA 2013, 110, 12078–12083. [Google Scholar] [CrossRef]
- Dasilva, M.; Brandt, C.; Gotthardt, S.; Gieselmann, M.A.; Distler, C.; Thiele, A. Cell class-specific modulation of attentional signals by acetylcholine in macaque frontal eye field. Proc. Natl. Acad. Sci. USA 2019, 116, 20180–20189. [Google Scholar] [CrossRef]
- Zhou, X.; Qi, X.L.; Douglas, K.; Palaninathan, K.; Kang, H.S.; Buccafusco, J.J.; Blake, D.T.; Constantinidis, C. Cholinergic modulation of working memory activity in primate prefrontal cortex. J. Neurophysiol. 2011, 106, 2180–2188. [Google Scholar] [CrossRef]
- Major, A.J.; Vijayraghavan, S.; Everling, S. Muscarinic Attenuation of Mnemonic Rule Representation in Macaque Dorsolateral Prefrontal Cortex during a Pro- and Anti-Saccade Task. J. Neurosci. 2015, 35, 16064–16076. [Google Scholar] [CrossRef]
- Galvin, V.C.; Yang, S.T.; Paspalas, C.D.; Yang, Y.; Jin, L.E.; Datta, D.; Morozov, Y.M.; Lightbourne, T.C.; Lowet, A.S.; Rakic, P.; et al. Muscarinic M1 Receptors Modulate Working Memory Performance and Activity via KCNQ Potassium Channels in the Primate Prefrontal Cortex. Neuron 2020, 106, 649–661.e4. [Google Scholar] [CrossRef]
- Major, A.J.; Vijayraghavan, S.; Everling, S. Cholinergic Overstimulation Attenuates Rule Selectivity in Macaque Prefrontal Cortex. J. Neurosci. 2018, 38, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Vijayraghavan, S.; Major, A.J.; Everling, S. Muscarinic M1 Receptor Overstimulation Disrupts Working Memory Activity for Rules in Primate Prefrontal Cortex. Neuron 2018, 98, 1256–1268.e1254. [Google Scholar] [CrossRef] [PubMed]
- Galvin, V.C.; Arnsten, A.F.T.; Wang, M. Involvement of Nicotinic Receptors in Working Memory Function. Curr. Top. Behav. Neurosci. 2020, 45, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.V.; Goldman-Rakic, P.S. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 1995, 376, 572–575. [Google Scholar] [CrossRef]
- Freund, H.J.; Kuhn, J.; Lenartz, D.; Mai, J.K.; Schnell, T.; Klosterkoetter, J.; Sturm, V. Cognitive functions in a patient with Parkinson-dementia syndrome undergoing deep brain stimulation. Arch. Neurol. 2009, 66, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Saper, C.B.; Fuller, P.M. Wake-sleep circuitry: An overview. Curr. Opin. Neurobiol. 2017, 44, 186–192. [Google Scholar] [CrossRef]
- Walker, L.C.; Price, D.L.; Young, W.S., 3rd. GABAergic neurons in the primate basal forebrain magnocellular complex. Brain Res. 1989, 499, 188–192. [Google Scholar] [CrossRef]
- Kim, T.; Thankachan, S.; McKenna, J.T.; McNally, J.M.; Yang, C.; Choi, J.H.; Chen, L.; Kocsis, B.; Deisseroth, K.; Strecker, R.E.; et al. Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc. Natl. Acad. Sci. USA 2015, 112, 3535–3540. [Google Scholar] [CrossRef]
- Semba, K. Multiple output pathways of the basal forebrain: Organization, chemical heterogeneity, and roles in vigilance. Behav. Brain Res. 2000, 115, 117–141. [Google Scholar] [CrossRef]
- Detari, L.; Rasmusson, D.D.; Semba, K. The role of basal forebrain neurons in tonic and phasic activation of the cerebral cortex. Prog. Neurobiol. 1999, 58, 249–277. [Google Scholar] [CrossRef] [PubMed]
- Granger, A.J.; Wang, W.; Robertson, K.; El-Rifai, M.; Zanello, A.F.; Bistrong, K.; Saunders, A.; Chow, B.W.; Nunez, V.; Turrero Garcia, M.; et al. Cortical ChAT(+) neurons co-transmit acetylcholine and GABA in a target- and brain-region-specific manner. eLife 2020, 9, e57749. [Google Scholar] [CrossRef] [PubMed]
- Granger, A.J.; Mulder, N.; Saunders, A.; Sabatini, B.L. Cotransmission of acetylcholine and GABA. Neuropharmacology 2016, 100, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Vitek, J.L. Mechanisms of deep brain stimulation: Excitation or inhibition. Mov. Disord. 2002, 17, S69–S72. [Google Scholar] [CrossRef]
- Mahlknecht, P.; Limousin, P.; Foltynie, T. Deep brain stimulation for movement disorders: Update on recent discoveries and outlook on future developments. J. Neurol. 2015, 262, 2583–2595. [Google Scholar] [CrossRef]
- Wichmann, T.; DeLong, M.R. Deep Brain Stimulation for Neurologic and Neuropsychiatric Disorders. Neuron 2006, 52, 197–204. [Google Scholar] [CrossRef]
- Montemurro, N.; Aliaga, N.; Graff, P.; Escribano, A.; Lizana, J. New Targets and New Technologies in the Treatment of Parkinson’s Disease: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 8799. [Google Scholar] [CrossRef]
- Popovych, V.O.; Lysyansky, B.; Tass, P.A. Closed-loop deep brain stimulation by pulsatile delayed feedback with increased gap between pulse phases. Sci. Rep. 2017, 7, 1033. [Google Scholar] [CrossRef]
- Kent, A.R.; Grill, W.M. Recording evoked potentials during deep brain stimulation: Development and validation of instrumentation to suppress the stimulus artefact. J. Neural Eng. 2012, 9, 036004. [Google Scholar] [CrossRef]
- Stoney, S.D.; Thompson, W.D.; Asanuma, H. Excitation of pyramidal tract cells by intracortical microstimulation: Effective extent of stimulating current. J. Neurophysiol. 1968, 31, 659–669. [Google Scholar] [CrossRef]
- Murasugi, C.M.; Salzman, C.D.; Newsome, W.T. Microstimulation in visual area MT: Effects of varying pulse amplitude and frequency. J. Neurosci. 1993, 13, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.C.; Mori, S.; Sherman, D.L.; Thakor, V.N.; Vitek, J.L. Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin. Neurophysiol. 2004, 115, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Grossman, N.; Bono, D.; Dedic, N.; Kodandaramaiah, S.B.; Rudenko, A.; Suk, H.J.; Cassara, A.M.; Neufeld, E.; Kuster, N.; Tsai, L.H.; et al. Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 2017, 169, 1029–1041.e1016. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Liu, D.; Shi, L.; Meng, F.; Fang, H.; Liu, H.; Zhang, H.; Yang, A.; Zhang, J. Differential Effects of Subthalamic Nucleus and Globus Pallidus Internus Deep Brain Stimulation on Motor Subtypes in Parkinson’s Disease. World Neurosurg. 2022, 164, e245–e255. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Crawford, J.; Callahan, P.M.; Terry, V.A.; Constantinidis, C.; Blake, D.T. Intermittent Stimulation of the Nucleus Basalis of Meynert Improves Working Memory in Adult Monkeys. Curr. Biol. 2017, 27, 2640–2646. [Google Scholar] [CrossRef]
- Liu, R.; Crawford, J.; Callahan, P.M.; Terry, A.V.; Constantinidis, C.; Blake, D.T. Intermittent stimulation in the nucleus basalis of meynert improves sustained attention in rhesus monkeys. Neuropharmacology 2018, 137, 202–210. [Google Scholar] [CrossRef]
- Chung, S.; Bava, J.; Wang, Z.; Garin, C.M.; Clemencich, K.; Pennington, K.R.; Bick, S.K.; Englot, D.J.; Blake, D.T.; Constantinidis, C. Intermittent stimulation of the nucleus basalis improves working memory in aged monkeys. Soc. Neurosci. Abstr. 2022, 654, 623. [Google Scholar]
- Qi, X.L.; Liu, R.; Singh, B.; Bestue, D.; Compte, A.; Vazdarjanova, A.I.; Blake, D.T.; Constantinidis, C. Nucleus basalis stimulation enhances working memory by stabilizing stimulus representations in primate prefrontal cortical activity. Cell Rep. 2021, 36, 109469. [Google Scholar] [CrossRef]
- Li, W.; Piech, V.; Gilbert, C.D. Perceptual learning and top-down influences in primary visual cortex. Nat. Neurosci. 2004, 7, 651–657. [Google Scholar] [CrossRef]
- Yang, T.; Maunsell, J.H. The effect of perceptual learning on neuronal responses in monkey visual area V4. J. Neurosci. 2004, 24, 1617–1626. [Google Scholar] [CrossRef]
- Raiguel, S.; Vogels, R.; Mysore, S.G.; Orban, G.A. Learning to see the difference specifically alters the most informative V4 neurons. J. Neurosci. 2006, 26, 6589–6602. [Google Scholar] [CrossRef]
- Sanayei, M.; Chen, X.; Chicharro, D.; Distler, C.; Panzeri, S.; Thiele, A. Perceptual learning of fine contrast discrimination changes neuronal tuning and population coding in macaque V4. Nat. Commun. 2018, 9, 4238. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.J.; Beck, J.M.; Latham, P.E.; Pouget, A. Bayesian inference with probabilistic population codes. Nat. Neurosci. 2006, 9, 1432–1438. [Google Scholar] [CrossRef]
- Zhang, K.; Sejnowski, T.J. Neuronal tuning: To sharpen or broaden? Neural Comput. 1999, 11, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Butts, D.A.; Goldman, M.S. Tuning curves, neuronal variability, and sensory coding. PLoS Biol. 2006, 4, e92. [Google Scholar] [CrossRef]
- Pouget, A.; Deneve, S.; Ducom, J.C.; Latham, P.E. Narrow versus wide tuning curves: What’s best for a population code? Neural Comput. 1999, 11, 85–90. [Google Scholar] [CrossRef]
- Stein, H.; Barbosa, J.; Compte, A. Towards biologically constrained attractor models of schizophrenia. Curr. Opin. Neurobiol. 2021, 70, 171–181. [Google Scholar] [CrossRef]
- Qi, X.L.; Meyer, T.; Stanford, T.R.; Constantinidis, C. Changes in Prefrontal Neuronal Activity after Learning to Perform a Spatial Working Memory Task. Cereb. Cortex 2011, 21, 2722–2732. [Google Scholar] [CrossRef]
- Qi, X.L.; Constantinidis, C. Neural changes after training to perform cognitive tasks. Behav. Brain Res. 2013, 241, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Thiele, A.; Herrero, J.L.; Distler, C.; Hoffmann, K.P. Contribution of cholinergic and GABAergic mechanisms to direction tuning, discriminability, response reliability, and neuronal rate correlations in macaque middle temporal area. J. Neurosci. 2012, 32, 16602–16615. [Google Scholar] [CrossRef]
- Minces, V.; Pinto, L.; Dan, Y.; Chiba, A.A. Cholinergic shaping of neural correlations. Proc. Natl. Acad. Sci. USA 2017, 114, 5725–5730. [Google Scholar] [CrossRef]
- Singh, B.; Qi, X.L.; Blake, D.T.; Constantinidis, C. Rhythmicity of Prefrontal Local Field Potentials after Nucleus Basalis Stimulation. eNeuro 2022, 9, ENEURO.0380-21.2022. [Google Scholar] [CrossRef]
- Kuhn, J.; Hardenacke, K.; Lenartz, D.; Gruendler, T.; Ullsperger, M.; Bartsch, C.; Mai, J.K.; Zilles, K.; Bauer, A.; Matusch, A.; et al. Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer’s dementia. Mol. Psychiatry 2015, 20, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Gratwicke, J.; Zrinzo, L.; Kahan, J.; Peters, A.; Beigi, M.; Akram, H.; Hyam, J.; Oswal, A.; Day, B.; Mancini, L.; et al. Bilateral Deep Brain Stimulation of the Nucleus Basalis of Meynert for Parkinson Disease Dementia: A Randomized Clinical Trial. JAMA Neurol. 2017, 75, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, S.; Cohn, M.; Harmsen, I.E.; Loh, A.; Cho, S.S.; Saenz-Farret, M.; Maciel, R.; Soh, D.; Boutet, A.; Germann, J.; et al. Single-Trajectory Multiple-Target Deep Brain Stimulation for Parkinsonian Mobility and Cognition. Mov. Disord. 2022, 37, 635–640. [Google Scholar] [CrossRef]
- Nombela, C.; Lozano, A.; Villanueva, C.; Barcia, J.A. Simultaneous Stimulation of the Globus Pallidus Interna and the Nucleus Basalis of Meynert in the Parkinson-Dementia Syndrome. Dement. Geriatr. Cogn. Disord. 2019, 47, 19–28. [Google Scholar] [CrossRef]
- Maltete, D.; Wallon, D.; Bourilhon, J.; Lefaucheur, R.; Danaila, T.; Thobois, S.; Defebvre, L.; Dujardin, K.; Houeto, J.L.; Godefroy, O.; et al. Nucleus Basalis of Meynert Stimulation for Lewy Body Dementia: A Phase I Randomized Clinical Trial. Neurology 2021, 96, e684–e697. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, T.S.; Chen, Y.C.; Guo, P.; Lian, T.H.; Liu, Y.Y.; Liu, W.; Bai, Y.T.; Zhang, Q.; Zhang, W.; et al. Deep brain stimulation of the nucleus basalis of Meynert modulates hippocampal-frontoparietal networks in patients with advanced Alzheimer’s disease. Transl. Neurodegener. 2022, 11, 51. [Google Scholar] [CrossRef]
- Bogdan, I.D.; Oterdoom, D.L.M.; van Laar, T.; Huitema, R.B.; Odekerken, V.J.; Boel, J.A.; de Bie, R.M.A.; van Dijk, J.M.C.; on behalf of the NSTAPS Study Group. Serendipitous Stimulation of Nucleus Basalis of Meynert—The Effect of Unintentional, Long-Term High-Frequency Stimulation on Cognition in Parkinson’s Disease. J. Clin. Med. 2022, 11, 337. [Google Scholar] [CrossRef]
- Gratwicke, J.; Oswal, A.; Akram, H.; Jahanshahi, M.; Hariz, M.; Zrinzo, L.; Foltynie, T.; Litvak, V. Resting state activity and connectivity of the nucleus basalis of Meynert and globus pallidus in Lewy body dementia and Parkinson’s disease dementia. NeuroImage 2020, 221, 117184. [Google Scholar] [CrossRef]


| Study First Author | Year of Publication | Type of Patients | Target | Number of Patients |
|---|---|---|---|---|
| Freund et al. [76] | 2009 | Parkinson’s–dementia | STN and NB | 1 |
| Kuhn et al. [113] | 2015 | Alzheimer’s | NB | 6 |
| Gratwicke et al. [114] | 2017 | Parkinson’s | NB | 6 |
| Nombela et al. [116] | 2019 | Parkinson’s–dementia | GPi and NB | 1 |
| Gratwicke et al. [120] | 2020 | Lewy body dementia | NB | 6 |
| Maltête et al. [117] | 2021 | Lewy body dementia | NB | 6 |
| Sasikumar et al. [115] | 2022 | Parkinson’s | GPi and NB | 6 |
| Jiang et al. [118] | 2022 | Alzheimer’s | NB | 8 |
| Bogdan et al. [119] | 2022 | Parkinson’s | GPi and NB | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bava, J.M.; Wang, Z.; Bick, S.K.; Englot, D.J.; Constantinidis, C. Improving Visual Working Memory with Cholinergic Deep Brain Stimulation. Brain Sci. 2023, 13, 917. https://doi.org/10.3390/brainsci13060917
Bava JM, Wang Z, Bick SK, Englot DJ, Constantinidis C. Improving Visual Working Memory with Cholinergic Deep Brain Stimulation. Brain Sciences. 2023; 13(6):917. https://doi.org/10.3390/brainsci13060917
Chicago/Turabian StyleBava, Janki M., Zhengyang Wang, Sarah K. Bick, Dario J. Englot, and Christos Constantinidis. 2023. "Improving Visual Working Memory with Cholinergic Deep Brain Stimulation" Brain Sciences 13, no. 6: 917. https://doi.org/10.3390/brainsci13060917
APA StyleBava, J. M., Wang, Z., Bick, S. K., Englot, D. J., & Constantinidis, C. (2023). Improving Visual Working Memory with Cholinergic Deep Brain Stimulation. Brain Sciences, 13(6), 917. https://doi.org/10.3390/brainsci13060917





