Abstract
Since 1998, when Schmahmann first proposed the concept of the “cognitive affective syndrome” that linked cerebellar damage to cognitive and emotional impairments, a substantial body of literature has emerged. Anatomical, neurophysiological, and functional neuroimaging data suggest that the cerebellum contributes to cognitive functions through specific cerebral–cerebellar connections organized in a series of parallel loops. The aim of this paper is to review the current findings on the involvement of the cerebellum in selective cognitive functions, using a psychophysiological perspective with event-related potentials (ERPs), alone or in combination with non-invasive brain stimulation techniques. ERPs represent a very informative method of monitoring cognitive functioning online and have the potential to serve as valuable biomarkers of brain dysfunction that is undetected by other traditional clinical tools. This review will focus on the data on attention, executive functions, and time processing obtained in healthy subjects and patients with varying clinical conditions, thus confirming the role of ERPs in understanding the role of the cerebellum in cognition and exploring the potential diagnostic and therapeutic implications of ERP-based assessments in patients.
1. Introduction
The cerebellum is widely known to be associated with motor control [1], but several studies of neuroanatomy, neuroimaging, neuropsychology, and non-invasive brain stimulation (NIBS), have implicated the cerebellum in specific cognitive domains [2,3,4,5] such as attention [6,7,8,9,10,11], executive functioning [12], temporal representation [13], language processing [14,15], visuospatial cognition and personality changes that may result in a general decline in intellectual functioning (so-called “cerebellar cognitive affective syndrome”, CCAS, [16]).
The cerebellar contribution to cognition is mediated by specific cerebral–cerebellar crossed connections [17], organized in parallel loops, with a prominent role played by the cortico-ponto-cerebello-dentato-thalamo-cortical pathway [2,18,19]. Output from the cerebellar cortex through the dentate nucleus reaches the cerebral cortex diffusely [20]. The main part of this output is for higher-level prefrontal and parietal cortices, but temporal areas also have reciprocal connections with the cerebellum mediated via the pons [21,22,23]. Cerebellar–cortical connections also depart from the interposed nucleus and the fastigial nucleus, and (through thalamic interconnections) reach brain areas involved in emotional control such as the amygdala, hippocampus and middle temporal gyrus [24,25].
Neuroimaging studies, including those adopting resting-state functional connectivity, have confirmed dense connections between the movement-related cerebellar areas (lobule I, IV, V and anterior area of lobule VI) and the contralateral primary motor and primary somatosensory cortex [26,27,28,29,30], but also strong connectivity between cerebellar regions, brain associative cortices and systems of interconnected neurons such as the salience network and the default-mode network [29,30,31,32]. Finally, right lobules VI and Crus I are involved in language processing, and left lobule VI is involved in visuospatial processing [3].
A considerable effort has been made to understand the cerebellar contribution to cognition, yielding interesting results from many neuroimaging studies as well as neuropsychological studies, including those on psychiatric and developmental disorders [14,15,33,34,35,36].
Physiologically, the main cerebellar function is the inhibitory firing toward the brain areas. This is called cerebellar brain inhibition tone [37,38], and it influences both motor and cognitive circuits via a synaptic relay in the ventral-lateral thalamus [39,40]. The dentate-thalamus pathway influences the functioning of parietal and the frontal cortices [39] involved in attention and in executive control. The cerebellum modulates the levels of activation or inhibition of cerebral cortical areas, thus acting as a “coordinator” that indirectly influences the cognitive processes. A specific cerebellar function is predictive coding, that is, the ability to make specific sensory predictions according to the anticipatory or feed-forward model [4,12]. Predictive coding works by evaluating temporal patterns of stimuli, errors or conflicting signals with the aim of improving the reliability of future predictions and producing online changes in behavior, thereby influencing inhibitory control.
The purpose of this review is to examine the use of event-related potential (ERP) techniques in the investigation of cerebellar contributions to cognitive functioning not related to motor preparation, control or execution.
When a stimulus, or a combination of stimuli, of any sensory modality (auditory, visual, somatosensory) activates a higher-level neural process, thus becoming a so-called “event,” the resulting flow of brain information can be traced by the ongoing electroencephalogram (EEG) recorded from the scalp [41]. A waveform representative of the changes in brain voltage related to the underlying neural process can be obtained by averaging the EEG activity over multiple trials. This waveform is called event-related potential (see Figure 1, Adapted from Woodman, 2010 [42]) and it consists of various components. Depending on the experimental conditions, ERP components can be related to events such as predicting errors (mismatch negativity (MMN), error-related negativity (ERN) and error-related positivity (Pe)), switching attention to task-irrelevant novel stimuli (P3a), categorizing target stimuli (P3b) [43], establishing the temporal contingency between stimuli (contingent negative variation (CNV)) [44] and so on, up to imagery processes [45]. ERP components are described by their polarity (positive or negative), their amplitude (reflecting how much neural activity is allocated to the current cognitive function), their latency (signaling the time at which the neural activity occurs) and their scalp distribution (the superficial projection of potentials generated by cortical structures) [46].
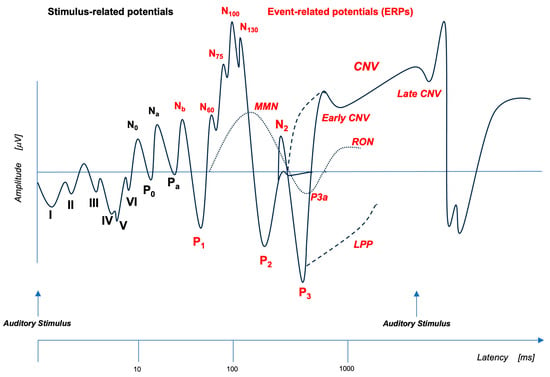
Figure 1.
Event-related potentials (ERPs) represent a measure of cortical electric activity evoked during a cognitive task and recorded from scalp with EEG. Here, a schematic representation of auditory ERPs detected after EEG signal averaging analysis is shown in logarithmic temporal sequence. Early responses to auditory stimuli (AEP) are depicted with black letters. Long latency waves, time-locked to the auditory event (ERPs), are depicted with red letters. Each component is described by a letter that indicates its polarity (P: positive and N: negative) and by a number/letter that indicates its position in the sequence or a number that indicates its latency. CNV: contingent negative variation; LPP: late positive potential; MMN: mismatch negativity; RON: reorienting negativity (Adapted from Woodman, 2010 [42]).
ERPs represent a reliable and non-invasive method to acquire real-time knowledge about how the human brain processes information with an exceptional temporal resolution, in healthy subjects and patients with neurological conditions.
The ability to track selective cognitive functions and monitor them through quantitative parameters such as amplitude, latency and distribution provides ERPs with a clinical value similar to that of short-latency evoked potentials, although the neural generators of the late components that form ERPs are much more complex of those that give rise to the short-latency evoked components [46].
2. Cerebellum and Attention
Psychophysiological studies of adult patients with acquired cerebellar lesions provided evidence for the cerebellum’s role in various aspects of attention [47,48,49,50]. We reported a reduction in P3 amplitude evoked by a classic auditory oddball paradigm in a man with a left posterior cerebellar ischemic stroke [49]. The P3 improved after 4 weeks. These observations align with a previous case report that described P3 alterations (also evoked by auditory stimuli) in a patient with a large cerebellar lesion [47]. The P3 changes observed in these patients likely reflected attentional dysfunction, thus supporting the contribution of the cerebellum to the attentional processing of the stimulus. Our observation that P3b amplitude restores after 4 weeks from stroke, which paralleled the recovery of the attentional cognitive functioning, suggests a possible role of the P3 component as a neurophysiological marker of functional cerebellar recovery. To confirm that the cerebellum contributes to attention, early and late ERP components (early posterior negativity (EPN) and late positive potential (LPP)) to highly and poorly emotionally arousing pictures (pleasant, unpleasant and neutral pictures of the International Affective Picture System), with and without competing attentional tasks, were recorded in a patient with an ischemic cerebellar infarct [50]. The EPN response to highly arousing emotional cues in the competing visual attention condition was absent, whereas the LPP response to highly arousing emotional cues showed augmentation over frontal areas. This pattern of ERP findings suggested specific neural dysfunction associated with emotional–behavioral disturbances following cerebellar lesions, pointing toward a role of the cerebellum in supporting emotional attention. Another study in patients with cerebellar lesions used a N100/N1 suppression paradigm combining self-initiated with externally triggered auditory stimuli, to explore the ability to allocate attention to stimuli related to the prediction of an expected sensory information [48]. Generally, the N1 is suppressed when sensory information matches the prediction of an expected stimulus so that the brain activity directed to the actual input is reduced. Self-initiated sensory stimuli are highly predictable, while externally triggered stimuli lead to an increased processing activity as external sensations may provide new and important information. Patients lacked N1 suppression in response to self-initiated sounds, thus suggesting that the cerebellum is essential for generating internal forward predictions [48].
Other interesting insights arise from studies on genetically determined cerebellar ataxias (CA) in which cognitive decline is a common non-motor feature. Using behavioral tests with concomitant EEG recordings, sensory predictive coding processes and response adaptations were examined [51]. Sensory prediction coding was tested with an auditory distraction paradigm and error-related behavioral adaptations were tested with a visual flanker task. Many ERPs were measured, including the P3a for the orientation of attention, the N2 and the ERN for the cognitive adaptation processes/consequences of response errors, the Pe for error awareness, the MMN for sensory predictive coding and automatic involuntary attention and the reorientation negativity (RON) for reorientation after unexpected events. ERPs related to voluntary cognitive processing, including attentional switching (P3a, RON) and error awareness (Pe), were abnormal in CA patients, whereas attentional functioning that generates internal automatic forward processes resulted largely intact (MMN, ERN/N2).
Attentional abnormalities were also observed in patients with spinocerebellar ataxia 2 (SCA2), who showed abnormal visual/cognitive processing measured by visually evoked potentials (VEPs) and P3 components elicited by a visual oddball task [52]. Similarly to what was observed with auditory stimuli, recordings of visual paradigms showed a lower P3 amplitude and prolonged P3 latency [53], thus suggesting attentional, discriminative and working memory abnormalities in these patients. Preclinical SCA2 carriers exhibited less severe but significant prolongation of P3 latencies. Overall, these findings provide evidence supporting the cerebellar involvement in attention and memory, and they show that psychophysiological measures may act as the biomarkers of the cognitive decline in SCA [53].
A recent P3 study evoked by a dual-task emotion perception task that adopted angry, happy and neutral facial expressions explored the presence of cognitive deficits in Type I Chiari malformation. This impairment is thought to be related directly to compression dynamics at the cervico-medullary junction and indirectly to long-term chronic pain experienced by patients [54]. Although patients had slower response times than normal, they did not differ from controls in the ability to allocate attentional resources. However, patients had an increased frontal representation of the P3 amplitude, reflecting compensatory neural recruitment.
Interestingly, the cerebellum emerged as one of the key dysfunctional brain cognitive nodes in specific pathological populations with neuropsychiatric diseases [55,56,57,58]. While investigating the neural basis of the abnormalities in eye gaze processing with source imaging analysis in patients with attention deficit hyperactivity disorder (ADHD), the P200 wave at the left/midline cerebellum was found to be reduced [59]. Based on the growing evidence of the cerebellar involvement in emotion recognition, theory of mind and empathy [60,61], the reduced cerebellar activity was thought to lead patients to dysfunctionally integrate social inputs to attentional executive tasks. In a double-blind placebo-controlled study, the efficacy of 3-week prefrontal-excitatory and cerebellar-inhibitory transcranial direct current stimulation (tDCS) on neurocognitive functioning was explored in patients with the euthymic bipolar disorder [62]. They showed improved executive functioning (trail making test—B) and visuospatial memory (Rey complex figure test delay recall) after an active tDCS session, accompanied by a decrease in the P3b latency. These findings suggest that cerebellar tDCS improves the attentional brain processing stream, and indicates that the prefrontal cortex, cerebellum and prefrontal–thalamic–cerebellar circuitry are implicated in cognitive processes, including those reported as dysfunctional in these patients with the euthymic bipolar disorder. Concomitant prefrontal-excitatory and cerebellar-inhibitory tDCS may represent a useful rehabilitative tool for better neurocognitive performance.
Cerebellar involvement in attention functioning was also explored in healthy subjects. The role in pre-attentive automatic change detection processes was suggested by ERP studies showing changes in N1 and MMN components after cerebellar modulation with non-invasive brain stimulation (NIBS) techniques [63,64]. Investigating the effects of tDCS delivered over the left cerebellar hemisphere in cathodal (inhibitory), anodal (excitatory) and sham sessions during a P3 Novelty auditory task, cathodal cerebellar tDCS alone reduced the amplitude of the N1, N2 and P3 components for both the target and novel stimuli, and shortened the N1 latency evoked by each stimulus (target, novel and standard) [65]. These ERP changes show that the cerebellum operates in different stages of the attentional processing of the stimulus, from the automatic involuntary detection (N1) and the early phase of the attention switching (N2) to the attentive discrimination of the stimuli (P300), by regulating the activation and inhibition levels of the brain cortical areas involved in attentional networks. The role of the cerebellum in the functioning of the attention networks was also demonstrated by using the attention network task combined with tDCS [66]. In this study, we reported a selective reduction in the efficiency of the executive network after cerebellar inhibition, specifically related to the ability to process complex stimuli in which conflict signals or errors are present.
Combining functional neuroimaging (fMRI) and ERP data during a modified auditory oddball paradigm for the elicitation of P3 components related to attention and working memory cognitive processes as well as the activation of fronto-parietal areas and the cerebellum was found in healthy children between the ages of 11 and 16 (Figure 2) [67].
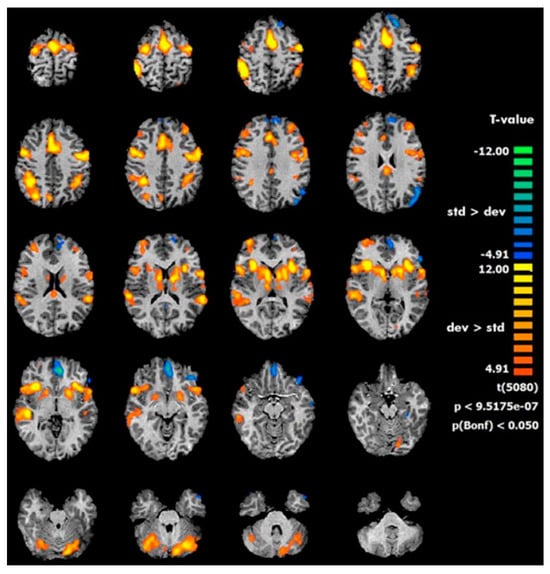
Figure 2.
fMRI activations during oddball paradigm in pediatric population (from Rusiniak et al., 2013) [67].
Lastly, when examining the neural mechanisms of emotional word processing in bilinguals with an ERP–fMRI registration, emotional processing activated a rapid and automatic attentional orienting response during left cerebellum activation [68].
3. Cerebellum and Executive Functioning
The term “executive functions” refers to the ability to coordinate different cognitive tasks to obtain specific goals [69]. It consists of various cognitive abilities, such as working memory, problem solving, cognitive flexibility, preparation and inhibitory control, necessary to plan and direct goal-oriented behavior. The prefrontal cortex is crucial in maintaining executive control, also thanks to a strong fronto-cerebellar connectivity, consisting of closed cortico-cerebellar loops in which the dorsolateral prefrontal cortex connects to the cerebellum via pontine nuclei, and the cerebellum sends projections back to the prefrontal cortex via the dentate nucleus and thalamus [70,71].
Cerebellar involvement in executive functioning has been documented by experimental ERP studies, primarily in patients with movement disorders, such as Parkinson’s disease.
To investigate the preparation of self-paced and externally cued movements in patients with Parkinson’s disease and essential tremors, motor-related potentials (MRP), such as the Bereitschaftpotential (BP) and the contingent negative variation (CNV), were recorded [72]. BP is recorded over the motor cortices and is best represented contralateral to the finger performing self-paced movements; CNV is recorded over fronto-parietal cortices bilaterally and reflects the attentional anticipation and motor preparation to externally cued movements [73]. Motor preparation goes under the control of distinct cortico-thalamo-cortical circuits according to various motor parameters including the type of cueing. The pattern of findings in BP and CNV in the two group of patients (having predominant basal ganglia vs. predominant cerebellar dysfunction) showed that the cortico-basal ganglia–thalamocortical circuit prepared both self-paced and externally cued movements, whereas the cerebello-dentato-thalamocortical pathway prepared only self-paced movements. This observation is especially interesting because many neurophysiological studies have shown that this pathway is involved in executing externally cued movements that, indeed, are relatively spared in patients with Parkinson’s disease [74]. A reasonable explanation for cerebellum involvement in preparing self-paced movements is that, during motor preparation, subjects were asked to determine the timing of the self-paced task, and cerebellum is known to manage the timing of movement preparation.
Some insights related to cerebellar involvement in cognitive flexibility were obtained from psychophysiological data in drug abusers [75]. By studying a conflict task designed to elicit a slow EEG potential (SP, a P3-like potential that emerges approximately 500 ms after stimulus onset) and using these data to localize the neural substrates of response dysregulation, the SP amplitude showed a normal spatial conflict effect for opioid-dependent and non-opioid-dependent subjects, but not for cocaine-dependent patients. Correlational analysis showed that abnormal SP was not related to quantity, frequency or recent cocaine use, but depended on comorbid alcohol use. A neuroanatomical localization algorithm applied to SP data showed that comorbid alcohol use disrupted normal task-related activation of the anterior cingulate, prefrontal cortex and cerebellum.
In one patient with a large cerebellar lesion, the P300 component was prolonged, reduced and changed in morphology, with two distinct peaks not normally elicited by the classical auditory oddball P300 paradigm. That patient performed poorly in neuropsychological tests, with difficulties in planning, abstract reasoning, set-shifting and perseveration [47]. Executive dysfunction was considered the consequence of changes in cerebello-frontal circuitry, since P300 is generated by cortical (especially prefrontal) and subcortical areas [76,77].
In a recent study aimed to evaluate the executive inhibitory control in cognitively intact APOE4 non-carrier elders, the EEG source analysis revealed greater cerebellar activity during the P300 time window in a stop-signal task that used visual stimuli. This activity was considered compensatory and was absent in healthy elderly APOE carriers, indicating that APOE carriers, even when asymptomatic, lack cerebellar compensatory mechanisms [78].
The cerebellar contribution to inhibitory executive control emerges from a study in which the profile of cognitive impairment was assessed in patients with cerebellar cortical atrophy [79]. To assess attentional performance and the ability to control a motor response, subjects were subjected to a comprehensive battery of neuropsychological tests along with a conventional auditory oddball task and a continuous performance task, i.e., execute (“Go”) or inhibit a motor reaction (“No Go”). Baseline-independent measures (global field power (GFP)) were determined, and low-resolution brain electromagnetic tomography (LORETA) was used to calculate the three-dimensional intracerebral distribution of electrical activity of the P3 component of Go and NoGo responses. Patients had prolonged GFP peak latency and attenuated GFP peak specifically in the NoGo condition, which is associated with LORETA evidence of low frontal NoGo P3 source activation. This pattern of findings suggests that cerebellar degeneration contributes to frontal executive dysfunction by impairing the inhibitory executive system.
A visual Go/NoGo task with concomitant LORETA was used to investigate P2/P3 potentials and brain generators in children with ADHD [80]. They had reduced Go and NoGo-P3 components due to a decreased contribution of frontal areas and dorsal ACC, respectively. Concomitantly, the increased NoGo-P2 amplitude was the result of the decreased contribution of the dorsolateral prefrontal cortex, the insula and the cerebellum. These findings suggest the fronto-cerebellar involvement in the automatic feature of inhibition processes.
Inhibitory control was analyzed in healthy subjects who underwent an auditory Go/NoGo task before and after cathodal and sham cerebellar tDCS in separate sessions [81]. Cathodal but not sham tDCS prolonged and reduced the N2-NoGo potential, indicating that tDCS-induced cerebellar inhibition worsened the ability to allocate attentional resources to stimuli containing hostile information and consequently impaired inhibitory control. Changes in the N2-NoGo potential suggest that the cerebellum contributes to regulating attentional mechanisms of stimulus orienting and inhibitory control both directly by predicting errors or error-related behavior and indirectly by controlling the functioning of the cortical areas involved in signal perception of conflict and the basal ganglia involved in the inhibitory control of movement.
The cerebellar role in executive functioning was also investigated in healthy subjects using repetitive transcranial magnetic stimulation technique during a visual 2-back task commonly used to explore working memory processes [82]. Further, 5 Hz and 20 Hz stimulation on the Crus II region of the cerebellum, respectively, increased N170 amplitude in the prefrontal areas and the P300 amplitude in the prefrontal and parietal sites. Cerebellar excitatory rTMS proved effective in modifying cognitive markers of working memory, bringing further evidence of the cerebellum’s contribution to cognition.
Various tasks using visual stimuli were delivered to musically trained and untrained normal young individuals to investigate the cerebellar involvement in executive functioning [83]. Neuropsychological, psychophysiological and fMRI evaluations proved “not normal” in musically trained young individuals. Specifically, the P3b component to incongruent target stimuli was more posteriorly distributed on the scalp, similarly to the adult P3b response, a finding thought to reflect an early maturity of updating and working memory functions related to target processing. During set-shifting tasks, the fMRI data showed less activity in frontal, parietal and occipital areas of the dorsal attention network and in the cerebellum, indicating that musically trained young individuals have a more efficient recruitment of neural resources after childhood.
4. Cerebellum and Timing Processing
As previously suggested by Purzner et al. [72], the cerebellum is also engaged in timing control of movement preparation. Cerebellar involvement in time perception has its roots in long-established clinical observations that motor coordination can be severely disrupted by cerebellar injury [84]. With the loss of precise timing information, motor acts and internal cognitive processes may no longer be appropriately selected and sequenced at a fine level. Thus, motorically, individuals may become less coordinated, and, cognitively, they may exhibit the so-called “dysmetria of thought” with problems in task shifting and other forms of executive cognitive control. The cerebellum is considered a critical substrate for the perceptual timing of single intervals [13] with a specific role in the discrimination of sub-second time range that is a more automatic system closely linked with motor circuits [85,86].
A hierarchical set of timing tasks has been created to examine the cerebellar role in perceptual timing. Specifically, two different levels of time measures were individuated: a more basic one related to the duration discrimination of single stimuli or intervals and a more complex one related to the discrimination of the rhythmic patterns of a temporal representation.
Few ERP studies investigated the cerebellar role in time control. An auditory mismatch paradigm was analyzed in patients with bilateral cerebellar degeneration to evaluate sensory prediction of temporal regularity [87]. Patients had a MMN of normal amplitude but prolonged latency for stimulus duration deviants alone, but not for pitch and location deviants. This finding reflects an impairment at the early stage of auditory processing (100–200 ms), which is the automatic phase of cognitive processing for temporal estimation, and provides support for a cerebellar contribution to the automatic, pre-attentive duration estimation of the stimuli.
A later study provided evidence that the voluntary processing of the temporal structure of events can also be influenced by the cerebellum [88]. During a P300 auditory oddball paradigm, cerebellar patients and healthy controls displayed a normally increased N2b response to deviant tones regardless of the temporal context. However, whereas healthy controls expectedly enhanced the P3b response to deviant tones in temporally regular sequences, patients unexpectedly decreased the response. These results indicate that structural damage to the cerebellum affects the predictive adaptation to the temporal structure of events and the updating of a mental model of the environment under voluntary attention. In support of this view, by combining magnetoencephalographic (MEG) and EEG recordings in normal subjects during the performance of intermittent electrical stimulation of the finger with random stimulus omissions, the violations of temporal expectancies in the somatosensory domain produced a localized physiological signal to the cerebellum [89].
Most recently, to explore how the cerebellum estimates the duration of time intervals, a CNV paradigm elicited by S1-S2 motor tasks was analyzed in healthy subjects before and after cathodal and sham cerebellar tDCS (Figure 3) [90]. The CNV task consisted of a duration discrimination task in which subjects had to determine whether the duration of a probe interval trial was shorter (800 ms), longer (1600 ms) or equal to the target interval of 1200 ms. CNV amplitude decreased only after cathodal tDCS for short and target interval trials, but not for the long interval trials, suggesting that cerebellar inhibition induced by tDCS impaired the perception of short timing intervals. These data point toward a selective involvement of the cerebellum in second and sub-second timing control, as is also shown by ERP findings in rat studies [91,92].
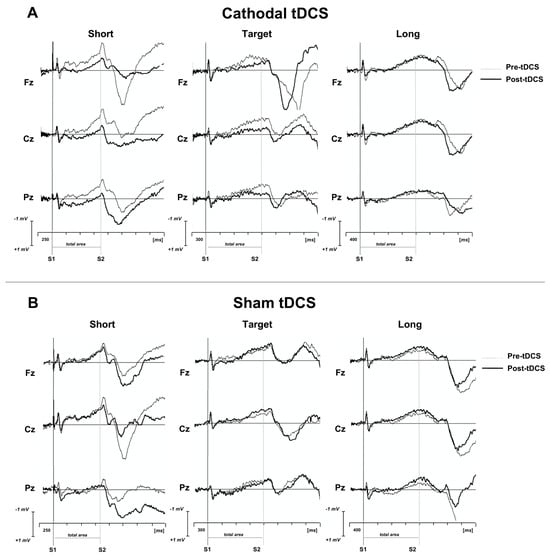
Figure 3.
Grand average CNV traces superimposed for pre- and post-tDCS for both (A) cathodal and (B) sham conditions for short, target and long time intervals. Short trial was 800 ms; target trial was 1200 ms; long trial was 1600 ms. S1: warning auditory stimulus (frequency: 1000 Hz; duration: 50 ms; intensity: 60 dB); S2: imperative auditory stimulus (frequency: 1500 Hz; duration: 50 ms; intensity: 60 dB). (from Mannarelli et al., 2023) [90].
5. Limitations and Future Directions
We have presented psychophysiological evidence from ERP studies on the cerebellum’s contribution to attention, executive functions and timing. The ERP technique has some limitations. First, ERP components may vary inter-individually in amplitude, due to differences in cortical folding patterns, or skull thickness [93]. The cognitive processes that ERPs explore are complex in nature, and more than one function at a time could be engaged during many tasks. Therefore, the psychophysiological components may be evoked by an overlap of various functions rather than a selective process [94], and the cerebellum’s contribution to a specific cognitive function may be difficult to unravel. As opposed to the high temporal resolution, the scarce spatial resolution offered by ERPs may be considered detrimental; however, analysis algorithms, such as LORETA, provide reliable information regarding the sources of activity.
Some of the studies reviewed were conducted on small-sized samples (Table 1), thus raising possible issues of statistical power. However, this was unavoidable for case reports and studies in patients with rare neurological conditions. The groups studied, the tasks performed, the sensory modalities of delivered stimuli, the recording techniques and the analysis procedures differed between studies; thus, the conclusions are not easily comparable. On the other hand, across the many small differences found using the research methods, the role of the cerebellum in the investigated cognitive process emerges anyway. Thus, further studies are needed to confirm the reviewed findings that include larger population samples and more standardized protocols.

Table 1.
Summary of the studies investigating the cerebellar contribution to attention, executive functions and timing using ERPs.
However, using ERPs as a research tool has numerous advantages. ERPs are non-invasive, making them safe and well tolerated by participants. They are relatively low cost compared to neuroimaging techniques; they have lower technological requirements than other research methods, which makes them accessible in various clinical and investigational contexts. Overall, ERPs provide a valuable and versatile tool for studying cognitive neural processing in the brain, possibly serving as a biomarker of brain dysfunctions not detected by traditional clinical tools [93].
The combination of ERP techniques with NIBS has proven to be extremely useful for testing specific cognitive functions in healthy subjects and for monitoring therapeutic benefit in neurological conditions. By inducing transient changes in local neural networks, NIBS can help distinguish the contribution of brain structures to individual stages of cognitive processes, as reflected by ERP components [95]. Finally, ERPs are particularly well suited to monitor the longer-lasting effect of stimulation and to test whether this effect may be beneficial in neurological conditions, including cerebellar involvement.
6. Conclusions
The psychophysiological evidence reviewed here indicates that ERPs detect the contribution of the cerebellum to cognition. The cerebellum coordinates the correct development of cognitive processes by regulating highly organized cerebro-cerebellar neural networks, and cerebellar dysfunction impairs cognitive processes such as attention, executive functions and timing.
Attention-related ERP components, especially P3, are reduced in neurological conditions characterized by cerebellar damage (including stroke, SCA, ADHD), and in subjects with virtual cerebellar lesions induced by NIBS. Executive functions-related ERPs have prolonged latency (for instance, P3, NoGo GFPm, NoGo N2) following cerebellar damage. Timing-related ERPs reveal that both degenerative and virtual cerebellar lesions prolong MMN latency or reduce CNV amplitude.
Despite their agreement in identifying psychophysiological abnormalities, ERP studies exploring the contribution of the cerebellum to cognition are scarce. This highlights the need for further research to confirm the reviewed findings, to gain a deeper understanding of the cerebellar cognitive mechanism and to explore the potential diagnostic and therapeutic implications of ERP-based assessments in patients with cerebellar disorders.
Author Contributions
Conceptualization, D.M., C.P., F.F. and A.C.; methodology, F.F.; investigation and resources, D.M. and C.P.; writing—original draft preparation, D.M. and C.P.; writing—review and editing, P.M., C.T., F.C. and A.C.; supervision, F.F. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Holmes, G.M. Clinical Symptoms of Cerebellar Disease and Their Interpretation. Lancet 1922, 2, 59–65. [Google Scholar]
- Ito, M. Control of Mental Activities by Internal Models in the Cerebellum. Nat. Rev. Neurosci. 2008, 9, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, C.J.; Schmahmann, J.D. Functional Topography in the Human Cerebellum: A Meta-Analysis of Neuroimaging Studies. Neuroimage 2009, 44, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D. The Role of the Cerebellum in Cognition and Emotion: Personal Reflections since 1982 on the Dysmetria of Thought Hypothesis, and Its Historical Evolution from Theory to Therapy. Neuropsychol. Rev. 2010, 20, 236–260. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, C.J.; Valera, E.M.; Schmahmann, J.D. Functional Topography of the Cerebellum for Motor and Cognitive Tasks: An FMRI Study. Neuroimage 2012, 59, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.; Buxton, R.B.; Wong, E.C.; Courchesne, E. Attentional Activation of the Cerebellum Independent of Motor Involvement. Science 1997, 275, 1940–1943. [Google Scholar] [CrossRef] [PubMed]
- Le, T.H.; Pardo, J.V.; Hu, X. 4 T-FMRI Study of Nonspatial Shifting of Selective Attention: Cerebellar and Parietal Contributions. J. Neurophysiol. 1998, 79, 1535–1548. [Google Scholar] [CrossRef]
- Schweizer, T.A.; Alexander, M.P.; Cusimano, M.; Stuss, D.T. Fast and Efficient Visuotemporal Attention Requires the Cerebellum. Neuropsychologia 2007, 45, 3068–3074. [Google Scholar] [CrossRef]
- Striemer, C.L.; Cantelmi, D.; Cusimano, M.D.; Danckert, J.A.; Schweizer, T.A. Deficits in Reflexive Covert Attention Following Cerebellar Injury. Front. Hum. Neurosci. 2015, 9, 428. [Google Scholar] [CrossRef]
- Gottwald, B.; Mihajlovic, Z.; Wilde, B.; Mehdorn, H.M. Does the Cerebellum Contribute to Specific Aspects of Attention? Neuropsychologia 2003, 41, 1452–1460. [Google Scholar] [CrossRef]
- Exner, C.; Weniger, G.; Irle, E. Cerebellar Lesions in the PICA but Not SCA Territory Impair Cognition. Neurology 2004, 63, 2132–2135. [Google Scholar] [CrossRef] [PubMed]
- Baillieux, H.; De Smet, H.J.; Paquier, P.F.; De Deyn, P.P.; Mariën, P. Cerebellar Neurocognition: Insights into the Bottom of the Brain. Clin. Neurol. Neurosurg. 2008, 110, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.M.C.; Ivry, R.B. Cerebellum and Timing. In Handbook of the Cerebellum and Cerebellar Disorders; Springer: Dordrecht, The Netherlands, 2013; pp. 1201–1219. [Google Scholar]
- Petersen, S.E.; Fox, P.T.; Posner, M.I.; Mintun, M.; Raichle, M.E. Positron Emission Tomographic Studies of the Processing of Singe Words. J. Cogn. Neurosci. 1989, 1, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Fiez, J.A.; Petersen, S.E.; Cheney, M.K.; Raichle, M.E. Impaired Non-Motor Learning and Error Detection Associated with Cerebellar Damage. Brain 1992, 115, 155–178. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D.; Sherman, J.C. The Cerebellar Cognitive Affective Syndrome. Brain 1998, 121 Pt 4, 561–579. [Google Scholar] [CrossRef]
- Brodal, P. The Pontocerebellar Projection in the Rhesus Monkey: An Experimental Study with Retrograde Axonal Transport of Horseradish Peroxidase. Neuroscience 1979, 4, 193–208. [Google Scholar] [CrossRef]
- Strick, P.L.; Dum, R.P.; Fiez, J.A. Cerebellum and Nonmotor Function. Annu. Rev. Neurosci. 2009, 32, 413–434. [Google Scholar] [CrossRef]
- Schmahmann, J.D. The Cerebellum and Cognition. Neurosci. Lett. 2019, 688, 62–75. [Google Scholar] [CrossRef]
- Dum, R.P.; Strick, P.L. An Unfolded Map of the Cerebellar Dentate Nucleus and Its Projections to the Cerebral Cortex. J. Neurophysiol. 2003, 89, 634–639. [Google Scholar] [CrossRef]
- Brodal, P. The Corticopontine Projection in the Rhesus Monkey Origin and Principles of Organization. Brain 1978, 101, 251–283. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Pandya, D.N. Projections to the Basis Pontis from the Superior Temporal Sulcus and Superior Temporal Region in the Rhesus Monkey. J. Comp. Neurol. 1991, 308, 224–248. [Google Scholar] [CrossRef] [PubMed]
- Glickstein, M.; Gerrits, N.; Kralj-Hans, I.; Mercier, B.; Stein, J.; Voogd, J. Visual Pontocerebellar Projections in the Macaque. J. Comp. Neurol. 1994, 349, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D.; Weilburg, J.B.; Sherman, J.C. The Neuropsychiatry of the Cerebellum—Insights from the Clinic. Cerebellum 2007, 6, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.G.; Harper, J.W. Ascending Projections of the Cerebellar Fastigial Nucleus to the Hippocampus, Amygdala, and Other Temporal Lobe Sites: Evoked Potential and Histological Studies in Monkeys and Cats. Exp. Neurol. 1974, 45, 268–287. [Google Scholar] [CrossRef] [PubMed]
- Grodd, W.; Hülsmann, E.; Lotze, M.; Wildgruber, D.; Erb, M. Sensorimotor Mapping of the Human Cerebellum: FMRI Evidence of Somatotopic Organization. Hum. Brain Mapp. 2001, 13, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Rijntjes, M.; Buechel, C.; Kiebel, S.; Weiller, C. Multiple Somatotopic Representations in the Human Cerebellum. Neuroreport 1999, 10, 3653–3658. [Google Scholar] [CrossRef]
- Nitschke, M.F.; Kleinschmidt, A.; Wessel, K.; Frahm, J. Somatotopic Motor Representation in the Human Anterior Cerebellum. A High-Resolution Functional MRI Study. Brain 1996, 119 Pt 3, 1023–1029. [Google Scholar] [CrossRef]
- Habas, C.; Kamdar, N.; Nguyen, D.; Prater, K.; Beckmann, C.F.; Menon, V.; Greicius, M.D. Distinct Cerebellar Contributions to Intrinsic Connectivity Networks. J. Neurosci. 2009, 29, 8586–8594. [Google Scholar] [CrossRef]
- Buckner, R.L.; Krienen, F.M.; Castellanos, A.; Diaz, J.C.; Thomas Yeo, B.T. The Organization of the Human Cerebellum Estimated by Intrinsic Functional Connectivity. J. Neurophysiol. 2011, 106, 2322–2345. [Google Scholar] [CrossRef]
- O’Reilly, J.X.; Beckmann, C.F.; Tomassini, V.; Ramnani, N.; Johansen-Berg, H. Distinct and Overlapping Functional Zones in the Cerebellum Defined by Resting State Functional Connectivity. Cereb. Cortex 2010, 20, 953–965. [Google Scholar] [CrossRef]
- Dobromyslin, V.I.; Salat, D.H.; Fortier, C.B.; Leritz, E.C.; Beckmann, C.F.; Milberg, W.P.; McGlinchey, R.E. Distinct Functional Networks within the Cerebellum and Their Relation to Cortical Systems Assessed with Independent Component Analysis. Neuroimage 2012, 60, 2073–2085. [Google Scholar] [CrossRef] [PubMed]
- Ivry, R.; Fiez, J.C.N. Cerebellar Contributions to Cognition and Imagery. In The Cognitive Neurosciences; MIT Press: Cambridge, MA, USA, 2000; pp. 999–1011. [Google Scholar]
- Leiner, H.C.; Leiner, A.L.; Dow, R.S. Cognitive and Language Functions of the Human Cerebellum. Trends Neurosci. 1993, 16, 444–447. [Google Scholar] [CrossRef]
- Raichle, M.E.; Fiez, J.A.; Videen, T.O.; MacLeod, A.-M.K.; Pardo, J.V.; Fox, P.T.; Petersen, S.E. Practice-Related Changes in Human Brain Functional Anatomy during Nonmotor Learning. Cereb. Cortex 1994, 4, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H.; Wildgruber, D.; Daum, I.; Grodd, W. Does the Cerebellum Contribute to Cognitive Aspects of Speech Production? A Functional Magnetic Resonance Imaging (FMRI) Study in Humans. Neurosci. Lett. 1998, 247, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.M.; Strick, P.L. Cerebellar Loops with Motor Cortex and Prefrontal Cortex of a Nonhuman Primate. J. Neurosci. 2003, 23, 8432–8444. [Google Scholar] [CrossRef] [PubMed]
- Galea, J.M.; Jayaram, G.; Ajagbe, L.; Celnik, P. Modulation of Cerebellar Excitability by Polarity-Specific Noninvasive Direct Current Stimulation. J. Neurosci. 2009, 29, 9115–9122. [Google Scholar] [CrossRef] [PubMed]
- Middleton, F.A.; Strick, P.L. Cerebellar Projections to the Prefrontal Cortex of the Primate. J. Neurosci. 2001, 21, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Middleton, F.A.; Strick, P.L. Cerebellar Output: Motor and Cognitive Channels. Trends Cogn. Sci. 1998, 2, 348–354. [Google Scholar] [CrossRef]
- Kappenman, E.S.; Luck, S.J. ERP Components: The Ups and Downs of Brainwave Recordings; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Woodman, G.F. A Brief Introduction to the use of Event-related potentials (ERPs) in studies of perception and attention. Atten. Percept. Psychophys. 2010, 72, 2031–2046. [Google Scholar] [CrossRef]
- Bao, H.; Xie, M.; Huang, Y.; Liu, Y.; Lan, C.; Lin, Z.; Wang, Y.; Qin, P. Specificity in the Processing of a Subject’s Own Name. Soc. Cogn. Affect. Neurosci. 2023, 18, nsad066. [Google Scholar] [CrossRef]
- Pauletti, C.; Mannarelli, D.; Grippo, A.; Currà, A.; Locuratolo, N.; De Lucia, M.C.; Fattapposta, F. Phasic Alertness in a Cued Double-Choice Reaction Time Task: A Contingent Negative Variation (CNV) Study. Neurosci. Lett. 2014, 581, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Proverbio, A.M.; Tacchini, M.; Jiang, K. What Do You Have in Mind? ERP Markers of Visual and Auditory Imagery. Brain Cogn. 2023, 166, 105954. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.C.; Barry, R.J.; Connolly, J.F.; Fischer, C.; Michie, P.T.; Näätänen, R.; Polich, J.; Reinvang, I.; Van Petten, C. Event-Related Potentials in Clinical Research: Guidelines for Eliciting, Recording, and Quantifying Mismatch Negativity, P300, and N400. Clin. Neurophysiol. 2009, 120, 1883–1908. [Google Scholar] [CrossRef] [PubMed]
- Paulus, K.S.; Magnano, I.; Conti, M.; Galistu, P.; D’Onofrio, M.; Satta, W.; Aiello, I. Pure Post–Stroke Cerebellar Cognitive Affective Syndrome: A Case Report. Neurol. Sci. 2004, 25, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Knolle, F.; Schröger, E.; Kotz, S.A. Cerebellar Contribution to the Prediction of Self-Initiated Sounds. Cortex 2013, 49, 2449–2461. [Google Scholar] [CrossRef] [PubMed]
- Mannarelli, D.; Pauletti, C.; De Lucia, M.C.; Currà, A.; Fattapposta, F. Insights from ERPs into Attention during Recovery after Cerebellar Stroke: A Case Report. Neurocase 2015, 21, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Adamaszek, M.; Olbrich, S.; Kirkby, K.C.; Woldag, H.; Willert, C.; Heinrich, A. Event-Related Potentials Indicating Impaired Emotional Attention in Cerebellar Stroke-a Case Study. Neurosci. Lett. 2013, 548, 206–211. [Google Scholar] [CrossRef]
- Tunc, S.; Baginski, N.; Lubs, J.; Bally, J.F.; Weissbach, A.; Baaske, M.K.; Tadic, V.; Brüggemann, N.; Bäumer, T.; Beste, C.; et al. Predictive Coding and Adaptive Behavior in Patients with Genetically Determined Cerebellar Ataxia-A Neurophysiology Study. Neuroimage Clin. 2019, 24, 102043. [Google Scholar] [CrossRef]
- Kremlacek, J.; Valis, M.; Masopust, J.; Urban, A.; Zumrova, A.; Talab, R.; Kuba, M.; Kubova, Z.; Langrova, J. An Electrophysiological Study of Visual Processing in Spinocerebellar Ataxia Type 2 (SCA2). Cerebellum 2011, 10, 32–42. [Google Scholar] [CrossRef]
- Rodríguez-Labrada, R.; Velázquez-Pérez, L.; Ortega-Sánchez, R.; Peña-Acosta, A.; Vázquez-Mojena, Y.; Canales-Ochoa, N.; Medrano-Montero, J.; Torres-Vega, R.; González-Zaldivar, Y. Insights into Cognitive Decline in Spinocerebellar Ataxia Type 2: A P300 Event-Related Brain Potential Study. Cerebellum Ataxias 2019, 6, 3. [Google Scholar] [CrossRef]
- Houston, J.R.; Hughes, M.L.; Lien, M.C.; Martin, B.A.; Loth, F.; Luciano, M.G.; Vorster, S.; Allen, P.A. An Electrophysiological Study of Cognitive and Emotion Processing in Type I Chiari Malformation. Cerebellum 2018, 17, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Krain, A.L.; Castellanos, F.X. Brain Development and ADHD. Clin. Psychol. Rev. 2006, 26, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, C.J. Distinct Regions of the Cerebellum Show Gray Matter Decreases in Autism, ADHD, and Developmental Dyslexia. Front. Syst. Neurosci. 2014, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Bruchhage, M.M.K.; Bucci, M.P.; Becker, E.B.E. Cerebellar Involvement in Autism and ADHD. Handb. Clin. Neurol. 2018, 155, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J.; Agid, Y.; Brüne, M.; Bullmore, E.T.; Carter, C.S.; Clayton, N.S.; Connor, R.; Davis, S.; Deakin, B.; DeRubeis, R.J.; et al. Cognitive Dysfunction in Psychiatric Disorders: Characteristics, Causes and the Quest for Improved Therapy. Nat. Rev. Drug Discov. 2012, 11, 141–168. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, C.; Pham, E.; Kumar, S.; Piguet, C.; Deiber, M.P.; Aubry, J.M.; Dayer, A.; Michel, C.M.; Perroud, N.; Berchio, C. Dysfunctional Temporal Stages of Eye-Gaze Perception in Adults with ADHD: A High-Density EEG Study. Biol. Psychol. 2022, 171, 108351. [Google Scholar] [CrossRef]
- Adamaszek, M.; Kirkby, K.C.; Dagata, F.; Olbrich, S.; Langner, S.; Steele, C.; Sehm, B.; Busse, S.; Kessler, C.; Hamm, A. Neural Correlates of Impaired Emotional Face Recognition in Cerebellar Lesions. Brain Res. 2015, 1613, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gerschcovich, E.R.; Cerquetti, D.; Tenca, E.; Leiguarda, R. The Impact of Bilateral Cerebellar Damage on Theory of Mind, Empathy and Decision Making. Neurocase 2011, 17, 270–275. [Google Scholar] [CrossRef]
- Bersani, F.S.; Minichino, A.; Bernabei, L.; Spagnoli, F.; Corrado, A.; Vergnani, L.; Mannarelli, D.; Pauletti, C.; Fattapposta, F.; Biondi, M.; et al. Prefronto-Cerebellar TDCS Enhances Neurocognition in Euthymic Bipolar Patients. Findings from a Placebo-Controlled Neuropsychological and Psychophysiological Investigation. J. Affect. Disord. 2017, 209, 262–269. [Google Scholar] [CrossRef]
- Ruggiero, F.; Ferrucci, R.; Bocci, T.; Nigro, M.; Vergari, M.; Marceglia, S.; Barbieri, S.; Priori, A. Spino-Cerebellar TDCS Modulates N100 Components of the P300 Event Related Potential. Neuropsychologia 2019, 135, 107231. [Google Scholar] [CrossRef]
- Andrew, D.; Ibey, R.J.; Staines, W.R. Transient Inhibition of the Cerebellum Impairs Change-Detection Processes: Cerebellar Contributions to Sensorimotor Integration. Behav. Brain Res. 2020, 378, 112273. [Google Scholar] [CrossRef] [PubMed]
- Mannarelli, D.; Pauletti, C.; de Lucia, M.C.; Delle Chiaie, R.; Bersani, F.S.; Spagnoli, F.; Minichino, A.; Currà, A.; Trompetto, C.; Fattapposta, F. Effects of Cerebellar Transcranial Direct Current Stimulation on Attentional Processing of the Stimulus: Evidence from an Event-Related Potentials Study. Neuropsychologia 2016, 84, 127–135. [Google Scholar] [CrossRef]
- Mannarelli, D.; Pauletti, C.; Currà, A.; Marinelli, L.; Corrado, A.; Delle Chiaie, R.; Fattapposta, F. The Cerebellum Modulates Attention Network Functioning: Evidence from a Cerebellar Transcranial Direct Current Stimulation and Attention Network Test Study. Cerebellum 2019, 18, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Rusiniak, M.; Lewandowska, M.; Wolak, T.; Pluta, A.; Milner, R.; Ganc, M.; Włodarczyk, A.; Senderski, A.; Śliwa, L.; Skarżyński, H. A Modified Oddball Paradigm for Investigation of Neural Correlates of Attention: A Simultaneous ERP-FMRI Study. Magn. Reson. Mater. Phys. Biol. Med. 2013, 26, 511–526. [Google Scholar] [CrossRef]
- Chen, P.; Lin, J.; Chen, B.; Lu, C.; Guo, T. Processing Emotional Words in Two Languages with One Brain: ERP and FMRI Evidence from Chinese-English Bilinguals. Cortex 2015, 71, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Logan, G.D. Executive Control of Thought and Action. Acta Psychol. 1985, 60, 193–210. [Google Scholar] [CrossRef]
- Schmahmann, J.D. The Cerebellum and Cognition; Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Heyder, K.; Suchan, B.; Daum, I. Cortico-Subcortical Contributions to Executive Control. Acta Psychol. 2004, 115, 271–289. [Google Scholar] [CrossRef]
- Purzner, J.; Paradiso, G.O.; Cunic, D.; Saint-Cyr, J.A.; Hoque, T.; Lozano, A.M.; Lang, A.E.; Moro, E.; Hodaie, M.; Mazzella, F.; et al. Involvement of the Basal Ganglia and Cerebellar Motor Pathways in the Preparation of Self-Initiated and Externally Triggered Movements in Humans. J. Neurosci. 2007, 27, 6029–6036. [Google Scholar] [CrossRef]
- Brunia, C.H.M.; van Boxtel, G.J.M. Wait and See. Int. J. Psychophysiol. 2001, 43, 59–75. [Google Scholar] [CrossRef]
- Currà, A.; Berardelli, A.; Agostino, R.; Modugno, N.; Puorger, C.C.; Accornero, N.; Manfredi, M. Performance of Sequential Arm Movements with and without Advance Knowledge of Motor Pathways in Parkinson’s Disease. Mov. Disord. 1997, 12, 646–654. [Google Scholar] [CrossRef]
- Bauer, L.O. Differential Effects of Alcohol, Cocaine, and Opioid Abuse on Event-Related Potentials Recorded during a Response Competition Task. Drug Alcohol. Depend. 2002, 66, 137–145. [Google Scholar] [CrossRef]
- Halgren, E.; Marinkovic, K.; Chauvel, P. Generators of the Late Cognitive Potentials in Auditory and Visual Oddball Tasks. Electroencephalogr. Clin. Neurophysiol. 1998, 106, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Polich, J.; Criado, J.R. Neuropsychology and Neuropharmacology of P3a and P3b. Int. J. Psychophysiol. 2006, 60, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Paitel, E.R.; Nielson, K.A. Cerebellar EEG Source Localization Reveals Age-related Compensatory Activity Moderated by Genetic Risk for Alzheimer’s Disease. Psychophysiology 2023, 60, e14395. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Harada, M.; Arai, M.; Hirata, K. Cognitive Dysfunction in Cortical Cerebellar Atrophy Correlates with Impairment of the Inhibitory System. Neuropsychobiology 2003, 47, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Zarka, D.; Cebolla, A.M.; Cevallos, C.; Palmero-Soler, E.; Dan, B.; Cheron, G. Caudate and Cerebellar Involvement in Altered P2 and P3 Components of GO/NoGO Evoked Potentials in Children with Attention-deficit/Hyperactivity Disorder. Eur. J. Neurosci. 2021, 53, 3447–3462. [Google Scholar] [CrossRef] [PubMed]
- Mannarelli, D.; Pauletti, C.; Petritis, A.; Delle Chiaie, R.; Currà, A.; Trompetto, C.; Fattapposta, F. Effects of Cerebellar TDCS on Inhibitory Control: Evidence from a Go/NoGo Task. Cerebellum 2020, 19, 788–798. [Google Scholar] [CrossRef]
- Yao, J.; Song, B.; Shi, J.; Yin, K.; Du, W. Effects of Repetitive Transcranial Magnetic Stimulation at the Cerebellum on Working Memory. Brain Sci. 2023, 13, 1158. [Google Scholar] [CrossRef]
- Saarikivi, K.; Chan, T.M.V.; Huotilainen, M.; Tervaniemi, M.; Putkinen, V. Enhanced Neural Mechanisms of Set Shifting in Musically Trained Adolescents and Young Adults: Converging FMRI, EEG, and Behavioral Evidence. Cereb. Cortex 2023, 33, 7237–7249. [Google Scholar] [CrossRef]
- Holmes, G. The Cerebellum of Man. Brain 1939, 62, 1–30. [Google Scholar] [CrossRef]
- Mauk, M.D.; Buonomano, D.V. The Neural Basis of Temporal Processing. Annu. Rev. Neurosci. 2004, 27, 307–340. [Google Scholar] [CrossRef] [PubMed]
- Hore, J.; Wild, B.; Diener, H.C. Cerebellar Dysmetria at the Elbow, Wrist, and Fingers. J. Neurophysiol. 1991, 65, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Moberget, T.; Karns, C.M.; Deouell, L.Y.; Lindgren, M.; Knight, R.T.; Ivry, R.B. Detecting Violations of Sensory Expectancies Following Cerebellar Degeneration: A Mismatch Negativity Study. Neuropsychologia 2008, 46, 2569–2579. [Google Scholar] [CrossRef] [PubMed]
- Kotz, S.A.; Stockert, A.; Schwartze, M. Cerebellum, Temporal Predictability and the Updating of a Mental Model. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130403. [Google Scholar] [CrossRef] [PubMed]
- Tesche, C.D.; Karhu, J.J. Anticipatory Cerebellar Responses during Somatosensory Omission in Man. Hum. Brain Mapp. 2000, 9, 119–142. [Google Scholar] [CrossRef]
- Mannarelli, D.; Pauletti, C.; Petritis, A.; Maffucci, A.; Currà, A.; Trompetto, C.; Marinelli, L.; Fattapposta, F. The Role of Cerebellum in Timing Processing: A Contingent Negative Variation Study. Neurosci. Lett. 2023, 808, 137301. [Google Scholar] [CrossRef]
- Onoda, K.; Takahashi, E.; Sakata, S. Event-Related Potentials in the Frontal Cortex, Hippocampus, and Cerebellum during a Temporal Discrimination Task in Rats. Cogn. Brain Res. 2003, 17, 380–387. [Google Scholar] [CrossRef]
- Onoda, K.; Sakata, S. An ERP Study of Temporal Discrimination in Rats. Behav. Process. 2006, 71, 235–240. [Google Scholar] [CrossRef]
- Luck, S.J. An Introduction to the Event-Related Potential Technique, 2nd ed.; The MIT Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Voss, J.L.; Paller, K.A. Neural Substrates of Remembering—Electroencephalographic Studies. In Learning and Memory: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2008; pp. 79–97. [Google Scholar]
- Mannarelli, D.; Pauletti, C.; Grippo, A.; Amantini, A.; Augugliaro, V.; Currà, A.; Missori, P.; Locuratolo, N.; De Lucia, M.C.; Rinalduzzi, S.; et al. The Role of the Right Dorsolateral Prefrontal Cortex in Phasic Alertness: Evidence from a Contingent Negative Variation and Repetitive Transcranial Magnetic Stimulation Study. Neural Plast. 2015, 2015, 137301. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).