Assessing the Effects of the Topical Application of L-Menthol on Pain-Related Somatosensory-Evoked Potentials Using Intra-Epidermal Stimulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Intraepidermal Electrical Stimulation
2.3. Agonist and Control Solutions
2.4. EEG Recording
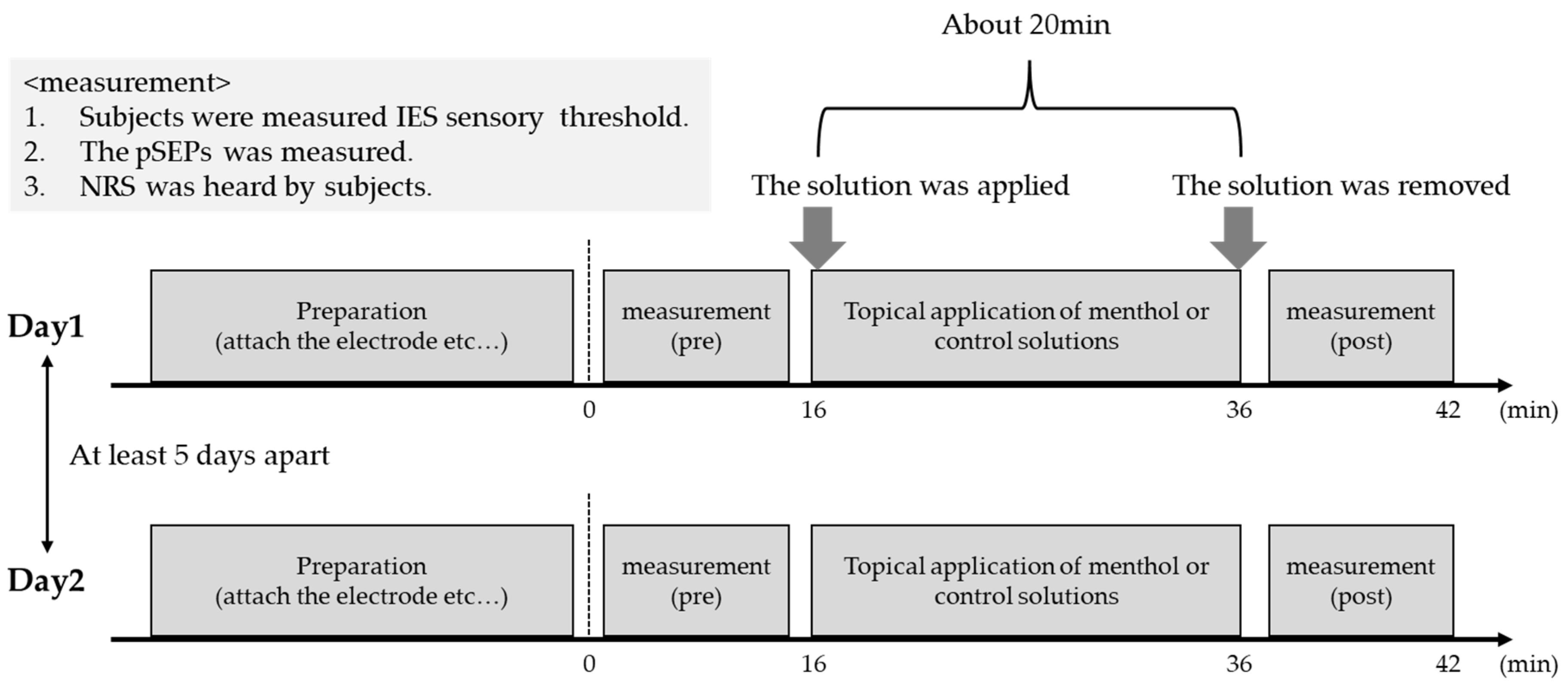
2.5. Experimental Procedures
2.6. Analyses
3. Results
3.1. Sensory Threshold
3.2. Effect of L-Menthol Application
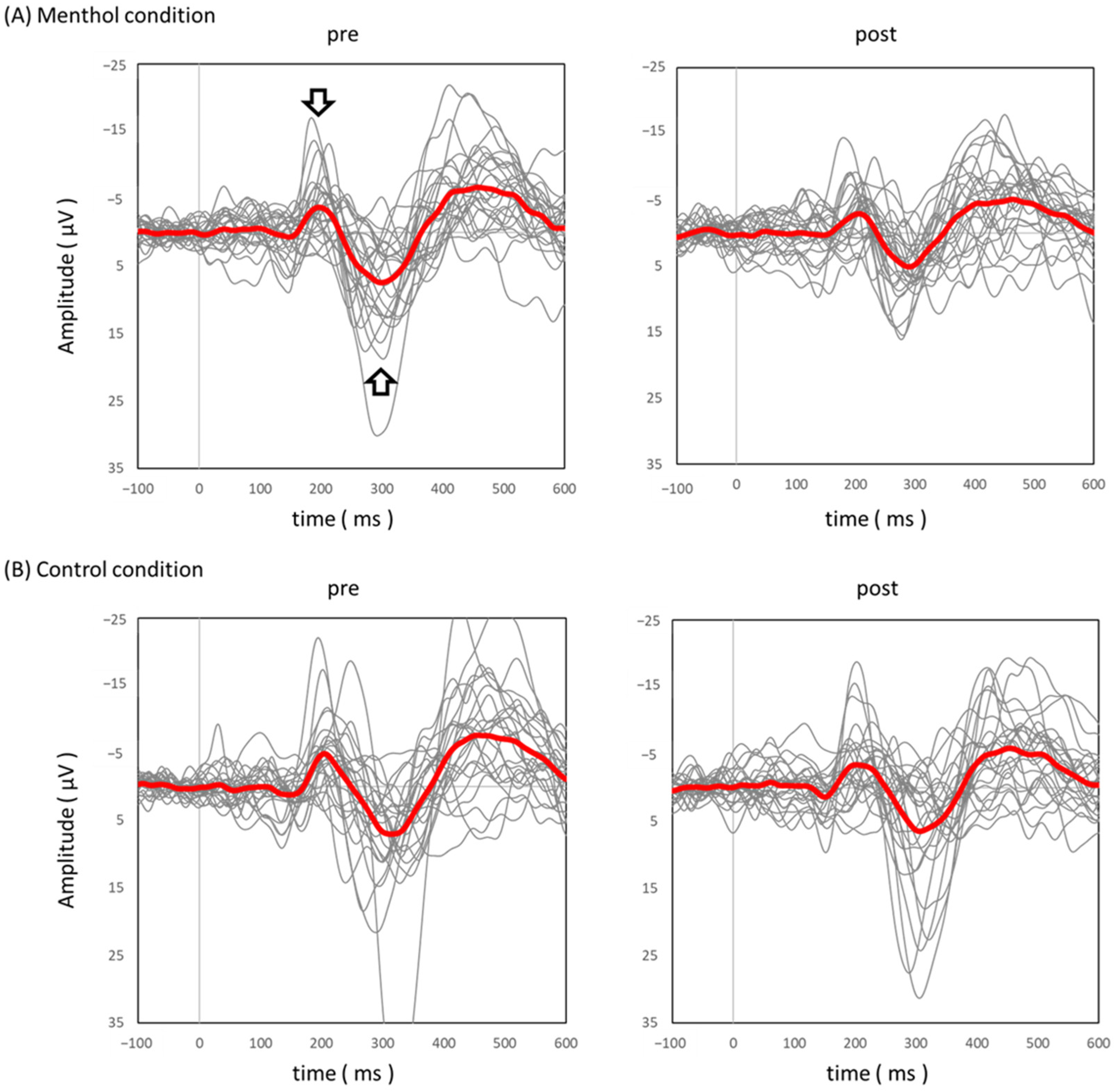
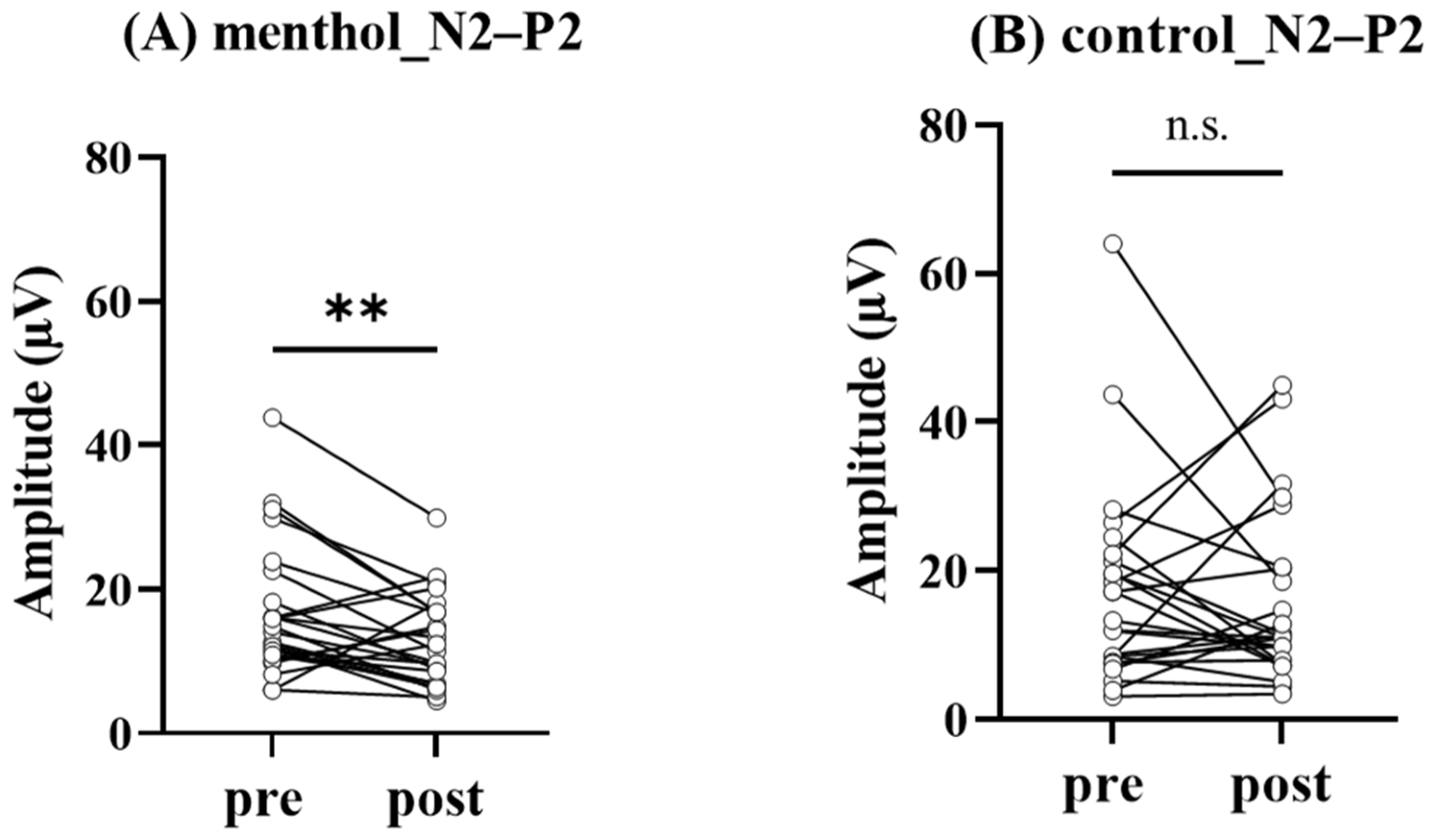
3.3. Effect of L-Menthol Application on pSEPs
4. Discussion
5. Limitation of This Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Mouraux, A.; Iannetti, G.D. The search for pain biomarkers in the human brain. Brain 2018, 141, 3290–3307. [Google Scholar] [CrossRef] [PubMed]
- Gaudioso, C.; Hao, J.; Martin-Eauclaire, M.F.; Gabriac, M.; Delmas, P. Menthol pain relief through cumulative inactivation of voltage-gated sodium channels. Pain 2012, 153, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef]
- Moran, M.M.; McAlexander, M.A.; Bíró, T.; Szallasi, A. Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.H.; Gazerani, P.; Arendt-Nielsen, L. High-Concentration L-Menthol Exhibits Counter-Irritancy to Neurogenic Inflammation, Thermal and Mechanical Hyperalgesia Caused by Trans-cinnamaldehyde. J. Pain 2016, 17, 919–929. [Google Scholar] [CrossRef]
- Liu, B.; Fan, L.; Balakrishna, S.; Sui, A.; Morris, J.B.; Jordt, S.E. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain 2013, 154, 2169–2177. [Google Scholar] [CrossRef]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP channel that senses cold stimuli and menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef]
- Courtin, A.S.; Mouraux, A. Combining Topical Agonists with the Recording of Event-Related Brain Potentials to Probe the Functional Involvement of TRPM8, TRPA1 and TRPV1 in Heat and Cold Transduction in the Human Skin. J. Pain 2022, 23, 754–771. [Google Scholar] [CrossRef]
- Dhaka, A.; Murray, A.N.; Mathur, J.; Earley, T.J.; Petrus, M.J.; Patapoutian, A. TRPM8 is required for cold sensation in mice. Neuron 2007, 54, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, C.J.; Garry, E.M.; Cottrell, D.F.; Rosie, R.; Anderson, H.; Robertson, D.C.; Fleetwood-Walker, S.M.; Mitchell, R. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr. Biol. CB 2006, 16, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, C.; Martín-Martínez, M.; Gómez-Monterrey, I.; González-Muñiz, R. TRPM8 Channels: Advances in Structural Studies and Pharmacological Modulation. Int. J. Mol. Sci. 2021, 22, 8502. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef]
- Knowlton, W.M.; Palkar, R.; Lippoldt, E.K.; McCoy, D.D.; Baluch, F.; Chen, J.; McKemy, D.D. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J. Neurosci. 2013, 33, 2837–2848. [Google Scholar] [CrossRef]
- Colburn, R.W.; Lubin, M.L.; Stone, D.J., Jr.; Wang, Y.; Lawrence, D.; D’Andrea, M.R.; Brandt, M.R.; Liu, Y.; Flores, C.M.; Qin, N. Attenuated cold sensitivity in TRPM8 null mice. Neuron 2007, 54, 379–386. [Google Scholar] [CrossRef]
- Gonzalez-Muniz, R.; Bonache, M.A.; Martin-Escura, C.; Gomez-Monterrey, I. Recent Progress in TRPM8 Modulation: An Update. Int. J. Mol. Sci. 2019, 20, 2618. [Google Scholar] [CrossRef]
- Andersen, H.H.; Olsen, R.V.; Moller, H.G.; Eskelund, P.W.; Gazerani, P.; Arendt-Nielsen, L. A review of topical high-concentration L-menthol as a translational model of cold allodynia and hyperalgesia. Eur. J. Pain 2014, 18, 315–325. [Google Scholar] [CrossRef]
- Wasner, G.; Schattschneider, J.; Binder, A.; Baron, R. Topical menthol—A human model for cold pain by activation and sensitization of C nociceptors. Brain 2004, 127, 1159–1171. [Google Scholar] [CrossRef]
- Pan, R.; Tian, Y.; Gao, R.; Li, H.; Zhao, X.; Barrett, J.E.; Hu, H. Central mechanisms of menthol-induced analgesia. J. Pharmacol. Exp. Ther. 2012, 343, 661–672. [Google Scholar] [CrossRef]
- Chung, M.K.; Caterina, M.J. TRP channel knockout mice lose their cool. Neuron 2007, 54, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, H.; Wang, Y.; Li, Y.; Li, Q.; Zhang, L. The distinctive role of menthol in pain and analgesia: Mechanisms, practices, and advances. Front. Mol. Neurosci. 2022, 15, 1006908. [Google Scholar] [CrossRef] [PubMed]
- Carlton, S.M.; Hargett, G.L.; Coggeshall, R.E. Localization of metabotropic glutamate receptors 2/3 on primary afferent axons in the rat. Neuroscience 2001, 105, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.R.; Pan, H.L. Distinct roles of group III metabotropic glutamate receptors in control of nociception and dorsal horn neurons in normal and nerve-injured Rats. J. Pharmacol. Exp. Ther. 2005, 312, 120–126. [Google Scholar] [CrossRef]
- Simmons, R.M.; Webster, A.A.; Kalra, A.B.; Iyengar, S. Group II mGluR receptor agonists are effective in persistent and neuropathic pain models in rats. Pharmacol. Biochem. Behav. 2002, 73, 419–427. [Google Scholar] [CrossRef]
- Tamaru, Y.; Nomura, S.; Mizuno, N.; Shigemoto, R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: Differential location relative to pre- and postsynaptic sites. Neuroscience 2001, 106, 481–503. [Google Scholar] [CrossRef]
- Oz, M.; El Nebrisi, E.G.; Yang, K.S.; Howarth, F.C.; Al Kury, L.T. Cellular and Molecular Targets of Menthol Actions. Front. Pharmacol. 2017, 8, 472. [Google Scholar] [CrossRef]
- Borhani Haghighi, A.; Motazedian, S.; Rezaii, R.; Mohammadi, F.; Salarian, L.; Pourmokhtari, M.; Khodaei, S.; Vossoughi, M.; Miri, R. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: A randomised, double-blind, placebo-controlled, crossed-over study. Int. J. Clin. Pract. 2010, 64, 451–456. [Google Scholar] [CrossRef]
- Wei, E.T.; Seid, D.A. AG-3-5: A chemical producing sensations of cold. J. Pharm. Pharmacol. 1983, 35, 110–112. [Google Scholar] [CrossRef]
- Namer, B.; Seifert, F.; Handwerker, H.O.; Maihöfner, C. TRPA1 and TRPM8 activation in humans: Effects of cinnamaldehyde and menthol. Neuroreport 2005, 16, 955–959. [Google Scholar] [CrossRef]
- Andersen, H.H.; Poulsen, J.N.; Uchida, Y.; Nikbakht, A.; Arendt-Nielsen, L.; Gazerani, P. Cold and L-menthol-induced sensitization in healthy volunteers--a cold hypersensitivity analogue to the heat/capsaicin model. Pain 2015, 156, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Inui, K.; Tran, T.D.; Hoshiyama, M.; Kakigi, R. Preferential stimulation of Aδ-fibers by intra-epidermal needle electrode in humans. Pain 2002, 96, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.; Shenoy, R.; Anand, P. A novel human volunteer pain model using contact heat evoked potentials (CHEP) following topical skin application of transient receptor potential agonists capsaicin, menthol and cinnamaldehyde. J. Clin. Neurosci. 2011, 18, 926–932. [Google Scholar] [CrossRef]
- Johar, P.; Grover, V.; Topp, R.; Behm, D.G. A comparison of topical menthol to ice on pain, evoked tetanic and voluntary force during delayed onset muscle soreness. Int. J. Sport. Phys. Ther. 2012, 7, 314–322. [Google Scholar]
- Inui, K.; Kakigi, R. Pain perception in humans: Use of intraepidermal electrical stimulation. J. Neurol. Neurosurg. Psychiatry 2012, 83, 551–556. [Google Scholar] [CrossRef]
- Garcia-Larrea, L. Objective pain diagnostics: Clinical neurophysiology. Neurophysiol. Clin. 2012, 42, 187–197. [Google Scholar] [CrossRef]
- Otsuru, N.; Inui, K.; Yamashiro, K.; Miyazaki, T.; Takeshima, Y.; Kakigi, R. Assessing Adelta fiber function with lidocaine using intraepidermal electrical stimulation. J. Pain 2010, 11, 621–627. [Google Scholar] [CrossRef]
- Smith, C.J.; Craighead, D.H.; Alexander, L.M. Effects of vehicle microdialysis solutions on cutaneous vascular responses to local heating. J. Appl. Physiol. 2017, 123, 1461–1467. [Google Scholar] [CrossRef]
- Yamashiro, K.; Sato, D.; Onishi, H.; Sugawara, K.; Nakazawa, S.; Shimojo, H.; Akatsuka, K.; Nakata, H.; Maruyama, A. Skill-Specific Changes in Somatosensory Nogo Potentials in Baseball Players. PLoS ONE 2015, 10, e0142581. [Google Scholar] [CrossRef]
- Yamashiro, K.; Sato, D.; Onishi, H.; Yoshida, T.; Horiuchi, Y.; Nakazawa, S.; Maruyama, A. Skill-specific changes in somatosensory-evoked potentials and reaction times in baseball players. Exp. Brain Res. 2013, 225, 197–203. [Google Scholar] [CrossRef]
- Yamashiro, K.; Yamazaki, Y.; Siiya, K.; Ikarashi, K.; Baba, Y.; Otsuru, N.; Onishi, H.; Sato, D. Modality-specific improvements in sensory processing among baseball players. Sci. Rep. 2021, 11, 2248. [Google Scholar] [CrossRef] [PubMed]
- Kodaira, M.; Ohashi, N.; Morita, H.; Sekijima, Y. Length-dependent truncal Adelta-fiber dysfunction in hereditary transthyretin amyloidosis: An intra-epidermal electrical stimulation study. Clin. Neurophysiol. 2019, 130, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Mouraux, A.; Iannetti, G.D.; Plaghki, L. Low intensity intra-epidermal electrical stimulation can activate Adelta-nociceptors selectively. Pain 2010, 150, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, T.; Wang, A.; Hurr, C. Topical Analgesic Containing Methyl Salicylate and L-Menthol Accelerates Heat Loss During Skin Cooling for Exercise-Induced Hyperthermia. Front. Physiol. 2022, 13, 945969. [Google Scholar] [CrossRef]
- Kakigi, R.; Shibasaki, H.; Ikeda, A. Pain-related somatosensory evoked potentials following CO2 laser stimulation in man. Electroencephalogr. Clin. Neurophysiol. 1989, 74, 139–146. [Google Scholar] [CrossRef]
- Shiroshita, Y.; Kirimoto, H.; Watanabe, T.; Yunoki, K.; Sobue, I. Event-related potentials evoked by skin puncture reflect activation of Abeta fibers: Comparison with intraepidermal and transcutaneous electrical stimulations. PeerJ 2021, 9, e12250. [Google Scholar] [CrossRef]
- Apkarian, A.V.; Bushnell, M.C.; Treede, R.D.; Zubieta, J.K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 2005, 9, 463–484. [Google Scholar] [CrossRef]
- Kramer, J.L.K.; Haefeli, J.; Jutzeler, C.R.; Steeves, J.D.; Curt, A. Improving the acquisition of nociceptive evoked potentials without causing more pain. Pain 2013, 154, 235–241. [Google Scholar] [CrossRef]
- Ploner, M.; Schmitz, F.; Freund, H.J.; Schnitzler, A. Parallel activation of primary and secondary somatosensory cortices in human pain processing. J. Neurophysiol. 1999, 81, 3100–3104. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Owsianik, G.; Nilius, B. TRP channels: An overview. Cell Calcium 2005, 38, 233–252. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makibuchi, T.; Yamashiro, K.; Anazawa, S.; Fujimoto, T.; Ochi, G.; Ikarashi, K.; Sato, D. Assessing the Effects of the Topical Application of L-Menthol on Pain-Related Somatosensory-Evoked Potentials Using Intra-Epidermal Stimulation. Brain Sci. 2023, 13, 918. https://doi.org/10.3390/brainsci13060918
Makibuchi T, Yamashiro K, Anazawa S, Fujimoto T, Ochi G, Ikarashi K, Sato D. Assessing the Effects of the Topical Application of L-Menthol on Pain-Related Somatosensory-Evoked Potentials Using Intra-Epidermal Stimulation. Brain Sciences. 2023; 13(6):918. https://doi.org/10.3390/brainsci13060918
Chicago/Turabian StyleMakibuchi, Taiki, Koya Yamashiro, Sayaka Anazawa, Tomomi Fujimoto, Genta Ochi, Koyuki Ikarashi, and Daisuke Sato. 2023. "Assessing the Effects of the Topical Application of L-Menthol on Pain-Related Somatosensory-Evoked Potentials Using Intra-Epidermal Stimulation" Brain Sciences 13, no. 6: 918. https://doi.org/10.3390/brainsci13060918
APA StyleMakibuchi, T., Yamashiro, K., Anazawa, S., Fujimoto, T., Ochi, G., Ikarashi, K., & Sato, D. (2023). Assessing the Effects of the Topical Application of L-Menthol on Pain-Related Somatosensory-Evoked Potentials Using Intra-Epidermal Stimulation. Brain Sciences, 13(6), 918. https://doi.org/10.3390/brainsci13060918





