Anatomical Variations of the Common Carotid Arteries and Neck Structures of the New Zealand White Rabbit and Their Implications for the Development of Preclinical Extracranial Aneurysm Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Animals
2.2. Approach and Measures
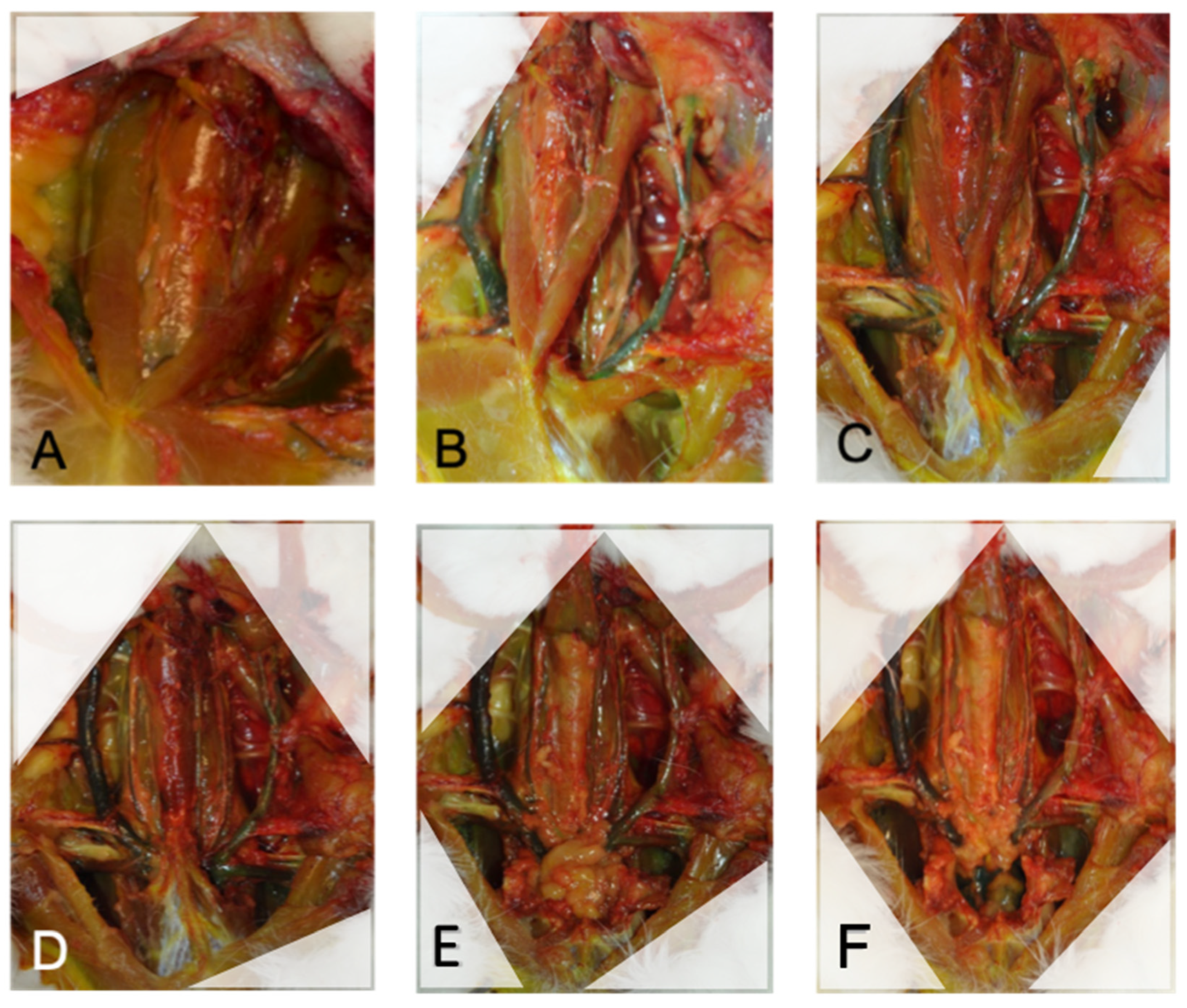
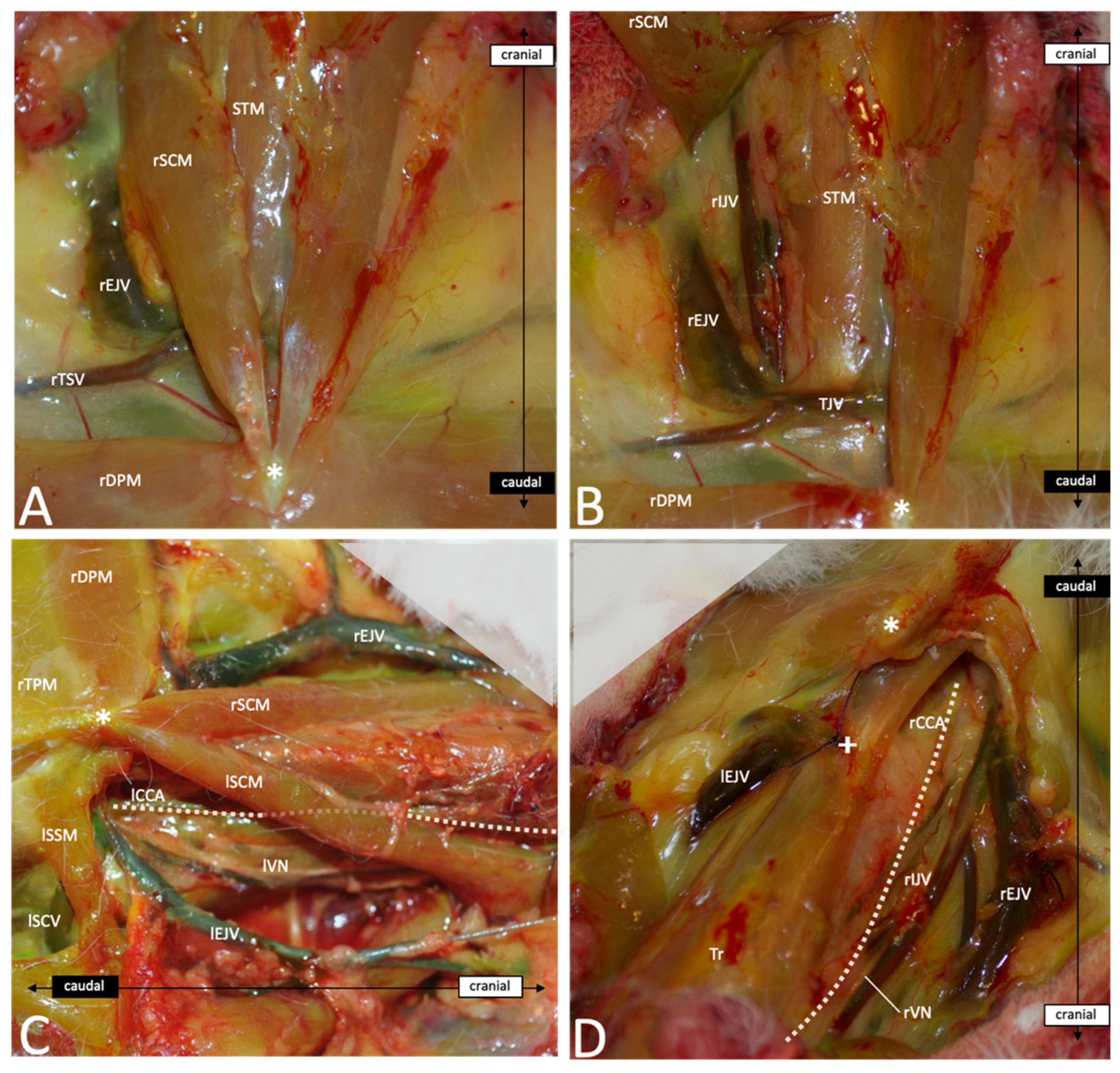
2.3. Neck Dissection
3. Results
3.1. Distance between rCCA’s Origin, Manubrium and lCCA’s Origin
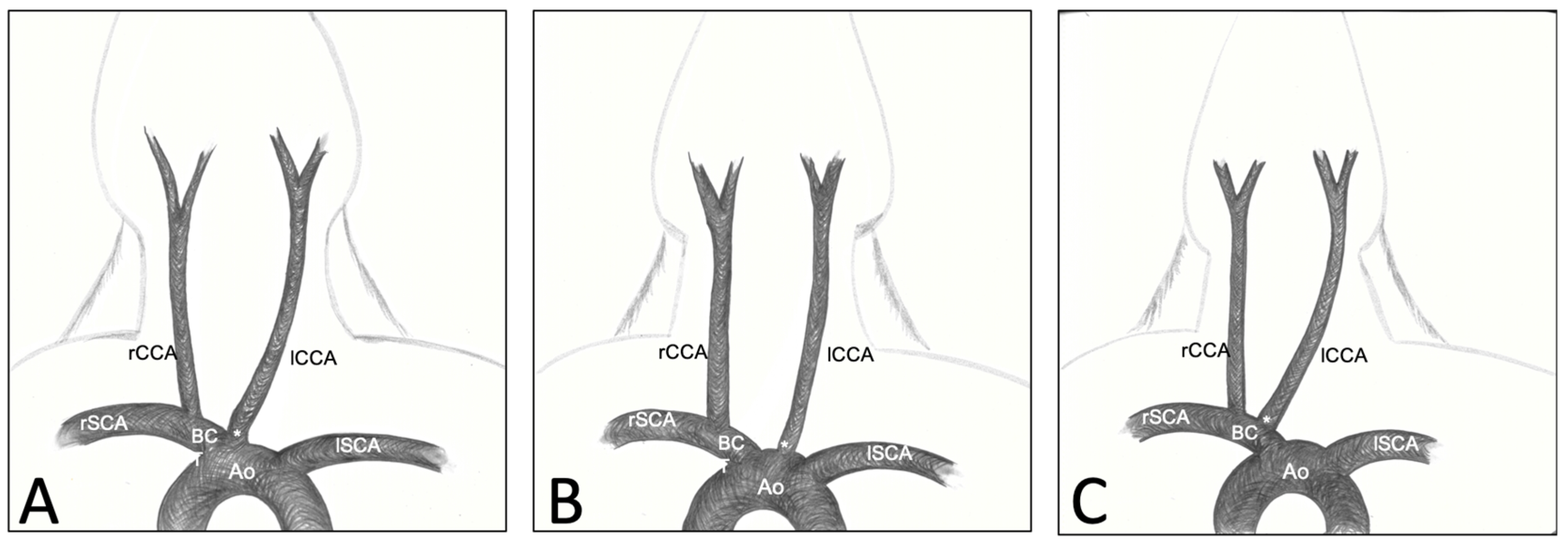
3.2. Variations of CCA’s Origin
3.3. Descriptive Anatomy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mapara, M.; Thomas, B.S.; Bhat, K.M. Rabbit as an animal model for experimental research. Dent. Res. J. 2012, 9, 111–118. [Google Scholar] [CrossRef]
- Marbacher, S.; Strange, F.; Frosen, J.; Fandino, J. Preclinical extracranial aneurysm models for the study and treatment of brain aneurysms: A systematic review. J. Cereb. Blood Flow Metab. 2020, 40, 922–938. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.W.; Elwardany, O.; McCarthy, D.J.; Sheinberg, D.L.; Alvarez, C.M.; Nada, A.; Snelling, B.M.; Chen, S.H.; Sur, S.; Starke, R.M. In vivo cerebral aneurysm models. Neurosurg. Focus 2019, 47, E20. [Google Scholar] [CrossRef]
- Bouzeghrane, F.; Naggara, O.; Kallmes, D.; Berenstein, A.; Raymond, J.; The International Consortium of Neuroendovascular Centres. In Vivo Experimental Intracranial Aneurysm Models: A Systematic Review. AJNR Am. J. Neuroradiol. 2010, 31, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Short, J.G.; Fujiwara, N.H.; Marx, W.F.; Helm, G.A.; Cloft, H.J.; Kallmes, D.F. Elastase-Induced Saccular Aneurysms in Rabbits: Comparison of Geometric Features with Those of Human Aneurysms. Am. J. Neuroradiol. 2001, 22, 1833–1837. [Google Scholar] [PubMed]
- Brinjikji, W.; Ding, Y.H.; Kallmes, D.F.; Kadirvel, R. From bench to bedside: Utility of the rabbit elastase aneurysm model in preclinical studies of intracranial aneurysm treatment. J. NeuroInterv. Surg. 2016, 8, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Kallmes, D.F.; Durka, M.J.; Ding, Y.; Lewis, D.; Kadirvel, R.; Robertson, A.M. Hemodynamics and Anatomy of Elastase-Induced Rabbit Aneurysm Models: Similarity to Human Cerebral Aneurysms? Am. J. Neuroradiol. 2011, 32, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Konczalla, J.; Wanderer, S.; Mrosek, J.; Gueresir, E.; Schuss, P.; Platz, J.; Seifert, V.; Vatter, H. Levosimendan, a new therapeutic approach to prevent delayed cerebral vasospasm after subarachnoid hemorrhage? Acta Neurochir. 2016, 158, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Hoh, B.L.; Rabinov, J.D.; Pryor, J.C.; Ogilvy, C.S. A modified technique for using elastase to create saccular aneurysms in animals that histologically and hemodynamically resemble aneurysms in human. Acta Neurochir. 2004, 146, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Altes, T.A.; Cloft, H.J.; Short, J.G.; DeGast, A.; Do, H.M.; Helm, G.A.; Kallmes, D.F. Creation of Saccular Aneurysms in the Rabbit: A model suitable for testing endovascular devices. Am. J. Roentgenol. 2000, 174, 349–354. [Google Scholar] [CrossRef]
- Krings, T.; Moller-Hartmann, W.; Hans, F.-J.; Thiex, R.; Brunn, A.; Scherer, K.; Meetz, A.; Dreeskamp, H.; Stein, K.-P.; Gilsbach, J.M.; et al. A refined method for creating saccular aneurysms in the rabbit. Neuroradiology 2003, 45, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.H.; Danielson, M.A.; Kadirvel, R.; Dai, D.; Lewis, D.A.; Cloft, H.J.; Kallmes, D.F. Modified technique to create morphologically reproducible elastase-induced aneurysms in rabbits. Neuroradiology 2006, 48, 528–532. [Google Scholar] [CrossRef]
- Möller-Hartmann, W.; Krings, T.; Stein, K.P.; Dreeskamp, A.; Meetz, A.; Thiex, R.; Hans, F.J.; Gilsbach, J.M.; Thron, A. Aberrant Origin of the Superior Thyroid Artery and the Tracheoesophageal Branch from the Common Carotid Artery: A Source of Failure in Elastase-Induced Aneurysms in Rabbits. Am. J. Roentgenol. 2003, 181, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Wanderer, S.; Waltenspuel, C.; Grüter, B.E.; Strange, F.; Sivanrupan, S.; Remonda, L.; Widmer, H.R.; Casoni, D.; Andereggen, L.; Fandino, J.; et al. Arterial Pouch Microsurgical Bifurcation Aneurysm Model in the Rabbit. J. Vis. Exp. 2020, 159, e61157. [Google Scholar] [CrossRef]
- Marbacher, S.; Erhardt, S.; Schläppi, J.-A.; Coluccia, D.; Remonda, L.; Fandino, J.; Sherif, C. Complex Bilobular, Bisaccular, and Broad-Neck Microsurgical Aneurysm Formation in the Rabbit Bifurcation Model for the Study of Upcoming Endovascular Techniques. Am. J. Neuroradiol. 2011, 32, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Sherif, C.; Fandino, J.; Erhardt, S.; Di Ieva, A.; Killer, M.; Kleinpeter, G.; Marbacher, S. Microsurgical Venous Pouch Arterial-Bifurcation Aneurysms in the Rabbit Model: Technical Aspects. J. Vis. Exp. 2011, 51, 2718. [Google Scholar] [CrossRef]
- Ding, Y.H.; Dai, D.; Layton, K.F.; Lewis, D.A.; Danielson, M.A.; Kadirvel, R.; Cloft, H.J.; Kallmes, D.F. Vascular Anatomic Variation in Rabbits. J. Vasc. Interv. Radiol. 2006, 17, 1031–1035. [Google Scholar] [CrossRef]
- Lee, J.S.; Hamilton, M.G.; Zabramski, J.M. Variations in the anatomy of the rabbit cervical carotid artery. Stroke 1994, 25, 501–503. [Google Scholar] [CrossRef]
- Popesko, P.; Rajtová, V.; Horák, J.i. A Colour Atlas of the Anatomy of Small Laboratory Animals; Wolfe Pub. Ltd.: London, UK, 1992. [Google Scholar]
- Barone, R. Atlas of Rabbit Anatomy; Masson: Paris, France, 1973; p. 219. [Google Scholar]
- Abruzzo, T.; Shengelaia, G.G.; Dawson, R.C.; Owens, D.S.; Cawley, C.M.; Gravanis, M.B. Histologic and morphologic comparison of experimental aneurysms with human intracranial aneurysms. Am. J. Neuroradiol. 1998, 19, 1309–1314. [Google Scholar]
- Aoki, T.; Nishimura, M. The Development and the Use of Experimental Animal Models to Study the Underlying Mechanisms of CA Formation. J. Biomed. Biotechnol. 2011, 2011, 535921. [Google Scholar] [CrossRef]
- Dai, D.; Ding, Y.H.; Danielson, M.A.; Kadirvel, R.; Lewis, D.A.; Cloft, H.J.; Kallmes, D.F. Histopathologic and Immunohistochemical Comparison of Human, Rabbit, and Swine Aneurysms Embolized with Platinum Coils. Am. J. Neuroradiol. 2005, 26, 2560–2568. [Google Scholar] [PubMed]
- Zaragoza, C.; Gomez-Guerrero, C.; Martin-Ventura, J.L.; Blanco-Colio, L.M.; Lavin, B.; Mallavia, B.; Tarin, C.; Mas, S.; Ortiz, A.; Egido, J. Animal Models of Cardiovascular Diseases. J. Biomed. Biotechnol. 2011, 2011, 497841. [Google Scholar] [CrossRef] [PubMed]
- Frösen, J.; Piippo, A.; Paetau, A.; Kangasniemi, M.; Niemelä, M.; Hernesniemi, J.; Jääskeläinen, J. Remodeling of Saccular Cerebral Artery Aneurysm Wall Is Associated with Rupture: Histological analysis of 24 unruptured and 42 ruptured cases. Stroke 2004, 35, 2287–2293. [Google Scholar] [CrossRef]
- Frösen, J.; Piippo, A.; Paetau, A.; Kangasniemi, M.; Niemelä, M.; Hernesniemi, J.; Jääskeläinen, J. Growth Factor Receptor Expression and Remodeling of Saccular Cerebral Artery Aneurysm Walls: Implications for Biological Therapy Preventing Rupture. Neurosurgery 2006, 58, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Stehbens, W.E. In re: Histologic and morphologic comparison of experimental aneurysms with human intracranial aneurysms. Am. J. Neuroradiol. 2000, 21, 1770. [Google Scholar]
- Ding, Y.; Dai, D.; Lewis, D.; Danielson, M.; Kadirvel, R.; Cloft, H.; Kallmes, D. Long-Term Patency of Elastase-Induced Aneurysm Model in Rabbits. Am. J. Neuroradiol. 2006, 27, 139–141. [Google Scholar] [PubMed]
- Marbacher, S.; Tastan, I.; Neuschmelting, V.; Erhardt, S.; Coluccia, D.; Sherif, C.; Remonda, L.; Fandino, J. Long-term patency of complex bilobular, bisaccular, and broad-neck aneurysms in the rabbit microsurgical venous pouch bifurcation model. Neurol. Res. 2012, 34, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Sherif, C.; Marbacher, S.; Erhardt, S.; Fandino, J. Improved Microsurgical Creation of Venous Pouch Arterial Bifurcation Aneurysms in Rabbits. Am. J. Neuroradiol. 2011, 32, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Sherif, C.; Marbacher, S.; Fandino, J. High-resolution three-dimensional 3 T magnetic resonance angiography for the evaluation of experimental aneurysm in the rabbit. Neurol. Res. 2009, 31, 869–872. [Google Scholar] [CrossRef] [PubMed]
- Strange, F.; Grüter, B.E.; Fandino, J.; Marbacher, S. Preclinical Intracranial Aneurysm Models: A Systematic Review. Brain Sci. 2020, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Origuchi, N.; Shigematsu, H.; Izumiyama, N.; Nakamura, K.; Toku, A.; Muto, T. Aneurysm induced by periarterial application of elastase heals spontaneously. Int. Angiol. 1998, 17, 113–119. [Google Scholar] [PubMed]
- Ho, J.P.; Galex, I.A.; Sadeghi, N.B.; Weledji, N.; Bermudez, S.I.C.; Mitchell, B.A.; Bush, D.M.; Yap, E.; Davis, N.C.; Catalino, M.P.; et al. Rabbit Elastase Aneurysm: Imaging and Histology Correlates for Inflammation and Healing. World Neurosurg. 2021, 148, e242–e251. [Google Scholar] [CrossRef]
- Sasaki, K.; Ujiie, H.; Higa, T.; Hori, T.; Shinya, N.; Uchida, T. Rabbit Aneurysm Model Mediated by the Application of Elastase. Neurol. Med. Chir. 2004, 44, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.; Sadasivan, C.; Onizuka, M.; Gounis, M.J.; Christian, F.; Miskolczi, L.; Wakhloo, A.K.; Lieber, B.B. Morphology of elastase-induced cerebral aneurysm model in rabbit and rapid prototyping of elastomeric transparent replicas. Biorheology 2005, 42, 345–361. [Google Scholar]
- Thiex, R.; Hans, F.J.; Scherer, K.; Krings, T.; Möller-Hartmann, W. Are the configuration and neck morphology of experimental aneurysms predictable? A technical approach. Neuroradiology 2004, 46, 571–576. [Google Scholar] [CrossRef]
- Killer, M.; Kallmes, D.; Jones, R.; Ding, Y.; Vestal, M.; Hauser, T.; Virmani, R.; Cruise, G. Long-Term Angiographic and Histological Results of a New Hydrogel-Containing Filling Coil in Experimental Rabbit Aneurysms. Min Minim. Invasive Neurosurg. 2010, 53, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Killer, M.; Kallmes, D.F.; McCoy, M.R.; Ding, Y.-H.; Shum, J.C.; Cruise, G.M. Angiographic and Histologic Comparison of Experimental Aneurysms Embolized with Hydrogel Filaments. Am. J. Neuroradiol. 2009, 30, 1488–1495. [Google Scholar] [CrossRef]
- Struffert, T.; Lang, S.; Adamek, E.; Engelhorn, T.; Strother, C.M.; Doerfler, A. Angiographic C-arm CT visualization of the Woven EndoBridge Cerebral Aneurysm Embolization Device (WEB): First Experience in an Animal Aneurysm Model. Clin. Neuroradiol. 2014, 24, 43–49. [Google Scholar] [CrossRef]
- Struffert, T.; Roth, C.; Romeike, B.; Grunwald, I.O.; Reith, W. Onyx in an experimental aneurysm model: Histological and angiographic results. J. Neurosurg. 2008, 109, 77–82. [Google Scholar] [CrossRef]
- Bai, Y.; Ding, X.; Zhao, Q.; Sun, H.; Li, T.; Li, Z.; Wang, H.; Zhang, L.; Zhang, C.; Xu, S. Development of an organic acid compound disinfectant to control food-borne pathogens and its application in chicken slaughterhouses. Poult. Sci. 2022, 101, 101842. [Google Scholar] [CrossRef]
- Wang, K.; Yuan, S.; Zhang, X.; Liu, Q.; Zhong, Q.; Zhang, R.; Lu, P.; Li, J. Biodegradable flow-diverting device for the treatment of intracranial aneurysm: Short-term results of a rabbit experiment. Neuroradiology 2013, 55, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Chavan, R.; Pons, S.; Gupta, V.; Hui, D.; Bose, A. Safety and performance of the Penumbra Liberty stent system in a rabbit aneurysm model. J. NeuroInterv. Surg. 2015, 7, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Nishi, S.; Nakayama, Y.; Ishibashi-Ueda, H.; Yoshida, M.; Yonetani, H. Treatment of rabbit carotid aneurysms by hybrid stents (microporous thin polyurethane-covered stents): Preservation of side-branches. J. Biomater. Appl. 2014, 28, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Ishii, A.; Ono, I.; Abekura, Y.; Ikeda, H.; Arai, D.; Yamao, Y.; Okawa, M.; Kikuchi, T.; Nakakura, A.; et al. Biodegradable Flow Diverter for the Treatment of Intracranial Aneurysms: A Pilot Study Using a Rabbit Aneurysm Model. J. Am. Heart Assoc. 2019, 8, e014074. [Google Scholar] [CrossRef]
- Grunwald, I.Q.; Romeike, B.; Eymann, R.; Roth, C.; Struffert, T.; Reith, W. An Experimental Aneurysm Model: A Training Model for Neurointerventionalists. Interv. Neuroradiol. 2006, 12, 17–24. [Google Scholar] [CrossRef]
- Tsumoto, T.; Terada, T.; Yamaga, H.; Itakura, T. Coil Embolization Training Using a Rabbit Saccular Aneurysm Model. Interv. Neuroradiol. 2006, 12, 57–60. [Google Scholar] [CrossRef]
- Lewis, D.A.; Ding, Y.H.; Dai, D.; Kadirvel, R.; Danielson, M.A.; Cloft, H.J.; Kallmes, D.F. Morbidity and Mortality Associated with Creation of Elastase-Induced Saccular Aneurysms in a Rabbit Model. Am. J. Neuroradiol. 2009, 30, 91–94. [Google Scholar] [CrossRef]
- Cesar, L.; Miskolczi, L.; Lieber, B.B.; Sadasivan, C.; Gounis, M.J.; Wakhloo, A.K. Neurological deficits associated with the elastase-induced aneurysm model in rabbits. Neurol. Res. 2009, 31, 414–419. [Google Scholar] [CrossRef]
- Villano, J.S.; Boehm, C.A.; Carney, E.L.; Cooper, T.K. Complications of elastase-induced arterial saccular aneurysm in rabbits: Case reports and literature review. Comp. Med. 2012, 62, 480–486. [Google Scholar]
- Wang, K.; Huang, Q.; Hong, B.; Xu, Y.; Zhao, W.; Chen, J.; Zhao, R.; Liu, J. Neck Injury Is Critical to Elastase-Induced Aneurysm Model. Am. J. Neuroradiol. 2009, 30, 1685–1687. [Google Scholar] [CrossRef]
- Grüter, B.E.; Croci, D.; Schöpf, S.; Nevzati, E.; D’Allonzo, D.; Lattmann, J.; Roth, T.; Bircher, B.; Muroi, C.; Dutilh, G.; et al. Systematic Review and Meta-analysis of Methodological Quality in In Vivo Animal Studies of Subarachnoid Hemorrhage. Transl. Stroke Res. 2020, 11, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Brunn, A.; Scherer, K.; Gilsbach, J.M.; Thron, A.; Krings, T.; Thiex, R.; Hans, F.J.; Möller-Hartmann, W. Haemorrhagic tracheal necrosis as a lethal complication of an aneurysm model in rabbits via endoluminal incubation with elastase. Acta Neurochir. 2004, 146, 285–289. [Google Scholar] [CrossRef]
- Boillat, G.; Franssen, T.; Grüter, B.; Wanderer, S.; Catalano, K.; Casoni, D.; Andereggen, L.; Marbacher, S. Creation of Two Saccular Elastase-digested Aneurysms with Different Hemodynamics in One Rabbit. J. Vis. Exp. 2021, e62518. [Google Scholar] [CrossRef]
- Ding, Y.H.; Dai, D.; Danielson, M.A.; Kadirvel, R.; Lewis, D.A.; Cloft, H.J.; Kallmes, D.F. Control of Aneurysm Volume by Adjusting the Position of Ligation During Creation of Elastase-Induced Aneurysms: A Prospective Study. Am. J. Neuroradiol. 2007, 28, 857–859. [Google Scholar] [PubMed]
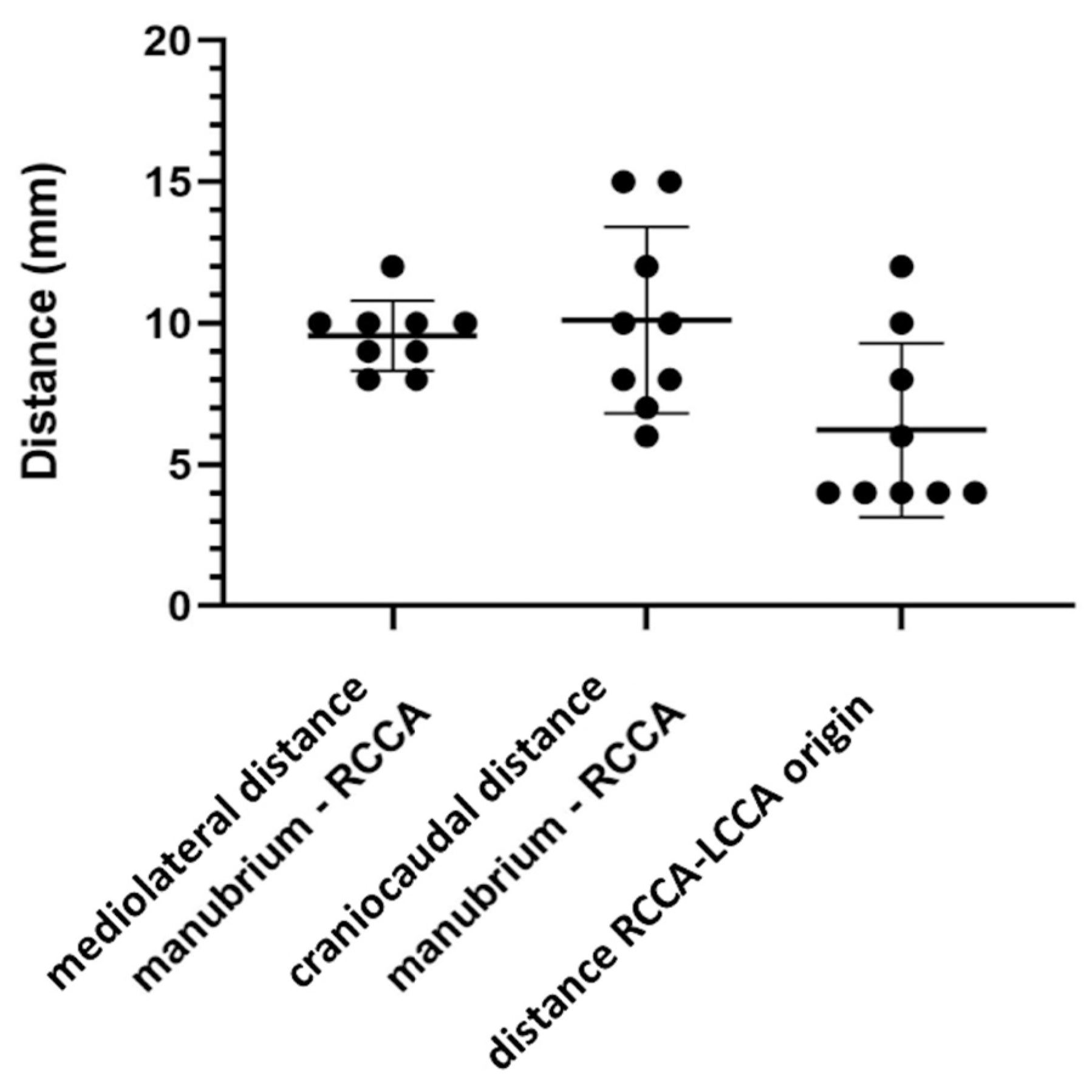

| Animal Number | Mediolateral Direction (mm) | Craniocaudal Direction (mm) |
|---|---|---|
| 1 | 10 | 8 |
| 2 | 10 | 7 |
| 3 | 10 | 10 |
| 4 | 10 | 8 |
| 5 | 8 | 6 |
| 6 | 12 | 15 |
| 7 | 9 | 12 |
| 8 | 9 | 15 |
| 9 | 8 | 10 |
| Mean (±SD) | 9.6 (±1.2) | 10.1 (±3.3) |
| Animal Number | Distance (mm) |
|---|---|
| 1 | 4 |
| 2 | 4 |
| 3 | 6 |
| 4 | 4 |
| 5 | 12 |
| 6 | 4 |
| 7 | 8 |
| 8 | 4 |
| 9 | 10 |
| Mean (±SD) | 6.2 (±3.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boillat, G.; Franssen, T.; Wanderer, S.; Rey, J.; Casoni, D.; Andereggen, L.; Marbacher, S.; Gruter, B.E. Anatomical Variations of the Common Carotid Arteries and Neck Structures of the New Zealand White Rabbit and Their Implications for the Development of Preclinical Extracranial Aneurysm Models. Brain Sci. 2023, 13, 222. https://doi.org/10.3390/brainsci13020222
Boillat G, Franssen T, Wanderer S, Rey J, Casoni D, Andereggen L, Marbacher S, Gruter BE. Anatomical Variations of the Common Carotid Arteries and Neck Structures of the New Zealand White Rabbit and Their Implications for the Development of Preclinical Extracranial Aneurysm Models. Brain Sciences. 2023; 13(2):222. https://doi.org/10.3390/brainsci13020222
Chicago/Turabian StyleBoillat, Gwendoline, Tim Franssen, Stefan Wanderer, Jeannine Rey, Daniela Casoni, Lukas Andereggen, Serge Marbacher, and Basil E. Gruter. 2023. "Anatomical Variations of the Common Carotid Arteries and Neck Structures of the New Zealand White Rabbit and Their Implications for the Development of Preclinical Extracranial Aneurysm Models" Brain Sciences 13, no. 2: 222. https://doi.org/10.3390/brainsci13020222
APA StyleBoillat, G., Franssen, T., Wanderer, S., Rey, J., Casoni, D., Andereggen, L., Marbacher, S., & Gruter, B. E. (2023). Anatomical Variations of the Common Carotid Arteries and Neck Structures of the New Zealand White Rabbit and Their Implications for the Development of Preclinical Extracranial Aneurysm Models. Brain Sciences, 13(2), 222. https://doi.org/10.3390/brainsci13020222







