Staged Strategies to Deal with Complex, Giant, Multi-Fossa Skull Base Tumors
Abstract
1. Introduction
2. Results
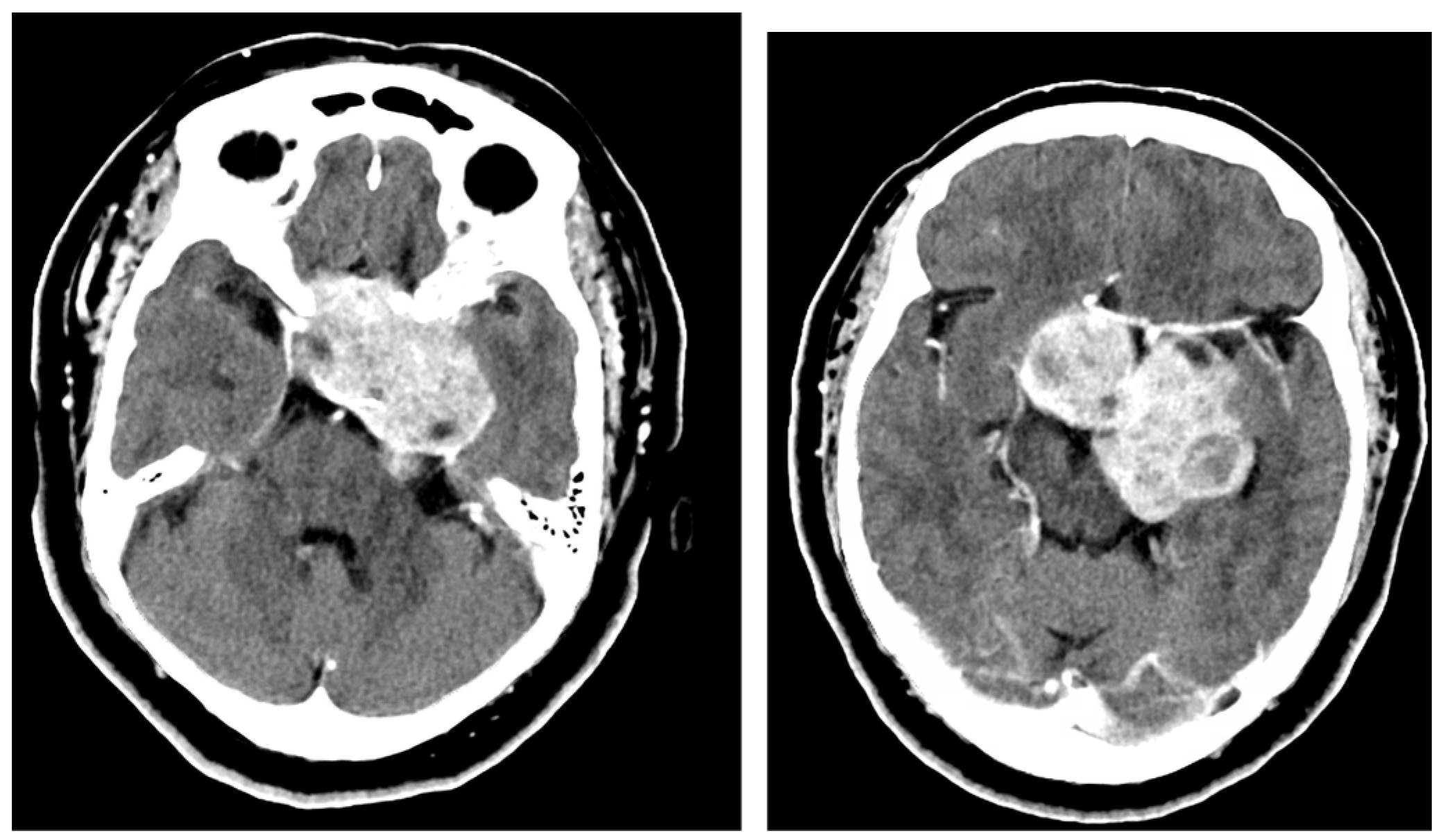
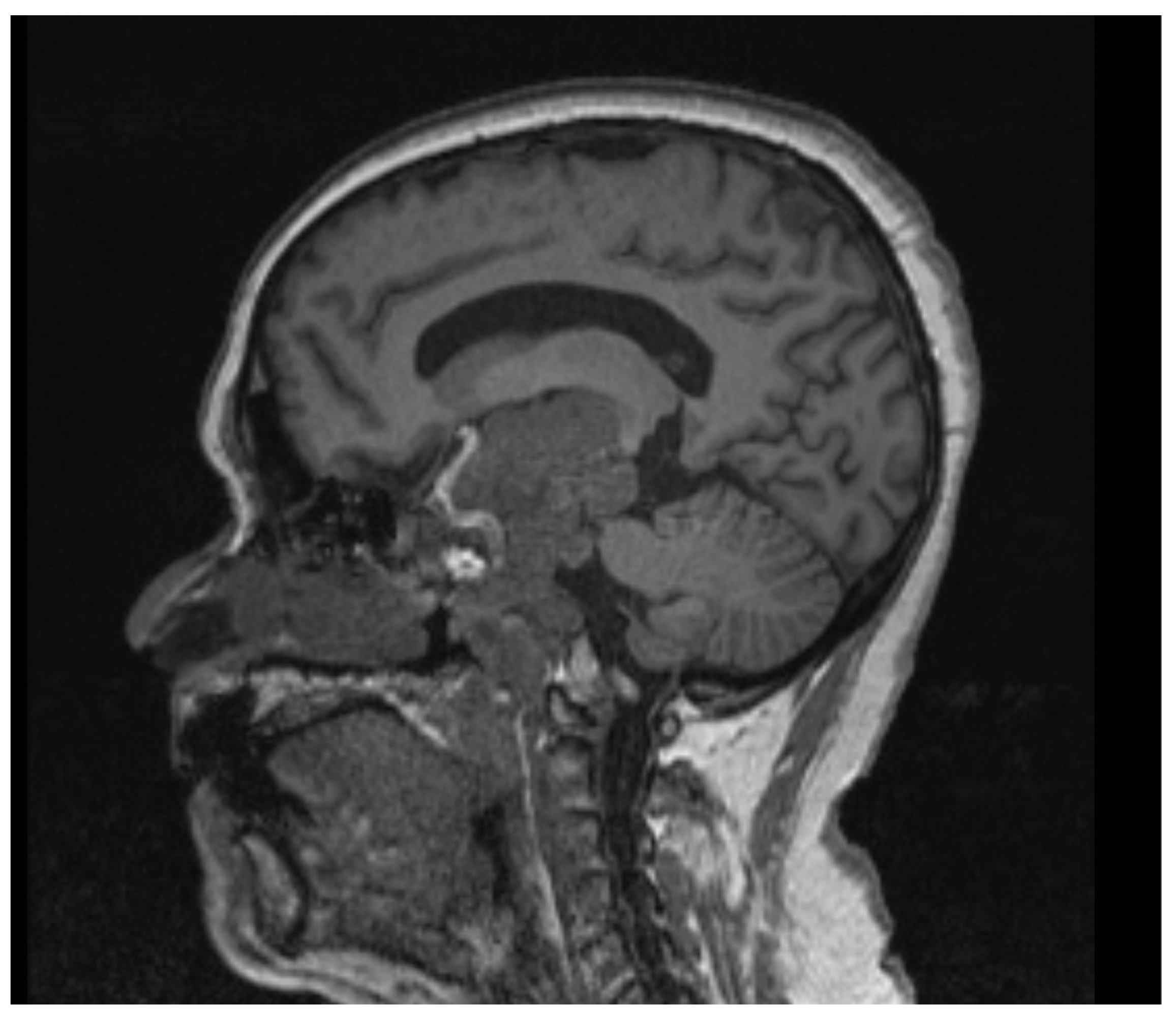
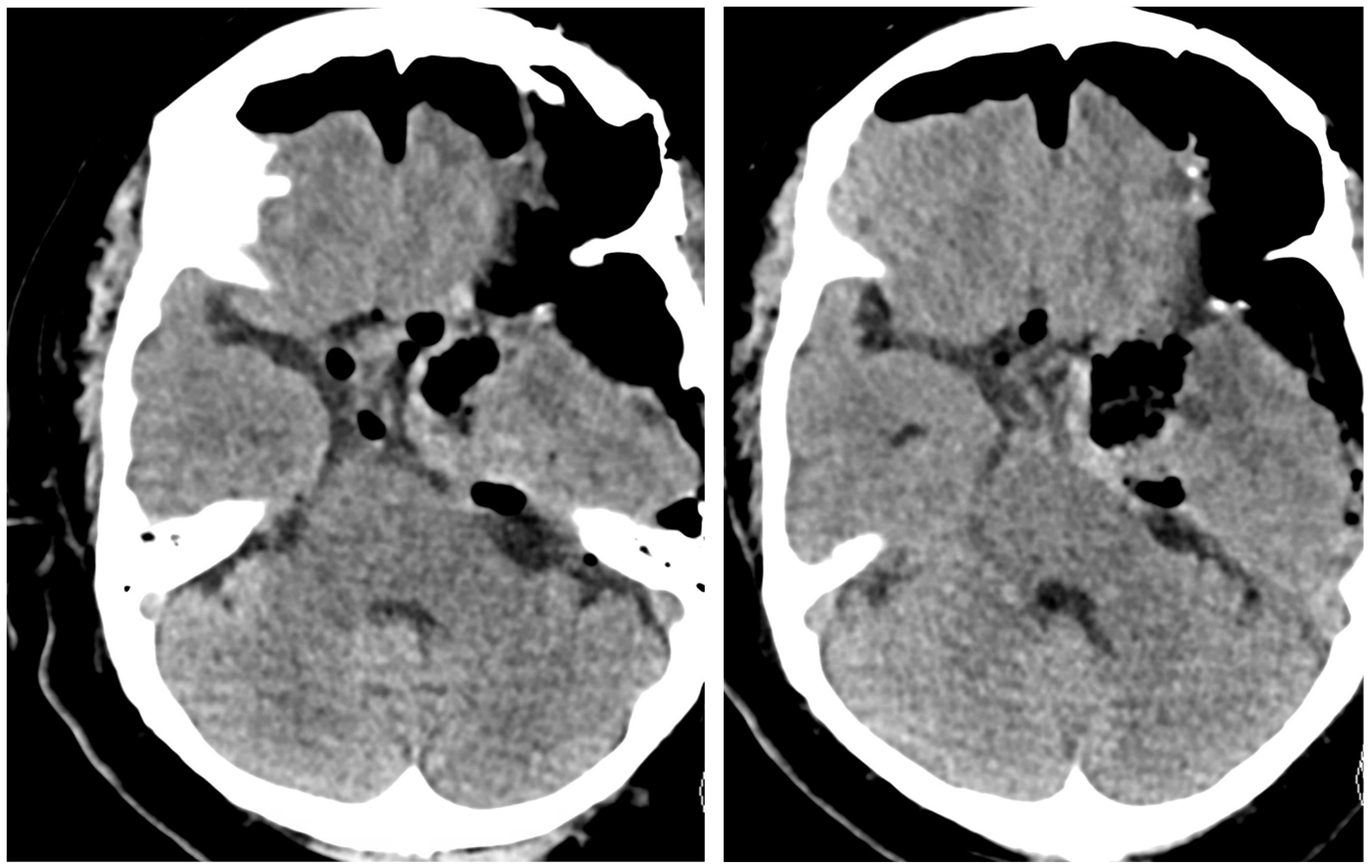
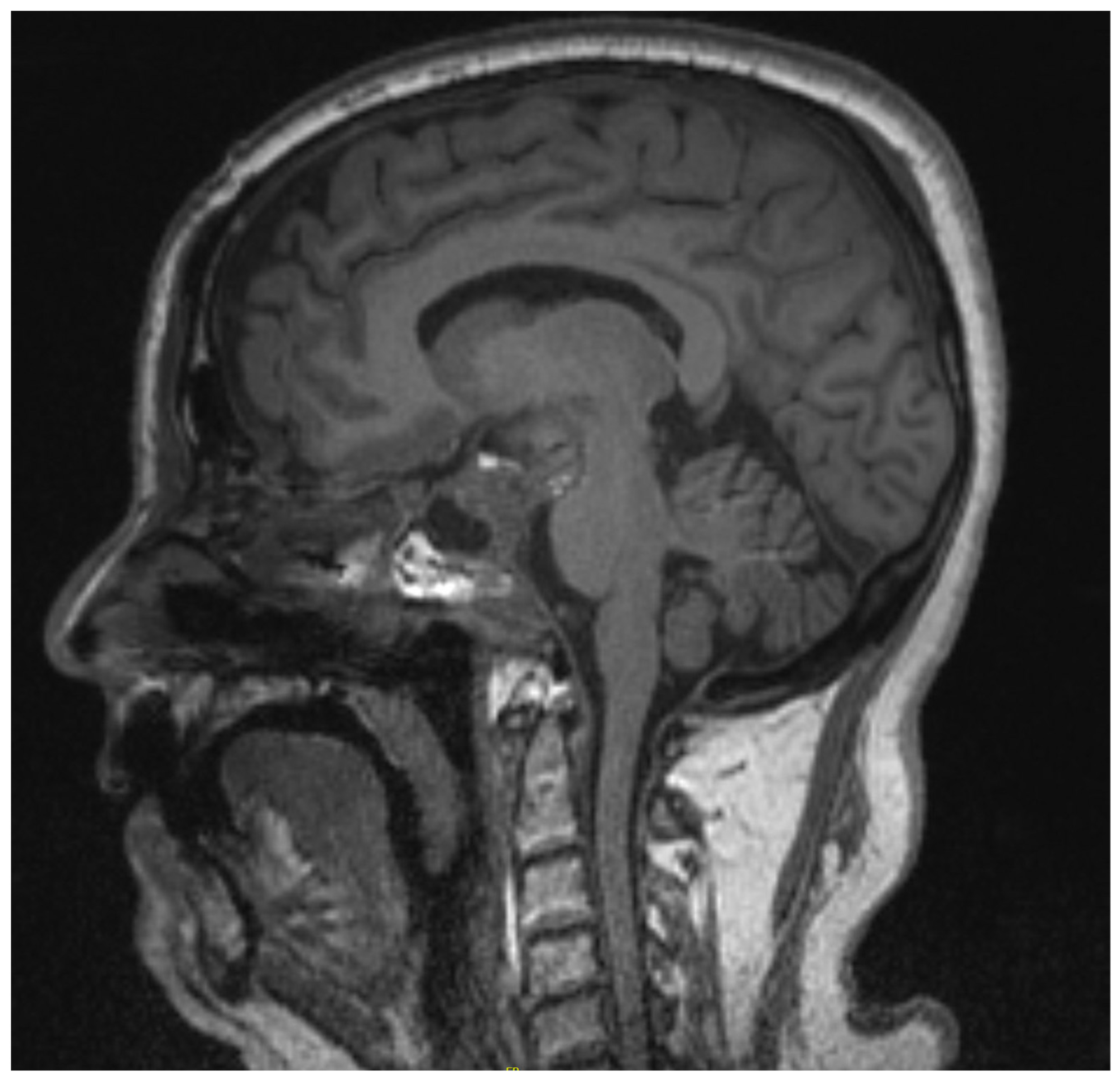
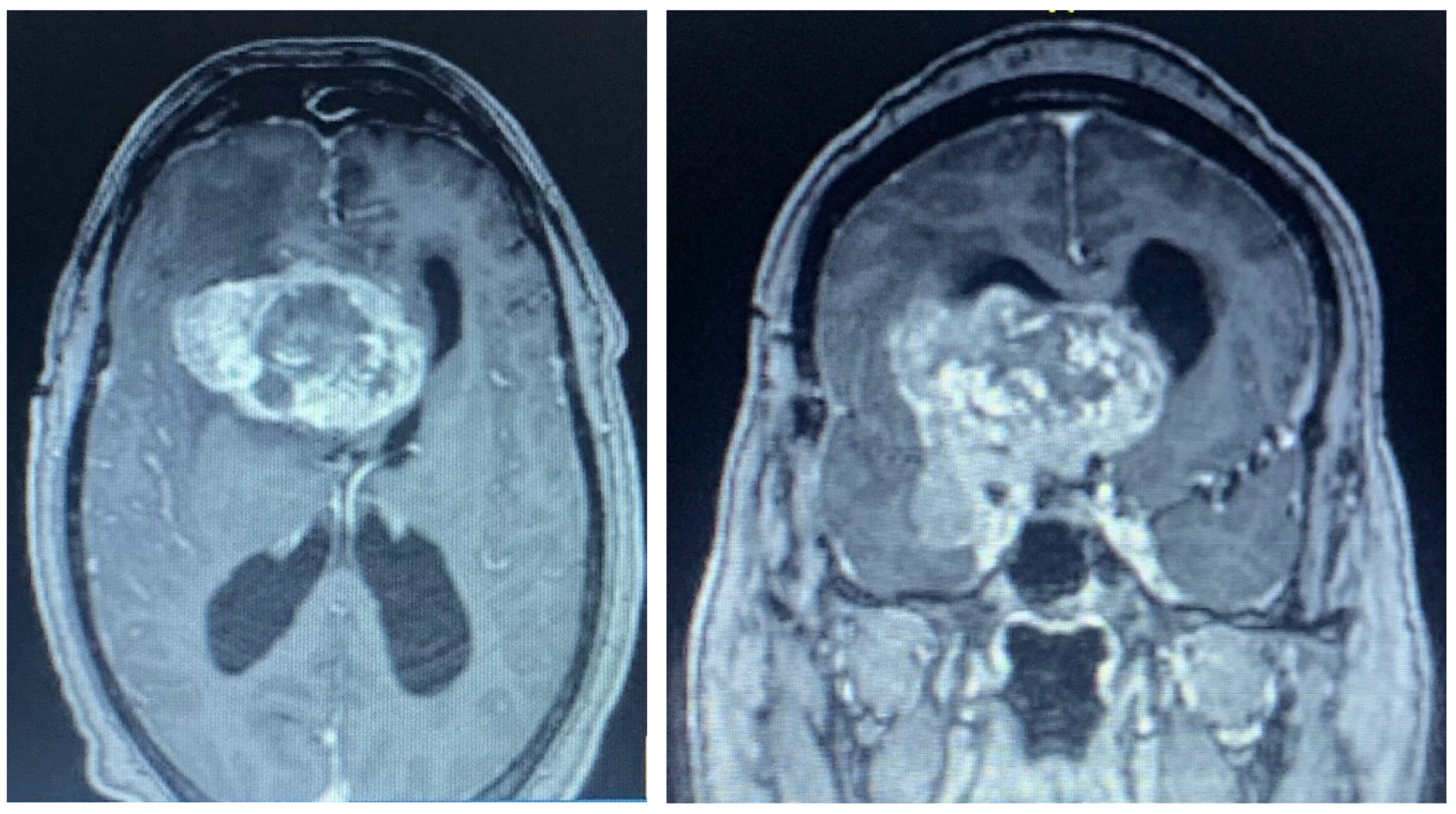
2.1. Case 1
2.2. Case 2
3. Discussion
3.1. Giant Pituitary Macroadenoma Surgery
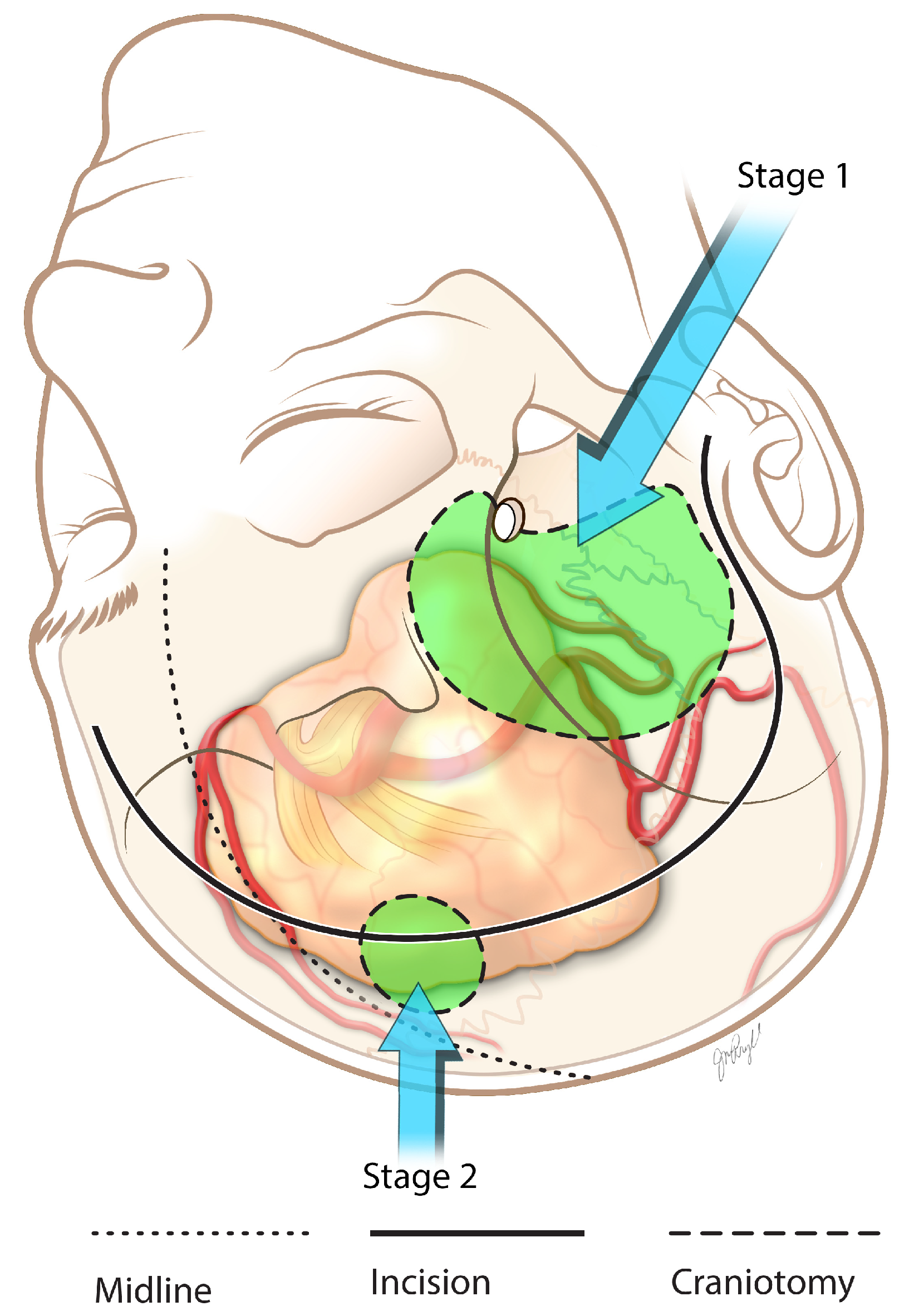
3.2. Surgical Pearls
3.3. Giant Anterior Clinoid Meningioma Surgery
3.4. Surgical Pearls
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seçkin, H.; Avci, E.; Uluç, K.; Niemann, D.; Başkaya, M.K. The work horse of skull base surgery: Orbitozygomatic approach. Technique, modifications, and applications. Neurosurg. Focus 2008, 25, E4. [Google Scholar] [CrossRef] [PubMed]
- Rubio, R.R.; Chae, R.; Vigo, V.; Abla, A.A.; McDermott, M. Immersive Surgical Anatomy of the Pterional Approach. Cureus 2019, 11, e5216. [Google Scholar] [CrossRef]
- Meybodi, A.T.; Mignucci-Jiménez, G.; Lawton, M.T.; Liu, J.K.; Preul, M.C.; Sun, H. Comprehensive microsurgical anatomy of the middle cranial fossa: Part I—Osseous and meningeal anatomy. Front. Surg. 2023, 10, 1132774. [Google Scholar] [CrossRef] [PubMed]
- Solari, D.; Villa, A.; De Angelis, M.; Esposito, F.; Cavallo, L.M.; Cappabianca, P. Anatomy and surgery of the en-doscopic endonasal approach to the skull base. Transl. Med.@ UniSa 2012, 2, 36–46. [Google Scholar]
- Goel, A.; Nadkarni, T.; Muzumdar, D.; Desai, K.; Phalke, U.; Sharma, P. Giant pituitary tumors: A study based on surgical treatment of 118 cases. Surg. Neurol. 2004, 61, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E. Diagnosis and Treatment of Pituitary Adenomas: A Review. JAMA 2017, 317, 516–524. [Google Scholar] [CrossRef]
- Meling, T.R.; Da Broi, M.; Scheie, D.; Helseth, E. Meningiomas: Skull base versus non-skull base. Neurosurg. Rev. 2018, 42, 163–173. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, W.; Hou, Y.; Wen, C.; Wang, J.; Wu, P.; Guo, Z. An Overview of Managements in Meningiomas. Front. Oncol. 2020, 10, 1523. [Google Scholar] [CrossRef]
- Asa, S.L.; Mete, O.; Perry, A.; Osamura, R.Y. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2022, 33, 6–26. [Google Scholar] [CrossRef]
- Serioli, S.; Doglietto, F.; Fiorindi, A.; Biroli, A.; Mattavelli, D.; Buffoli, B.; Ferrari, M.; Cornali, C.; Rodella, L.; Maroldi, R.; et al. Pituitary Adenomas and Invasiveness from Anatomo-Surgical, Radiological, and Histological Perspectives: A Systematic Literature Review. Cancers 2019, 11, 1936. [Google Scholar] [CrossRef]
- Raghib, M.F.; Salim, A.; Angez, M.; Ghazi, S.M.; Hashmi, S.; Tariq, M.B.; Hashmi, F.; Bin Anis, S.; Shamim, M.S.; Tanwir, A.; et al. Prognostic implication of size on outcomes of pituitary macroadenoma: A comparative analysis of giant adenoma with non-giant macroadenoma. J. Neuro-Oncol. 2022, 160, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Cincu, R.; Goel, A. Current concepts and controversies in the management of non-functioning giant pituitary macroadenomas. Clin. Neurol. Neurosurg. 2007, 109, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Koutourousiou, M.; Gardner, P.A.; Fernandez-Miranda, J.C.; Paluzzi, A.; Wang, E.W.; Snyderman, C.H. Endoscopic endonasal surgery for giant pituitary adenomas: Advantages and limitations. J. Neurosurg. 2013, 118, 621–631. [Google Scholar] [CrossRef]
- Butterfield, J.T.; Araki, T.; Guillaume, D.; Tummala, R.; Caicedo-Granados, E.; Tyler, M.A.; Venteicher, A.S. Estimating Risk of Pituitary Apoplexy after Resection of Giant Pituitary Adenomas. J. Neurol. Surg. Part B Skull Base 2021, 83 (Suppl. S2), e152–e159. [Google Scholar] [CrossRef] [PubMed]
- Couldwell, W.T. Transsphenoidal and transcranial surgery for pituitary adenomas. J. Neuro-Oncol. 2004, 69, 237–256. [Google Scholar] [CrossRef]
- Thakur, B.; Jesurasa, A.R.; Ross, R.; Carroll, T.A.; Mirza, S.; Sinha, S. Transnasal trans-sphenoidal endoscopic repair of CSF leak sec-ondary to invasive pituitary tumours using a nasoseptal flap. Pituitary 2011, 14, 163–167. [Google Scholar] [CrossRef]
- Shah, R.N.; Surowitz, J.B.; Patel, M.R.; Huang, B.Y.; Snyderman, C.H.; Carrau, R.L.; Kassam, A.B.; Germanwala, A.V.; Zanation, A.M. Endoscopic pedicled nasoseptal flap recon-struction for pediatric skull base defects. Laryngoscope 2009, 119, 1067–1075. [Google Scholar] [CrossRef]
- Cavallo, L.M.; Prevedello, D.M.; Solari, D.; Gardner, P.A.; Esposito, F.; Snyderman, C.H.; Carrau, R.L.; Kassam, A.B.; Cappabianca, P. Extended endoscopic endonasal transsphenoidal approach for residual or recurrent craniopharyngiomas. J. Neurosurg. 2009, 111, 578–589. [Google Scholar] [CrossRef]
- Zhang, X.; Fei, Z.; Zhang, J.N.; Fu, L.A.; Cao, W.D.; Qu, Y. Minimally invasive neurosurgery for removal of pituitary adenomas by neuroendoscope aided with sellar floor reconstruction. Zhonghua Yi Xue Za Zhi 2010, 90, 2342–2344. [Google Scholar]
- Torp, S.H.; Solheim, O.; Skjulsvik, A.J. The WHO 2021 Classification of Central Nervous System tumours: A practical update on what neurosurgeons need to know—A minireview. Acta Neurochir. 2022, 164, 2453–2464. [Google Scholar] [CrossRef]
- Huntoon, K.; Toland, A.M.S.; Dahiya, S. Meningioma: A Review of Clinicopathological and Molecular Aspects. Front. Oncol. 2020, 10, 579599. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Greenwald, N.F.; Abedalthagafi, M.; Wala, J.; Gibson, W.J.; Agarwalla, P.K.; Horowitz, P.; Schumacher, S.E.; Esaulova, E.; Mei, Y.; et al. Genomic landscape of high-grade meningiomas. NPJ Genom. Med. 2017, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.T.; Young, J.S.; Chae, R.; Aghi, M.K.; Theodosopoulos, P.V.; McDermott, M.W. Relationship between tumor location, size, and WHO grade in meningioma. Neurosurg. Focus 2018, 44, E4. [Google Scholar] [CrossRef] [PubMed]
- Christine, M.; Marco, H.; Karl, R.; Michele, R.; Milena, S.; Elena, M.; Charles, V. Meningioma. Crit. Rev. Oncol. Hematol. 2008, 67, 153–171. [Google Scholar] [CrossRef]
- Attia, M.; Umansky, F.; Paldor, I.; Dotan, S.; Shoshan, Y.; Spektor, S. Giant anterior clinoidal meningiomas: Surgical technique and outcomes. J. Neurosurg. 2012, 117, 654–665. [Google Scholar] [CrossRef]
- Al-Mefty, O. Clinoidal meningiomas. J. Neurosurg. 1990, 73, 840–849. [Google Scholar] [CrossRef]
- De Jesús, O.; Toledo, M.M. Surgical management of meningioma en plaque of the sphenoid ridge. Surg. Neurol. 2001, 55, 265–269. [Google Scholar] [CrossRef]
- Tomasello, F.; De Divitiis, O.; Angileri, F.; Salpietro, F.M.; D’Avella, D. Large sphenocavernous meningiomas: Is there still a role for the intradural approach via the pterional-transsylvian route? Acta Neurochir. 2003, 145, 273–282. [Google Scholar] [CrossRef]
- van Loveren, H.R.; Keller, J.T.; El-Kalliny, M.; Scodary, D.J.; Tew, J.M., Jr. The Dolenc technique for cavernous sinus exploration (cadaveric prosection). J. Neurosurg. 1991, 74, 837–844. [Google Scholar] [CrossRef]
- Jankowski, R.; Auque, J.; Simon, C.; Marchai, J.C.; Hepner, H.; Wayoff, M. Endoscopic Pituitary Tumor Surgery. Laryngoscope 1992, 102, 198–202. [Google Scholar] [CrossRef]
- Liu, J.K.; Das, K.; Weiss, M.H.; Laws, E.R., Jr.; Couldwell, W.T. The history and evolution of transsphenoidal surgery. J. Neurosurg. 2001, 95, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, N.; Eisenberg, A.A.; Cohan, P.; Chaloner, C.B.; Kelly, D.F. Value of endoscopy for maximizing tumor removal in endonasal transsphenoidal pituitary adenoma surgery. J. Neurosurg. 2013, 118, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Juraschka, K.; Khan, O.H.; Godoy, B.L.; Monsalves, E.; Kilian, A.; Krischek, B.; Ghare, A.; Vescan, A.; Gentili, F.; Zadeh, G. Endoscopic endonasal transsphenoidal approach to large and giant pituitary adenomas: Institutional experience and predictors of extent of resection. J. Neurosurg. 2014, 121, 75–83. [Google Scholar] [CrossRef]
- Elshazly, K.; Kshettry, V.R.; Farrell, C.J.; Nyquist, G.; Rosen, M.; Evans, J.J. Clinical Outcomes After Endoscopic Endonasal Resection of Giant Pituitary Adenomas. World Neurosurg. 2018, 114, e447–e456. [Google Scholar] [CrossRef]
- Rahimli, T.; Hidayetov, T.; Yusifli, Z.; Memmedzade, H.; Rajabov, T.; Aghayev, K. Endoscopic Endonasal Approach to Giant Pituitary Adenomas: Surgical Outcomes and Review of the Literature. World Neurosurg. 2021, 149, e1043–e1055. [Google Scholar] [CrossRef] [PubMed]
- Chibbaro, S.; Signorelli, F.; Milani, D.; Cebula, H.; Scibilia, A.; Bozzi, M.T.; Messina, R.; Zaed, I.; Todeschi, J.; Ollivier, I.; et al. Primary Endoscopic Endonasal Management of Giant Pituitary Adenomas: Outcome and Pitfalls from a Large Prospective Multicenter Experience. Cancers 2021, 13, 3603. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Lucifero, A.G.; Rabski, J.; Kadri, P.A.S.; Al-Mefty, O. The Party Wall: Redefining the Indications of Transcranial Approaches for Giant Pituitary Adenomas in Endoscopic Era. Cancers 2023, 15, 2235. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Wang, Y.; Yin, Y.-H.; Fei, Z.-M.; Luo, Q.-Z.; Jiang, J.-Y. Surgical management of anterior clinoidal meningiomas: A 26-case report. Surg. Neurol. 2007, 68 (Suppl. S2), S6–S10. [Google Scholar] [CrossRef]
- Sampirisi, L.; D’angelo, L.; Palmieri, M.; Pesce, A.; Santoro, A. Extradural Clinoidectomy in Clinoidal Meningiomas: Analysis of the Surgical Technique and Evaluation of the Clinical Outcome. Tomography 2022, 8, 2360–2368. [Google Scholar] [CrossRef]
- Czernicki, T.; Kunert, P.; Nowak, A.; Marchel, A. Results of surgical treatment of anterior clinoidal meningiomas—Our experiences. Neurol. i Neurochir. Polska 2015, 49, 29–35. [Google Scholar] [CrossRef]
- Nanda, A.; Konar, S.K.; Maiti, T.K.; Bir, S.C.; Guthikonda, B. Stratification of predictive factors to assess resectability and surgical outcome in clinoidal meningioma. Clin. Neurol. Neurosurg. 2016, 142, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Starnoni, D.; Tuleasca, C.; Giammattei, L.; Cossu, G.; Bruneau, M.; Berhouma, M.; Cornelius, J.F.; Cavallo, L.; Froelich, S.; Jouanneau, E.; et al. Surgical management of anterior clinoidal meningiomas: Consensus statement on behalf of the EANS skull base section. Acta Neurochir. 2021, 163, 3387–3400. [Google Scholar] [CrossRef]
- Bonnal, J.; Thibaut, A.; Brotchi, J.; Born, J. Invading meningiomas of the sphenoid ridge. J. Neurosurg. 1980, 53, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, A.; Przybylowski, C.; Chen, C.-J.; Ding, D.; Foreman, P.M.; Buell, T.J.; Taylor, D.G.; Kalani, M.Y.; Park, M.S. Preoperative embolization of skull base meningiomas: A systematic review. J. Clin. Neurosci. 2018, 59, 259–264. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeun, S.S.; Evans, J.; Kosmorsky, G. Surgical management of clinoidal meningiomas. Neurosurgery 2001, 48, 1012–1019. [Google Scholar] [PubMed]
- Linsler, S.; Fischer, G.; Skliarenko, V.; Stadie, A.; Oertel, J. Endoscopic Assisted Supraorbital Keyhole Approach or Endoscopic Endonasal Approach in Cases of Tuberculum Sellae Meningioma: Which Surgical Route Should Be Favored? World Neurosurg. 2017, 104, 601–611. [Google Scholar] [CrossRef]
- Giammattei, L.; Starnoni, D.; Levivier, M.; Messerer, M.; Daniel, R.T. Surgery for Clinoidal Meningiomas: Case Series and Meta-Analysis of Outcomes and Complications. World Neurosurg. 2019, 129, e700–e717. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edelbach, B.; Lopez-Gonzalez, M.A. Staged Strategies to Deal with Complex, Giant, Multi-Fossa Skull Base Tumors. Brain Sci. 2023, 13, 916. https://doi.org/10.3390/brainsci13060916
Edelbach B, Lopez-Gonzalez MA. Staged Strategies to Deal with Complex, Giant, Multi-Fossa Skull Base Tumors. Brain Sciences. 2023; 13(6):916. https://doi.org/10.3390/brainsci13060916
Chicago/Turabian StyleEdelbach, Brandon, and Miguel Angel Lopez-Gonzalez. 2023. "Staged Strategies to Deal with Complex, Giant, Multi-Fossa Skull Base Tumors" Brain Sciences 13, no. 6: 916. https://doi.org/10.3390/brainsci13060916
APA StyleEdelbach, B., & Lopez-Gonzalez, M. A. (2023). Staged Strategies to Deal with Complex, Giant, Multi-Fossa Skull Base Tumors. Brain Sciences, 13(6), 916. https://doi.org/10.3390/brainsci13060916




