Eyes-Open and Eyes-Closed Resting State Network Connectivity Differences
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset 1 Berlin
2.1.1. Participants
2.1.2. MRI Data Acquisition
2.2. Dataset 2 New Taipei City
2.2.1. Participants
2.2.2. MRI Data Acquisition
2.2.3. Data Preprocessing
2.2.4. Functional Connectivity Analysis
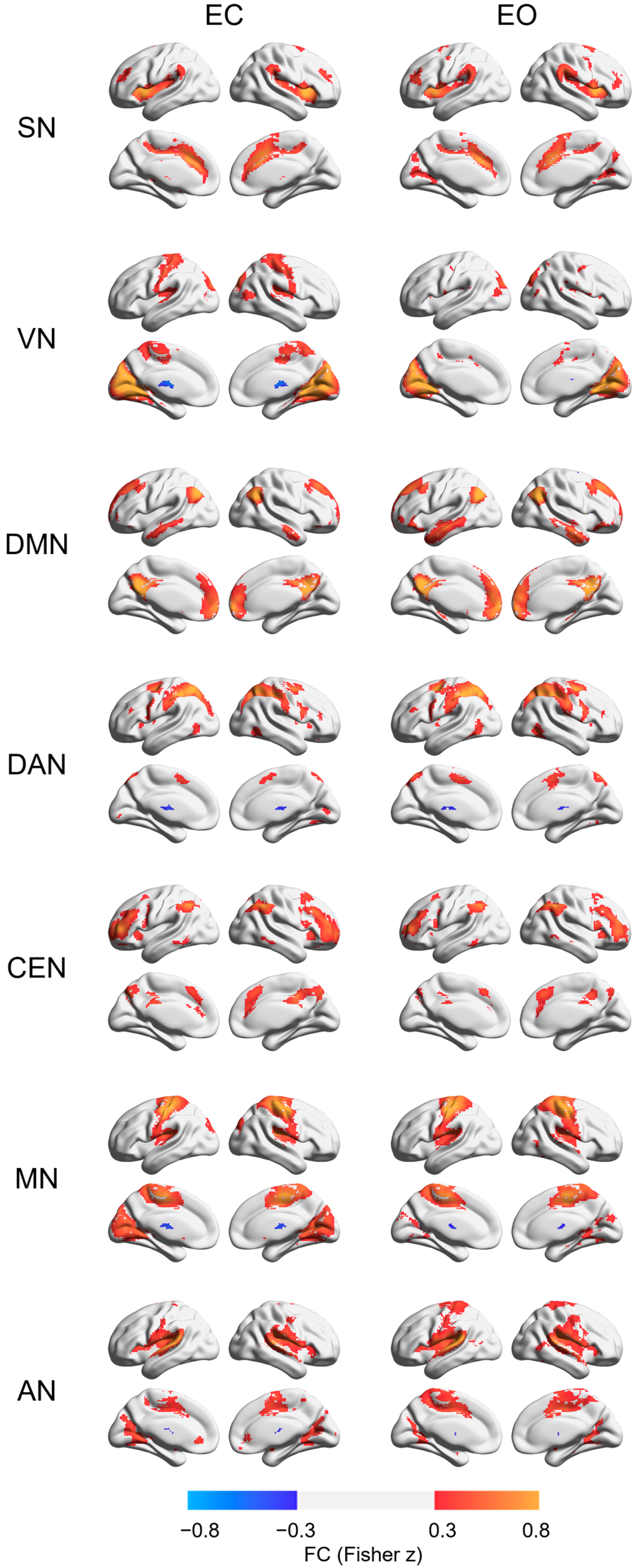
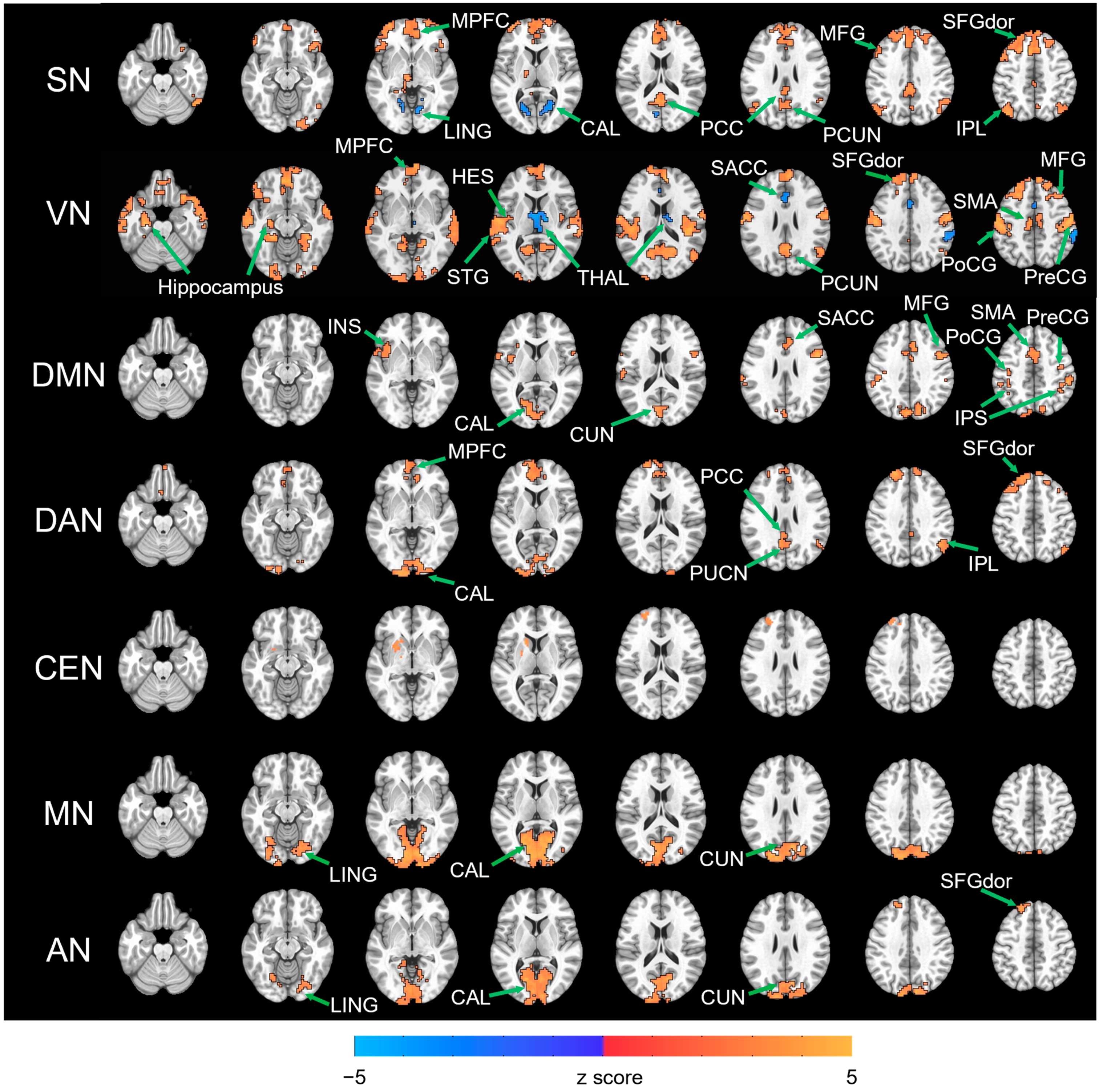
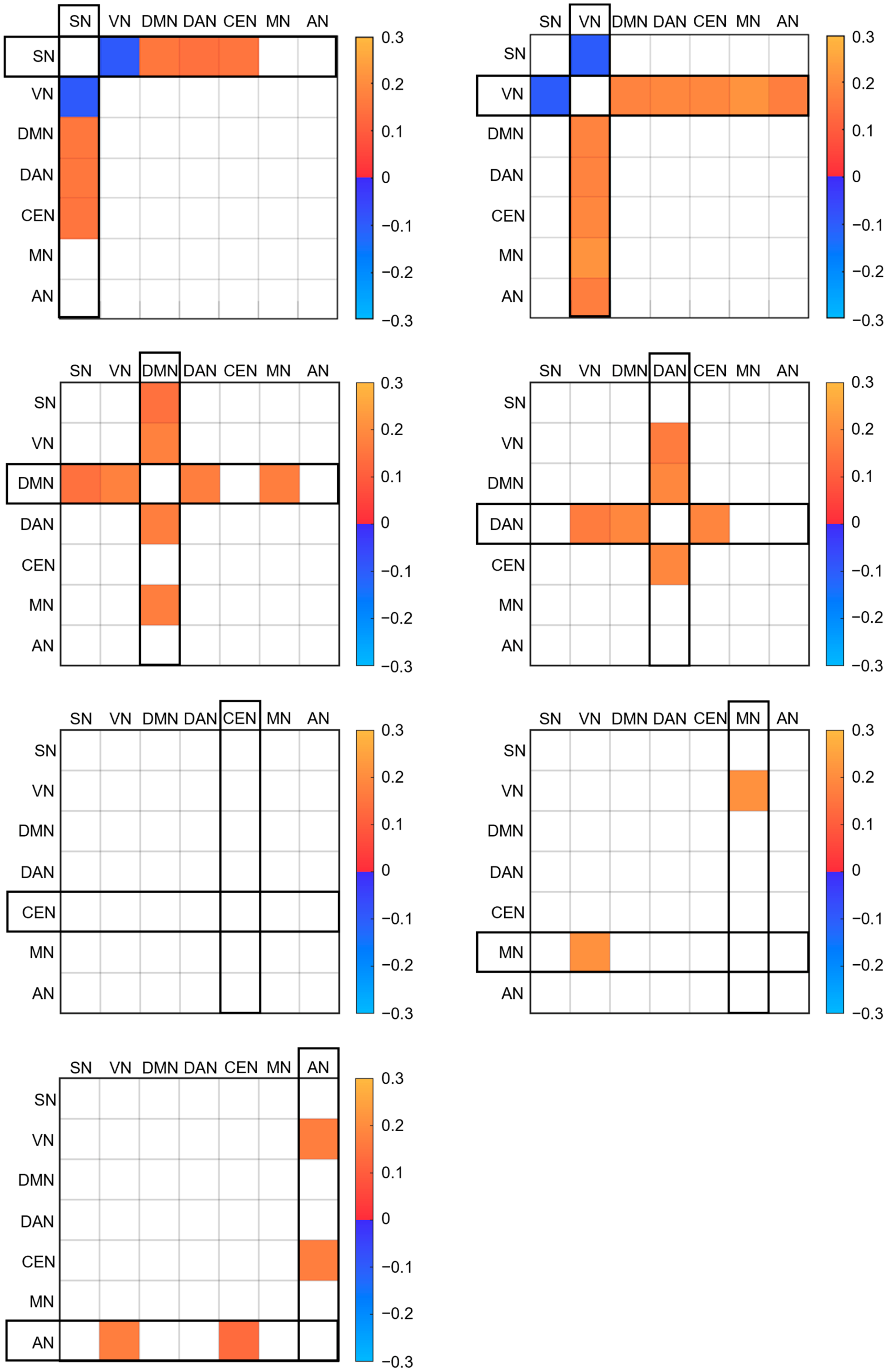
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Raichle, M.E. The Restless Brain: How Intrinsic Activity Organizes Brain Function. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140172. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, J.; Schooler, J.W. The Science of Mind Wandering: Empirically Navigating the Stream of Consciousness. Annu. Rev. Psychol. 2015, 66, 487–518. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J.S.; Rombouts, S.A.R.B.; Barkhof, F.; Scheltens, P.; Stam, C.J.; Smith, S.M.; Beckmann, C.F. Consistent Resting-State Networks across Healthy Subjects. Proc. Natl. Acad. Sci. USA 2006, 103, 13848–13853. [Google Scholar] [CrossRef] [PubMed]
- Agcaoglu, O.; Wilson, T.W.; Wang, Y.; Stephen, J.; Calhoun, V.D. Resting State Connectivity Differences in Eyes Open versus Eyes Closed Conditions. Hum. Brain Mapp. 2019, 40, 2488–2498. [Google Scholar] [CrossRef]
- McAvoy, M.; Larson-Prior, L.; Nolan, T.S.; Vaishnavi, S.N.; Raichle, M.E.; D’Avossa, G. Resting States Affect Spontaneous BOLD Oscillations in Sensory and Paralimbic Cortex. J. Neurophysiol. 2008, 100, 922–931. [Google Scholar] [CrossRef]
- Wu, L.; Eichele, T.; Calhoun, V.D. Reactivity of Hemodynamic Responses and Functional Connectivity to Different States of Alpha Synchrony: A Concurrent EEG-FMRI Study. NeuroImage 2010, 52, 1252–1260. [Google Scholar] [CrossRef]
- Xu, P.; Huang, R.; Wang, J.; Van Dam, N.T.; Xie, T.; Dong, Z.; Chen, C.; Gu, R.; Zang, Y.F.; He, Y.; et al. Different Topological Organization of Human Brain Functional Networks with Eyes Open versus Eyes Closed. NeuroImage 2014, 90, 246–255. [Google Scholar] [CrossRef]
- Yan, C.; Liu, D.; He, Y.; Zou, Q.; Zhu, C.; Zuo, X.; Long, X.; Zang, Y. Spontaneous Brain Activity in the Default Mode Network Is Sensitive to Different Resting-State Conditions with Limited Cognitive Load. PLoS ONE 2009, 4, e5743. [Google Scholar] [CrossRef]
- Patriat, R.; Molloy, E.K.; Meier, T.B.; Kirk, G.R.; Nair, V.A.; Meyerand, M.E.; Prabhakaran, V.; Birn, R.M. The Effect of Resting Condition on Resting-State FMRI Reliability and Consistency: A Comparison between Resting with Eyes Open, Closed, and Fixated. NeuroImage 2013, 78, 463–473. [Google Scholar] [CrossRef]
- Jao, T.; Vértes, P.E.; Alexander-Bloch, A.F.; Tang, I.N.; Yu, Y.C.; Chen, J.H.; Bullmore, E.T. Volitional Eyes Opening Perturbs Brain Dynamics and Functional Connectivity Regardless of Light Input. NeuroImage 2013, 69, 21–34. [Google Scholar] [CrossRef]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, Switching, Attention and Control: A Network Model of Insula Function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A Default Mode of Brain Function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef]
- Fox, M.D.; Corbetta, M.; Snyder, A.Z.; Vincent, J.L.; Raichle, M.E. Spontaneous Neuronal Activity Distinguishes Human Dorsal and Ventral Attention Systems. Proc. Natl. Acad. Sci. USA 2006, 103, 10046–10051. [Google Scholar] [CrossRef]
- Menon, V. Salience Network. In Brain Mapping; Elsevier: Amsterdam, The Netherlands, 2015; pp. 597–611. ISBN 978-0-12-397316-0. [Google Scholar]
- Qin, P.; Grimm, S.; Duncan, N.W.; Holland, G.; Shen Guo, J.; Fan, Y.; Weigand, A.; Baudewig, J.; Bajbouj, M.; Northoff, G. Self-Specific Stimuli Interact Differently than Non-Self-Specific Stimuli with Eyes-Open versus Eyes-Closed Spontaneous Activity in Auditory Cortex. Front. Hum. Neurosci. 2013, 7, 437. [Google Scholar] [CrossRef]
- Christoff, K.; Irving, Z.C.; Fox, K.C.R.; Spreng, R.N.; Andrews-Hanna, J.R. Mind-Wandering as Spontaneous Thought: A Dynamic Framework. Nat. Rev. Neurosci. 2016, 17, 718–731. [Google Scholar] [CrossRef]
- Qin, P.; Duncan, N.W.; Chen, D.Y.T.; Chen, C.J.; Huang, L.K.; Huang, Z.; Lin, C.Y.E.; Wiebking, C.; Yang, C.M.; Northoff, G.; et al. Vascular-Metabolic and GABAergic Inhibitory Correlates of Neural Variability Modulation. A Combined FMRI and PET Study. Neuroscience 2018, 379, 142–151. [Google Scholar] [CrossRef]
- Cox, R.W. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput. Biomed. Res. 1996, 29, 162–173. [Google Scholar] [CrossRef]
- Qin, P.; Wu, X.; Huang, Z.; Duncan, N.W.; Tang, W.; Wolff, A.; Hu, J.; Gao, L.; Jin, Y.; Wu, X.; et al. How Are Different Neural Networks Related to Consciousness? Ann. Neurol. 2015, 78, 594–605. [Google Scholar] [CrossRef]
- Power, J.D.; Cohen, A.L.; Nelson, S.M.; Wig, G.S.; Barnes, K.A.; Church, J.A.; Vogel, A.C.; Laumann, T.O.; Miezin, F.M.; Schlaggar, B.L.; et al. Functional Network Organization of the Human Brain. Neuron 2011, 72, 665–678. [Google Scholar] [CrossRef]
- Brier, M.R.; Thomas, J.B.; Snyder, A.Z.; Benzinger, T.L.; Zhang, D.; Raichle, M.E.; Holtzman, D.M.; Morris, J.C.; Ances, B.M. Loss of Intranetwork and Internetwork Resting State Functional Connections with Alzheimer’s Disease Progression. J. Neurosci. 2012, 32, 8890–8899. [Google Scholar] [CrossRef]
- Xia, M.; Wang, J.; He, Y. BrainNet Viewer: A Network Visualization Tool for Human Brain Connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef]
- Rolls, E.T.; Huang, C.C.; Lin, C.P.; Feng, J.; Joliot, M. Automated Anatomical Labelling Atlas 3. NeuroImage 2020, 206, 116189. [Google Scholar] [CrossRef]
- Raichle, M.E. The Restless Brain. Brain Connect. 2011, 1, 3–12. [Google Scholar] [CrossRef]
- Tononi, G.; Edelman, G.M.; Sporns, O. Complexity and Coherency: Integrating Information in the Brain. Trends Cogn. Sci. 1998, 2, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Dong, Z.; Zuo, X.; Wang, J.; Zang, Y. Eyes-Open/Eyes-Closed Dataset Sharing for Reproducibility Evaluation of Resting State FMRI Data Analysis Methods. Neuroinformatics 2013, 11, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Marx, E.; Stephan, T.; Nolte, A.; Deutschländer, A.; Seelos, K.C.; Dieterich, M.; Brandt, T. Eye Closure in Darkness Animates Sensory Systems. NeuroImage 2003, 19, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Goulden, N.; Khusnulina, A.; Davis, N.J.; Bracewell, R.M.; Bokde, A.L.; McNulty, J.P.; Mullins, P.G. The Salience Network Is Responsible for Switching between the Default Mode Network and the Central Executive Network: Replication from DCM. NeuroImage 2014, 99, 180–190. [Google Scholar] [CrossRef]
- Sridharan, D.; Levitin, D.J.; Menon, V. A Critical Role for the Right Fronto-Insular Cortex in Switching between Central-Executive and Default-Mode Networks. Proc. Natl. Acad. Sci. USA 2008, 105, 12569–12574. [Google Scholar] [CrossRef]
- Watson, K.K.; Jones, T.K.; Allman, J.M. Dendritic Architecture of the von Economo Neurons. Neuroscience 2006, 141, 1107–1112. [Google Scholar] [CrossRef]
- Allman, J.M.; Watson, K.K.; Tetreault, N.A.; Hakeem, A.Y. Intuition and Autism: A Possible Role for Von Economo Neurons. Trends Cogn. Sci. 2005, 9, 367–373. [Google Scholar] [CrossRef]
- Christoff, K.; Gordon, A.M.; Smallwood, J.; Smith, R.; Schooler, J.W. Experience Sampling during FMRI Reveals Default Network and Executive System Contributions to Mind Wandering. Proc. Natl. Acad. Sci. USA 2009, 106, 8719–8724. [Google Scholar] [CrossRef]
- Mason, M.F.; Norton, M.I.; Van Horn, J.D.; Wegner, D.M.; Grafton, S.T.; Macrae, C.N. Wandering Minds: The Default Network and Stimulus-Independent Thought. Science 2007, 315, 393–395. [Google Scholar] [CrossRef]
- Johnson, S.C.; Baxter, L.C.; Wilder, L.S.; Pipe, J.G.; Heiserman, J.E.; Prigatano, G.P. Neural Correlates of Self-Reflection. Brain 2002, 125, 1808–1814. [Google Scholar] [CrossRef]
- Lane, T.J. The Minimal Self Hypothesis. Conscious. Cogn. 2020, 85, 103029. [Google Scholar] [CrossRef]
- Qin, P.; Northoff, G. How Is Our Self Related to Midline Regions and the Default-Mode Network? NeuroImage 2011, 57, 1221–1233. [Google Scholar] [CrossRef]
- Diana, R.A.; Yonelinas, A.P.; Ranganath, C. Imaging Recollection and Familiarity in the Medial Temporal Lobe: A Three-Component Model. Trends Cogn. Sci. 2007, 11, 379–386. [Google Scholar] [CrossRef]
- Schacter, D.L.; Addis, D.R.; Buckner, R.L. Remembering the Past to Imagine the Future: The Prospective Brain. Nat. Rev. Neurosci. 2007, 8, 657–661. [Google Scholar] [CrossRef]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional Connectivity in the Resting Brain: A Network Analysis of the Default Mode Hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef]
- Balaguer, J.; Spiers, H.; Hassabis, D.; Summerfield, C. Neural Mechanisms of Hierarchical Planning in a Virtual Subway Network. Neuron 2016, 90, 893–903. [Google Scholar] [CrossRef]
- Kumaran, D.; Maguire, E.A. The Human Hippocampus: Cognitive Maps or Relational Memory? J. Neurosci. 2005, 25, 7254–7259. [Google Scholar] [CrossRef]
- Hassabis, D.; Maguire, E.A. Deconstructing Episodic Memory with Construction. Trends Cogn. Sci. 2007, 11, 299–306. [Google Scholar] [CrossRef]
- Rilling, J.K.; Dagenais, J.E.; Goldsmith, D.R.; Glenn, A.L.; Pagnoni, G. Social Cognitive Neural Networks during In-Group and out-Group Interactions. NeuroImage 2008, 41, 1447–1461. [Google Scholar] [CrossRef]
- Sidlauskaite, J.; Wiersema, J.R.; Roeyers, H.; Krebs, R.M.; Vassena, E.; Fias, W.; Brass, M.; Achten, E.; Sonuga-Barke, E. Anticipatory Processes in Brain State Switching-Evidence from a Novel Cued-Switching Task Implicating Default Mode and Salience Networks. NeuroImage 2014, 98, 359–365. [Google Scholar] [CrossRef]
- Han, J.; Wu, X.; Wu, H.; Wang, D.; She, X.; Xie, M.; Zhang, F.; Zhang, D.; Zhang, X.; Qin, P. Eye-Opening Alters the Interaction Between the Salience Network and the Default-Mode Network. Neurosci. Bull. 2020, 36, 1547–1551. [Google Scholar] [CrossRef]
- Bonnelle, V.; Ham, T.E.; Leech, R.; Kinnunen, K.M.; Mehta, M.A.; Greenwood, R.J.; Sharp, D.J. Salience Network Integrity Predicts Default Mode Network Function after Traumatic Brain Injury. Proc. Natl. Acad. Sci. USA 2012, 109, 4690–4695. [Google Scholar] [CrossRef]
- Riedl, V.; Bienkowska, K.; Strobel, C.; Tahmasian, M.; Grimmer, T.; Förster, S.; Friston, K.J.; Sorg, C.; Drzezga, A. Local Activity Determines Functional Connectivity in the Resting Human Brain: A Simultaneous FDG-PET/FMRI Study. J. Neurosci. 2014, 34, 6260–6266. [Google Scholar] [CrossRef]
- Riedl, V.; Utz, L.; Castrillón, G.; Grimmer, T.; Rauschecker, J.P.; Ploner, M.; Friston, K.J.; Drzezga, A.; Sorg, C. Metabolic Connectivity Mapping Reveals Effective Connectivity in the Resting Human Brain. Proc. Natl. Acad. Sci. USA 2016, 113, 428–433. [Google Scholar] [CrossRef]
- Zhang, D.; Liang, B.; Wu, X.; Wang, Z.; Xu, P.; Chang, S.; Liu, B.; Liu, M.; Huang, R. Directionality of Large-Scale Resting-State Brain Networks during Eyes Open and Eyes Closed Conditions. Front. Hum. Neurosci. 2015, 9, 81. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, Z.; Liang, B.; Li, J.; Cai, Y.; Wang, Z.; Gao, M.; Jiao, B.; Huang, R.; Liu, M. Eyes Closed Elevates Brain Intrinsic Activity of Sensory Dominance Networks: A Classifier Discrimination Analysis. Brain Connect. 2019, 9, 221–230. [Google Scholar] [CrossRef]
- Hsu, T.-Y.; Zhou, J.-F.; Yeh, S.-L.; Northoff, G.; Lane, T.J. Intrinsic Neural Activity Predisposes Susceptibility to a Body Illusion. Cereb. Cortex Commun. 2022, 3, tgac012. [Google Scholar] [CrossRef]
- Friston, K.J.; Kahan, J.; Biswal, B.; Razi, A. A DCM for Resting State FMRI. NeuroImage 2014, 94, 396–407. [Google Scholar] [CrossRef]
- Stephan, K.E.; Penny, W.D.; Moran, R.J.; den Ouden, H.E.M.; Daunizeau, J.; Friston, K.J. Ten Simple Rules for Dynamic Causal Modeling. NeuroImage 2010, 49, 3099–3109. [Google Scholar] [CrossRef]
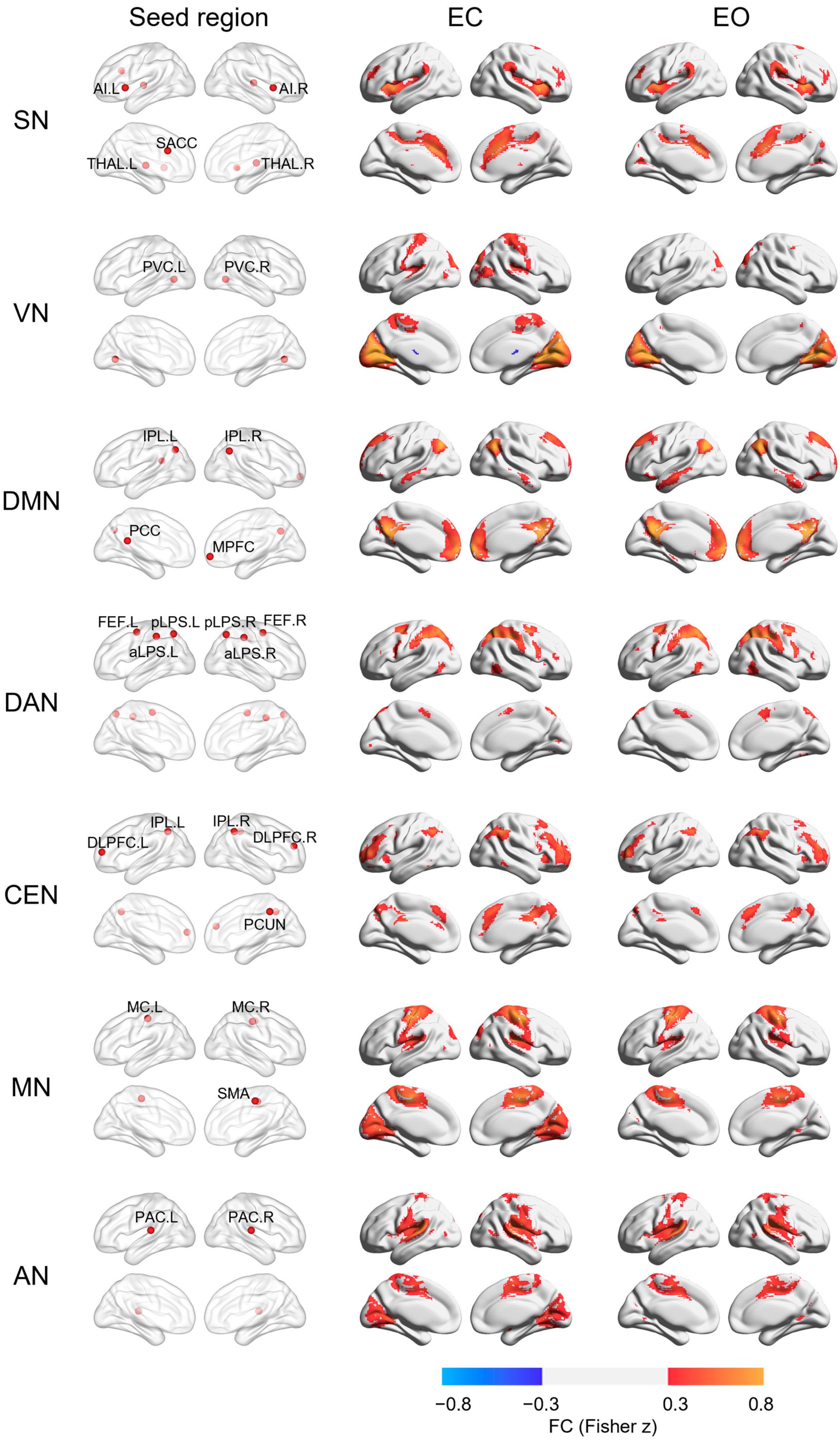
| Regions | Location | Coordinate (MNI) | References | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| SN | [20] | ||||
| SACC | M | −1 | 20 | 28 | |
| AI | L | −42 | 14 | 0 | |
| AI | R | 40 | 12 | 0 | |
| THAL | L | −12 | −16 | 4 | |
| THAL | R | 14 | −20 | 8 | |
| VN | [21] | ||||
| PVC | R | 20 | −66 | 2 | |
| PVC | L | −20 | −66 | 2 | |
| DMN | [20] | ||||
| PCC | M | 0 | −46 | 20 | |
| MPFC | M | 0 | 56 | −6 | |
| IPL | L | −42 | −68 | 38 | |
| IPL | R | 50 | −60 | 36 | |
| DAN | [21] | ||||
| pIPS | L | −26 | −65 | 52 | |
| pIPS | R | 28 | −65 | 51 | |
| FEF | L | −29 | −5 | 55 | |
| FEF | R | 31 | −5 | 54 | |
| aLPS | R | 43 | −36 | 46 | |
| aLPS | L | −45 | −37 | 48 | |
| CEN | [20] | ||||
| DLPFC | L | −36 | 52 | 10 | |
| DLPFC | R | 34 | 46 | 20 | |
| IPL | L | −40 | −56 | 44 | |
| IPL | R | 46 | −52 | 44 | |
| PCUN | M | 4 | −42 | 44 | |
| MN | [22] | ||||
| MC | L | −40 | −23 | 53 | |
| MC | R | 41 | −22 | 48 | |
| SMA | M | 1 | −18 | 49 | |
| AN | [22] | ||||
| PAC | L | −64 | −28 | 13 | |
| PAC | R | 62 | −24 | 13 | |
| RSN | Cluster (Voxel) | Peak Coordinate (MNI) | Regions within Cluster | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| SN | EC > EO | ||||
| 1523 | 1 | 25 | 52 | MPFC, MFG, SFGdor, MCC, SACC | |
| 384 | −2 | −62 | 25 | PCC, PCUN | |
| 230 | −41 | 58 | 4 | MFG, SFGdor | |
| 227 | −41 | −74 | 49 | ANG | |
| 164 | 55 | −59 | −23 | ITG, IOG | |
| 137 | 46 | −65 | 49 | IPL, ANG | |
| 136 | 49 | 25 | −8 | IFGord | |
| EO > EC | |||||
| 134 | 22 | −59 | 4 | CAL, LING | |
| 105 | −8 | −77 | 16 | CAL, LING | |
| VN | EC > EO | ||||
| 4713 | −56 | −23 | 55 | PreCG, PoCG, SMA, HES, STG | |
| 1573 | −8 | 49 | −11 | MPFC, SMA, SFGdor, MFG | |
| 943 | 13 | −65 | 19 | PCUN, CUN | |
| 253 | −20 | −104 | −2 | CAL | |
| 251 | −53 | 13 | −26 | MTP | |
| 174 | 10 | −89 | −5 | CAL | |
| 152 | 49 | −59 | 25 | ANG | |
| 98 | −23 | −17 | −20 | Hippocampus | |
| EO > EC | |||||
| 161 | 13 | −11 | 13 | THAL | |
| 107 | 1 | 31 | 22 | SACC | |
| 96 | 61 | −35 | 46 | SMG | |
| DMN | EC > EO | ||||
| 459 | −14 | −80 | 10 | CAL, CUN | |
| 342 | −38 | −56 | 61 | IPS, PreCG, PoCG | |
| 313 | 1 | 7 | 46 | SMA, SACC, MCC | |
| 163 | −41 | 1 | 7 | INS, ROL, TPOsup | |
| 152 | 31 | −56 | 55 | IPS, SMG | |
| 143 | 49 | 7 | 28 | PreCG, IFGoperc | |
| 119 | 49 | −5 | 52 | PreCG, MFG | |
| DAN | EC > EO | ||||
| 1129 | −11 | 40 | 52 | MPFC, SFGdor, MFG | |
| 513 | −14 | −107 | −5 | CAL, IOG | |
| 135 | −8 | −56 | 28 | PCC, PCUN | |
| 116 | 49 | −59 | 37 | IPL, ANG | |
| CEN | EC > EO | ||||
| 118 | −17 | 19 | −2 | CAU, PUT | |
| 109 | −23 | 49 | 40 | SFGdor | |
| MN | EC > EO | ||||
| 2487 | 13 | −101 | 5 | CAL, CUN, LING | |
| AN | EC > EO | ||||
| 1061 | −11 | −86 | 4 | CAL, CUN, LING | |
| 110 | −11 | 43 | 49 | SFGdor | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Zhou, L.; Wu, H.; Huang, Y.; Qiu, M.; Huang, L.; Lee, C.; Lane, T.J.; Qin, P. Eyes-Open and Eyes-Closed Resting State Network Connectivity Differences. Brain Sci. 2023, 13, 122. https://doi.org/10.3390/brainsci13010122
Han J, Zhou L, Wu H, Huang Y, Qiu M, Huang L, Lee C, Lane TJ, Qin P. Eyes-Open and Eyes-Closed Resting State Network Connectivity Differences. Brain Sciences. 2023; 13(1):122. https://doi.org/10.3390/brainsci13010122
Chicago/Turabian StyleHan, Junrong, Liwei Zhou, Hang Wu, Yujuan Huang, Mincong Qiu, Likai Huang, Chia Lee, Timothy Joseph Lane, and Pengmin Qin. 2023. "Eyes-Open and Eyes-Closed Resting State Network Connectivity Differences" Brain Sciences 13, no. 1: 122. https://doi.org/10.3390/brainsci13010122
APA StyleHan, J., Zhou, L., Wu, H., Huang, Y., Qiu, M., Huang, L., Lee, C., Lane, T. J., & Qin, P. (2023). Eyes-Open and Eyes-Closed Resting State Network Connectivity Differences. Brain Sciences, 13(1), 122. https://doi.org/10.3390/brainsci13010122






